Abstract
Both magnetic relaxometry and magnetic resonance imaging (MRI) can be used to detect and locate targeted magnetic nanoparticles, non-invasively and without ionizing radiation. Magnetic relaxometry offers advantages in terms of its specificity (only nanoparticles are detected) and the linear dependence of the relaxometry signal on the number of nanoparticles present. In this study, detection of single-core iron oxide nanoparticles by Superconducting Quantum Interference Device (SQUID)-detected magnetic relaxometry and standard 4.7 T MRI are compared. The nanoparticles were conjugated to a Her2 monoclonal antibody and targeted to Her2-expressing MCF7/Her2-18 breast cancer cells); binding of the nanoparticles to the cells was assessed by magnetic relaxometry and iron assay. The same nanoparticle-labeled cells, serially diluted, were used to assess the detection limits and MR relaxivities. The detection limit of magnetic relaxometry was 125,000 nanoparticle-labeled cells at 3 cm from the SQUID sensors. T2-weighted MRI yielded a detection limit of 15,600 cells in a 150 μl volume, with r1 = 1.1 mM−1s−1 and r2 = 166 mM−1s−1. Her2-targeted nanoparticles were directly injected into xenograft MCF7/Her2-18 tumors in nude mice, and magnetic relaxometry imaging and 4.7 T MRI were performed, enabling direct comparison of the two techniques. Co-registration of relaxometry images and MRI of mice resulted in good agreement. A method for obtaining accurate quantification of microgram quantities of iron in the tumors and liver by relaxometry was also demonstrated. These results demonstrate the potential of SQUID-detected magnetic relaxometry imaging for the specific detection of breast cancer and the monitoring of magnetic nanoparticle-based therapies.
Keywords: Magnetite, Nanoparticle, Magnetorelaxometry, SQUID, Magnetic Resonance Imaging, Magnetometry, Magnetic Susceptibility, Antibody targeting
1. Introduction
Although mammography has been successful at detecting breast cancer earlier and reducing breast cancer deaths (1), the ability of mammography to distinguish between benign and malignant lesions remains limited (2). Further, imaging the breasts of women with radiographically dense breast tissue, itself a risk factor for breast cancer, is a particular challenge due to the reduced x-ray contrast between malignant and normal radio-dense tissue (3). Thus, new breast cancer detection methods which are more specific and independent of radiographic density would be particularly beneficial. Although the detection of targeted radioisotopes by PET or SPECT is both sensitive and specific, nuclear medicine techniques are not ideal for screening or repeated imaging of the same patient (for example, to monitor the course of therapy) due to the cancer risk associated with the radiation dose (4). The use of imaging methods that avoid ionizing radiation, such as ultrasound and gadolinium-enhanced MRI, is becoming increasingly common in clinical breast imaging (5). Here we investigate the potential for detecting breast cancer using targeted iron oxide nanoparticles and magnetic relaxometry, a novel imaging modality. Iron oxide particles with biocompatible coatings have low toxicity (6), tissue is transparent to low-frequency (< kHz) magnetic fields, and the magnetic nanoparticles may also serve as therapeutic agents – for example, as drug carriers or mediators of localized hyperthermia (7). Methods for detecting magnetic nanoparticles include magnetic resonance imaging (MRI) (8, 9), magnetic relaxometry (10-12), magnetic particle imaging (MPI) (13, 14), and electron paramagnetic resonance (EPR) (15,16).
As a MRI contrast agent, magnetic nanoparticles are detected indirectly due to their influence on the relaxation rates of proton nuclear spins, which are an abundant signal source due to the high water content of soft tissue. Magnetic relaxometry, on the other hand, directly measures the magnetization of the nanoparticles themselves. To detect magnetic particles by magnetic relaxometry, the sample is exposed to a short DC magnetic field pulse (of order 50 G with a duration of order 1 second), which aligns the magnetic moments of the nanoparticles. After the field pulse is turned off, the relaxation (i.e., decay) of the net magnetization is detected using sensitive magnetic field sensors, such as SQUIDs. Other magnetic field detectors, such as atomic magnetometers (17) or fluxgate magnetometers (18,19) may also be used, although low-Tc SQUIDs currently have the highest sensitivity.
Magnetic relaxometry of nanoparticles for biomedical applications is a rapidly growing area of research, with recent work aimed at both in vitro bioassay (19,20) and in vivo applications (11,21-24). A particular strength of magnetic relaxometry is that the relaxation rates of bound and unbound particles differ, typically by several orders of magnitude, enabling the binding of ligand-targeted particles to their receptors to be specifically detected and quantified (20, 25, 26).
The long-term goal of the work presented here is in vivo detection and imaging of breast cancer by magnetic relaxometry, after systemic delivery of targeted nanoparticles to the patient. Achieving this goal will require continued optimization of both the nanoparticles (magnetic properties and biodistribution) and the detection method (hardware and imaging protocols). This study aims to address the latter, namely magnetic relaxometry detection and imaging performance. In the work presented here, our goal is to assess the sensitivity and imaging capability of a prototype Superconducting Quantum Interference Device (SQUID) magnetic relaxometry system, relative to standard 4.7 T MRI, for detecting and imaging magnetic nanoparticles. The sensitivity comparison was performed using in vitro samples, consisting of Her2 antibody-conjugated magnetic particles targeted to a Her2-expressing human cancer cell line (MCF7/Her2-18) embedded in an MR-visible medium. This cell line was chosen based on a previous study (24) demonstrating Her2-dependent binding of these nanoparticles to various in vitro tumor cell lines and ex vivo tumor tissue. To compare imaging by magnetic relaxometry and MRI, and demonstrate image co-registration, images of mice bearing MCF7/Her2-18 xenograft tumors were obtained after intratumoral injection of Her-2 antibody-conjugated nanoparticles.
2. Results
2.1. Nanoparticle Characterization
Size analysis (Fig. 1) of transmission electron microscopy images (Fig. 1 inset) of the SHP-20 nanoparticles indicates low size polydispersity, with a mean core diameter of 22 nm and a standard deviation of 2.3 nm. The low size polydispersity is important for the sensitivity of the magnetic relaxometry measurement, because the characteristic relaxation time of bound nanoparticles depends exponentially on the magnetic core volume (27). The Néel relaxation time is given by
| (1) |
where τ0 is customarily taken to be 10−10 s, K is the anisotropy energy density of the magnetic material (1.35 × 104 J/T for bulk magnetite), V is the volume of the magnetic core, k is Boltzmann's constant, and T is the absolute temperature. Only particles whose core diameter falls in a narrow size range, theoretically 24 ± 1 nm for magnetite, exhibit the appropriate relaxation time (on the order of 1 s) for detection by magnetic relaxometry according to Eq. 1. Fig. 1 shows that the mean diameter of the Ocean SHP-20 particles is slightly smaller than ideal, but the nanoparticle size distribution has significant intensity in the ideal size range, resulting in a detectable relaxometry signal.
Figure 1.
The iron oxide core size distribution of the Ocean SHP-20 8-30A particles indicates a mean Feret diameter of 22.1 nm with a standard deviation of 2.3 nm. The size distribution was determined from TEM images; a representative image is shown in the inset.
After conjugation of the SHP-20 nanoparticles to anti-Her2 antibodies, the particles were immobilized (by drying on a cotton swab) for magnetic characterization. Fig. 2A shows the magnetization (per unit mass of Fe3O4) as a function of the applied magnetic field. The data are consistent with superparamagnetic behavior, showing a saturation magnetization of 96 J/T/kg[Fe3O4]. Given a 5% uncertainty in the mass of nanoparticles in the sample, the saturation magnetization obtained agrees with the bulk value for magnetite (92 J/T/kg) within the experimental uncertainty. The high saturation magnetization suggests that the material is highly ordered, which is also supported by the observation of slight faceting of the particles (see TEM inset Fig. 1), suggestive of high crystallinity.
Figure 2.
Magnetic characterization of immobilized Her2-conjugated SHP-20 nanoparticles. A, M vs. B determined by DC susceptometry. The solid line is a guide to the eye. B, Imaginary component of the AC susceptibility (X″) vs. frequency. The relaxometry signal is proportional to the amplitude of X″ at ~1 Hz.
Fig. 2B shows the frequency response of the nanoparticles at temperatures ranging from body temperature (310 K) down to 273 K, measured by AC susceptometry. The relaxation times detectable by relaxometry (tens of milliseconds up to several seconds, as shown in Fig. 3 below) correspond to frequencies in the range of approximately 0.1 – 3 Hz. Ideally, the AC response would be a maximum in that frequency range at the temperature of the application. In this case, because the average nanoparticle size is slightly too small, the average particle has a relaxation rate that is too high, resulting in a maximum AC response at higher frequencies (1 kHz at body temperature). These data show that the AC loss in the 0.1 – 3 Hz range, which correlates with the relaxometry signal strength (28), is fairly strong and somewhat temperature-dependent. Slightly larger particles with even lower polydispersity would place the AC loss peak in the ideal frequency range, resulting in an even higher detected relaxometry signal.
Figure 3.
A, Diagram of relaxometry experimental scheme. B, Relaxation curves of Her2-conjugated nanoparticles in solution (gray symbols) and the same nanoparticles bound to MCF7/Her2-18 breast cancer cells (black symbols). The small signal from the nanoparticles alone suggests that a very small fraction of the nanoparticles are agglomerated, resulting in detectable relaxation times. C, Light microscopy (200× magnification, scale bar = 50 μm) of nanoparticle-labeled MCF7/Her2-18 cells. Prussian blue staining reveals the presence of iron. D, Relaxometry-detected magnetic moment vs. applied magnetic field of Her2-conjugated nanoparticles bound to 2 million MCF7/Her2-18 cells. The data show that a 4.8 mT magnetic field pulse of 0.75 s duration is adequate to saturate the observable magnetization.
2.2. In Vitro Detection by Magnetic Relaxometry and MRI
Fig. 3 shows a schematic of the magnetic relaxometry experiment (Fig. 3A), as well as representative signals for bound and unbound magnetic nanoparticles (Fig. 3B). As shown in Fig. 3A, the nanoparticles are exposed to a brief magnetizing pulse, and then, after a 40 ms settling time, the SQUID sensors may be turned on to record the decaying magnetization. In Fig. 3B, Her2-conjugated SHP-20 nanoparticles were measured by relaxometry in a cell-free solution (gray symbols), and the same quantity of particles was measured after incubation in a solution containing Her2+ breast cancer cells (black symbols). A microscope image of the nanoparticle-labeled cells is shown in Fig. 3C, with Prussian Blue stain used to show the presence of iron. In this case, the relaxation of unbound particles is dominated by Brownian rotation (29) of the particle with respect to the medium. The Brownian time constants for nanoparticles of this size (< 100 nm) are < 1 ms, much shorter than the 40 ms settling time, such that their relaxation is not detected. By contrast, a strong signal is detected after the nanoparticles bind to the cells via antibody-antigen interactions, which quenches Brownian relaxation. The immobilized particles decay by the Néel mechanism (27) involving rotation of the electronic magnetic moment in the absence of physical rotation, which is slower for sufficiently large particles, resulting in a detectable decay, as shown in Fig. 3B. Previously we have shown, using a range of cell lines, that the magnitude of the relaxometry signal from Her2-conjugated nanoparticles depends on the level of Her-2 expression, as expected (24).
The very small relaxometry signal detected from the cell-free solution likely arises from nanoparticle agglomerates. Because the Brownian relaxation time is linear in the hydrodynamic volume (29), agglomerates of sufficient size (of order 1 μm or larger) will exhibit Brownian relaxation times detectable by our method. Some agglomerates of this size are visible in Fig. 3C. The small amplitude of the relaxometry signal from the cell-free solution (relative to the signal amplitude from the cell sample) suggests that only a small fraction of the nanoparticles in the cell-free sample formed large agglomerates. In Fig. 3B, the cell-free solution consisted of PBS with 0.5% FBS, which contains only monovalent cations. Previously, we demonstrated a similarly low level of agglomeration of the same nanoparticle preparation suspended in RPMI-1640 media, which contains divalent cations (Ca and Mg) (24). Together, these results suggest that the colloidal stability of this nanoparticle preparation is not adversely affected by the presence of divalent cations, which is an important consideration for in vivo use.
Fig. 3D shows the dependence of the amplitude of the relaxometry signal on the applied magnetic field. A larger applied field amplitudes result in a greater degree of alignment of the nanoparticle moments up to ~5 mT (50 Gauss), where the relaxometry-detected magnetization saturates. Averaging the three highest values, the detected moment observed from 2 million cells is 7.05 × 104 pJ/T. An iron assay of the cells indicated that they contained 0.024 mg[Fe]. Thus, the relaxometry-detected moment per kg[Fe] is 2.9 J/T/kg[Fe] (equivalent to 2.1 J/T/kg[Fe3O4]) for the SHP-20 particles bound to cells at room temperature, comparable to the moment per kg observed from similar single-core particles studied previously (28). Theoretically, for a monodisperse ensemble of nanoparticles exhibiting a Néel relaxation time of 1 s, the relaxometry-detected moment would nearly equal the saturation magnetization of magnetite (92 J/T/kg[Fe3O4]). The moment detected experimentally by relaxometry is therefore only a few percent of the theoretical value, indicating that the majority of particles have Néel relaxation times that are much shorter or much longer than 1 s and are not detected within the acquisition window of our measurement (40 ms – 2.215 s after the magnetizing pulse). Although the nanoparticles used in this study are relatively uniform in size, further reductions in the nanoparticle polydispersity could yield an order of magnitude improvement in the sensitivity of the relaxometry method.
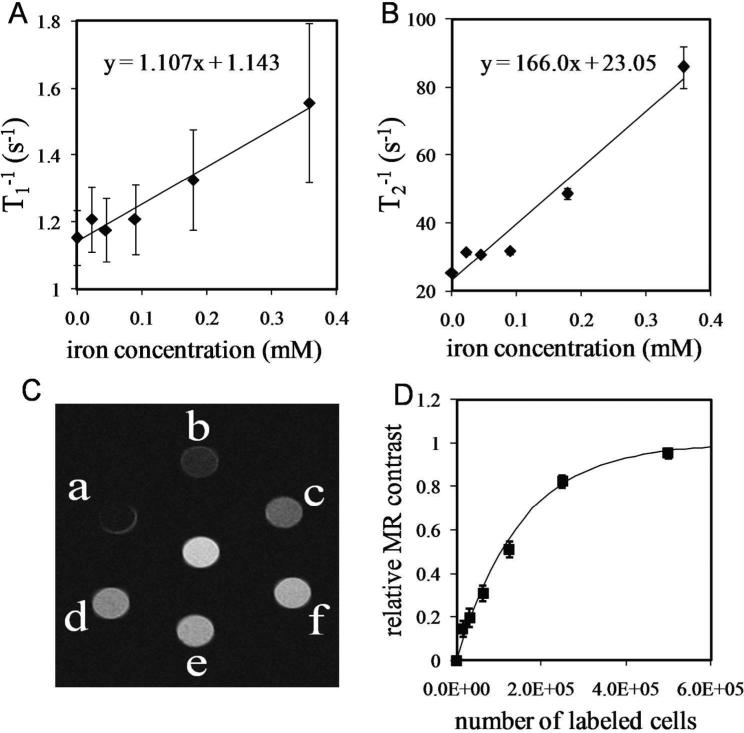
Figs. 4A and 4B show the magnetic resonance relaxation rates (T1−1 and T2−1) as a function of iron concentration for samples prepared with different numbers of MCF7/Her2-18 breast cancer cells, labeled with Her2-conjugated SHP-20 nanoparticles and embedded in agarose gel. The samples measured to produce Figs. 4A and 4B correspond to the samples b-f shown in Fig. 4C. The relaxation rates were determined by acquiring a series of images with different TR values (for T1−1) and different TE values (for T2−1) and fitting the TR- or TE-dependence of the signal intensities. The error bars represent the uncertainty in the fitted values computed by the fitting algorithm. Sample “a” from Fig. 4C was excluded from the analysis, because it was not possible to set TE short enough to obtain adequate signal-to-noise. The change in the transverse relaxation rate due to a MRI contrast agent is given by
| (2) |
where T2 is the transverse relaxation time in the presence of the nanoparticles, T2’ is the transverse relaxation time in the absence of the nanoparticles, r2 is the transverse relaxivity of the nanoparticles (mM−1s−1), and C is the iron concentration (mM). Thus, a plot of 1/T2 vs. C, as shown in Fig. 4B, has a slope equal to r2 and a y-intercept equal to 1/T2’. The longitudinal relaxivity r1 is defined similarly, by substituting a 1 for each of the subscripts in Eq. 2. As seen from the linear fits in Figs. 4A and 4B, the background relaxation times (T1’ and T2’) for the agarose gel in the absence of nanoparticles were found to be 0.90 s and 43 ms, respectively. We obtained relaxivities r1 = 1.1 mM−1s−1 and r2 = 166 mM−1s−1, yielding an r2/r1 ratio of 150. A typical r2 value for small iron oxide nanoparticles (diameter < 50 nm) is of order 100 mM−1s−1 (30-31).
Figure 4.
Magnetic resonance data obtained from in vitro samples containing different numbers of nanoparticle-labeled breast cancer cells (a = 5.0 × 105, b = 2.5 × 105, c = 1.25 × 105, d = 6.25 × 104, e = 3.13 × 104, f = 1.56 × 104 cells) in 150 μl of agarose gel. A, Longitudinal relaxation rates T1−1 and B, transverse relaxation rates T2−1 as a function of iron concentration, for samples b - f. The slopes of the linear fits are the relaxivities r1 and r2 for cell-bound SHP-20 nanoparticles. C, T2-weighted MRI (TE = 28 ms) of samples a - f. The sample at the center contains nanoparticle-free agarose gel. D, A plot of MR contrast vs. cell number for the image in 4C shows that the MR contrast (solid symbols) is sensitive and approximately linear over the 10,000 - 100,000 cell range, but saturates at higher cell number. The solid line was computed using TE = 28 ms and the r1 and r2 values obtained from Figs. 4A and 4B.
Fig. 4C shows a T2-weighted MR image of the magnetically-labeled cell samples (a-f) and a nanoparticle-free sample (center). In T2-weighted MR imaging, the signal intensity in each voxel is given by
| (3) |
where S0 is proportional to both the spin density and T1-weighting factor in the voxel, and TE is the echo time (28 ms, in this case). Relative contrast, which is plotted as a function of cell number in Fig. 4D, is calculated as
| (4) |
where S and S’ are the signal intensities of a magnetically-labeled cell sample and a nanoparticle-free sample, respectively. The solid symbols in Fig. 4D were computed using Eq. 4, with S values measured directly from the image data (Fig. 4C), using ImageJ. S was taken to be the mean value of a circular ROI centered on each tube, with the error bars determined from the standard deviation of each ROI. The solid line in Fig. 4D was calculated (without any free parameters) using Eq. 4, by calculating the S and S’ values from Eqs. 2 and 3, using the relaxivities and background relaxation rates obtained from Figs. 4A and 4B.
The relative MR contrast is useful for quantifying the iron concentration in the range where contrast varies significantly with concentration, in this case (using TE = 28 ms) for concentrations up to ~0.35 mM, corresponding to about 250,000 labeled cells. Although TE can be shortened to some extent to permit the determination of higher iron concentrations, TE values < 5 ms are not typically accessible, which places an upper limit on the iron concentration that can be accurately assessed.
The magnetic moments of samples containing between 1.6 × 104 and 2.0 × 106 cells, prepared in triplicate, were measured by magnetic relaxometry. (One of these three sets of samples was used to obtain the data in Fig. 4.) The relaxometry results are presented in Fig. 5, where each data point is the mean of three measurements (one measurement from each of three samples prepared at a given concentration), and the error bars represent the standard deviation of the three measurements. The measurement of the lowest concentration sample (1.56 × 104 cells) was excluded from the plot, due to unacceptably high uncertainty in the dipole fit. The data in Fig. 5 demonstrate the linearity of the relaxometry-detected signal. Note that the MR contrast was nearly saturated at 250,000 cells; for the samples containing 1 million and 2 million labeled cells, accurate magnetic resonance T1 and T2 values could not be obtained (T2 was too short compared to the shortest TE available), so the MR results from those samples were not included in the data presented in Fig. 4. Fig. 5 also indicates that magnetic relaxometry detection limit is currently ~125,000 MCF7/Her2-18 cells, at a distance of 3 cm from the sensors, using 10 signal averages. We note that the detection limit depends on the source-to-sensor distance, because the strength of the magnetic field from a dipole source is proportional to the inverse cube of the distance. In Fig. 4, sample f, which contains 15,600 cells, showed a detectable change in T2 contrast. Thus, at present, 4.7 T MRI has approximately an order of magnitude higher sensitivity for detecting the SHP-20 nanoparticles compared to SQUID-detected magnetic relaxometry.
Figure 5.
Detected magnetic moment vs. cell number determined by magnetic relaxometry for magnetically-labeled MCF7/Her2-18 breast cancer cells, prepared in triplicate. The data points represent the mean of three measurements, and the error bars are the standard deviation. These data indicate a detection limit of approximately 125,000 magnetically-labeled cells.
2.3. Comparison of Magnetic Relaxometry Imaging and MRI
To demonstrate imaging, MCF7/Her2-18 xenograft tumors (two per animal) were grown on the flanks of nude mice. Because magnetic relaxometry is sensitive only to immobilized nanoparticles, Her2-targeted magnetic nanoparticles were injected directly into the tumors to bind the particles to the tumor cells through antibody-antigen interactions. The intent of this experiment was to provide magnetic sources of an appropriate order of magnitude for demonstrating magnetic relaxometry imaging, based on the results of the in vitro sensitivity measurements presented above. Although physical trapping after intratumoral injection (e.g., due to clotting proteins in the needle track) can lead to some degree of nanoparticle immobilization, we have previously demonstrated that the magnetic relaxometry signal obtained with Her2-conjugated nanoparticles is significantly greater than the signal obtained from unconjugated nanoparticles, after intratumoral injection into ex vivo MCF7/Her2-18 xenografts (24).
Fig. 6 shows the results of magnetic relaxometry imaging and MRI obtained (post-mortem) from a mouse with two xenograft MCF7/Her2-18 tumors, as shown in the photograph (Fig. 6A). Each tumor was injected post-mortem with Her2-conjugated magnetic nanoparticles (0.09 mg[Fe] per tumor) to ensure a known quantity of nanoparticles would be present in the tumors. In the T2-weighted MR image (Fig. 6B), the positions of the xenograft tumors are visible as large signal voids. The magnetic relaxometry image (Fig. 6C) shows the locations of only the two magnetic sources. To obtain the magnetic relaxometry image, 2 measurements were performed at each of 5 sample positions (with 10 signal averages per position to improve signal-to-noise) resulting in a total data acquisition time of 5 minutes. By comparison, the total MR imaging time was 12.8 minutes. The relaxometry data were then fit using a dipole model, as described in the methods section, to simultaneously determine the number of magnetic sources and the strength and position of each source. The fit indicated the presence of two magnetic dipole sources; the fit parameters are summarized in Table 1. The sizes of the false color regions in Fig. 6C represent the 68% confidence intervals (i.e., ± 1 standard deviation) in the determination of the X and Y coordinates.
Figure 6.
Imaging of a nude mouse with two xenograft MCF7/Her2-18 tumors injected with Her2-conjugated magnetic nanoparticles. A, Photograph of the mouse on the relaxometry imaging stage. B, T2-weighted MRI after intratumoral injection of nanoparticles (normal gray scale). C, SQUID-detected magnetic relaxometry confidence intervals are centered at the positions of the detected dipole sources. The size of the confidence intervals indicates the uncertainty in the determination of the source position in the X and Y directions. D, Co-registry of the relaxometry confidence intervals and the MRI (reverse gray scale). The MRI (4 cm FOV) was scaled to the correct size relative to the relaxometry coordinate grid. The MRI was then translated to the same origin used for the relaxometry measurement (X=0 line coincides with the spine, Y=0 line bisects tumors).
Table 1.
Fit parameters for the relaxometry image in Fig. 6. The uncertainties are the 68% confidence intervals in each parameter.
| X (cm) | Y (cm) | Z (cm) | M (pJ/T) | |
|---|---|---|---|---|
| Right Tumor | −0.79 ± 0.29 | 0.10 ± 0.10 | 2.79 ± 0.23 | (6.36 ± 2.89) × 104 |
| Left Tumor | 0.86 ± 0.21 | 0.20 ± 0.07 | 2.58 ± 0.23 | (6.54 ± 2.77) × 104 |
The images in Fig. 6 were obtained post-mortem to ensure that the position of the mouse and the concentration of nanoparticles in the tumors did not change during transfer between the two imaging systems. Co-registration of the magnetic relaxometry and MR images (Fig. 6D) shows good agreement of the nanoparticle source positions. The source strengths can only be obtained from the relaxometry data (see Table 1), as the MR contrast is saturated.
Fig. 7 shows images acquired in vivo of a mouse with two xenograft tumors, which were injected with Her2-conjugated magnetic nanoparticles. In the in vivo case, the nanoparticles were not completely retained in the tumors after injection. At necropsy, it was observed that both tumors contained large central blood vessels, and the left tumor, in particular, showed significant necrosis at the center by histology. The necrosis and unusually large vessels are evidently the reason for non-uniform contrast observed in the tumor centers in the pre-contrast in vivo MRI (Fig. 7A). Fig. 7A, obtained before injecting the nanoparticles, demonstrates that non-uniform MR contrast (which is similar in appearance to the contrast expected from nanoparticles) can arise from endogenous causes; this underscores the need for both pre- and post-contrast MRI to properly identify the contrast arising from the magnetic particles. The post-contrast MRI (Fig. 7B) shows larger signal voids in the tumors, due to the injected nanoparticles (indicated by the blue and green ovals), as well as a decrease in the signal intensity of the liver (indicated by the red oval) compared to the pre-contrast image. Evidently nanoparticles entered the bloodstream, via the tumor vasculature, and were then sequestered in the liver. This was confirmed by in vivo magnetic relaxometry imaging (Fig. 7C) performed 1 hour after the completion of the MRI study. The magnetic relaxometry imging revealed three sources, corresponding to the positions of the tumors and the liver. The magnetic moment detected at the position of the liver suggests that roughly 1/3 of the injected nanoparticles reached the liver (Table 2).
Figure 7.
In vivo imaging of mouse with two Her2+ xenograft tumors injected intratumorally with Her2 Ab-conjugated magnetic nanoparticles. A, Pre-contrast T2-weighted MRI. The signal void (left tumor) and signal enhancements (right and left tumors) are associated with necrosis and large vessels in the tumor cores, which were subsequently observed by histology. B, Post-contrast T2-weighted MRI, showing larger signal voids and susceptibility artifacts (high intensity spots) in the tumors due to the injected nanoparticles. The approximate distribution of the nanoparticles is indicated by the blue and green dashed ovals. A post-contrast reduction in signal intensity in the liver (red dashed oval) indicates that some of the intra-tumorally injected nanoparticles entered the bloodstream. C, Post-contrast relaxometry imaging shows three magnetic sources corresponding to the positions of the left tumor (blue), right tumor (green), and liver (red); the size of the ovals corresponds to the position uncertainty (see Table 2). D, Co-registered magnetic relaxometry and post-contrast MRI (reverse gray scale). The MRI (3.84 cm FOV) was scaled to the appropriate size, and rotated and translated to put X = 0 along the spine and Y= 0 on a line bisecting the tumors.
Table 2.
Fit parameters for the relaxometry image in Fig. 7. The uncertainties are the 68% confidence intervals in each parameter.
| X (cm) | Y (cm) | Z (cm) | M (pJ/T) | |
|---|---|---|---|---|
| Left Tumor | −0.47 ± 0.48 | 0.16 ± 0.55 | 3.55 ± 0.53 | (9.2 ± 7.8) × 104 |
| Right Tumor | 1.00 ± 0.29 | 0.15 ± 0.18 | 2.10 ± 0.83 | (3.3 ± 4.9) × 104 |
| Liver | −0.24 ± 0.27 | 2.13 ± 0.75 | 3.17 ± 0.78 | (6.36 ± 2.89) × 104 |
Compared to the post-mortem (295 K) relaxometry imaging results (Fig. 6C, Table 1), the in vivo (310 K) relaxometry data (Fig. 7C, Table 2) show higher uncertainty in the tumor positions and the magnetic moments, due to the reduced signal-to-noise (SNR) of each source. Both the loss of particles to the liver and the temperature-dependence of the relaxometry signal (see Fig. 2B) contribute to the reduced SNR. Nevertheless, the relaxometry image correctly identifies the number of magnetic sources, and the positions are in reasonable agreement with the nanoparticle locations identified by MRI.
To complement the in vivo relaxometry imaging results, the magnetic moments of the tumors and liver were measured post-mortem, by excising the individual tissues and measuring the relaxometry-detected magnetic moment of each tissue at room temperature (295 K) and at body temperature (310 K) using a non-magnetic heating pad. As shown in Fig. 8, the precision of the post-mortem measurements is significantly improved compared to the in vivo imaging measurement, and the post-mortem measurements confirm that the detected moment decreases at 310 K (relative to 295 K), as expected based on Fig. 2B. The higher precision of the post-mortem quantification can be attributed to two factors: 1) the excised tissue was positioned closer to the SQUID sensor array, resulting in a larger magnetic field at the sensors, and 2) a simpler (single dipole source) model was sufficient to analyze the data.
Figure 8.
SQUID-detected relaxometry measurements of the magnetic moment of the tumors and the liver from the mouse shown in Fig. 7. Measurements were obtained post-mortem at room temperature (black bars), and at body temperature (dark gray bars) for comparison to the in vivo results (light gray bars). The post-mortem measurements are more accurate than the in vivo quantification due to the smaller distance between the sensors and the source. The post-mortem measurements confirm the temperature-dependence of the relaxometry signal predicted by Fig. 2B.
The magnetic moments measured by relaxometry are converted to iron content by dividing the measured magnetic moment by the relaxometry-detected moment per kg[Fe] determined from a calibration sample (at the same temperature). In this study, the magnetic moment for 2 million cells was measured by relaxometry at room temperature, and the iron content was determined by iron assay, as described above, resulting in a calibration factor of 2.9 J/T/kg[Fe]. The post-mortem magnetic moments of the excised tissues (measured at room temperature) therefore correspond to 12, 25, and 24 μg[Fe] for the left tumor, right tumor, and liver, respectively. Thus, magnetic relaxometry is a potentially useful laboratory tool for magnetic nanoparticle development, capable of quantitatively assessing nanoparticle biodistribution in animal models.
3. Discussion
In MRI, the presence of magnetic nanoparticles is detected due to a change in 1H NMR relaxation rates (1/T1 and 1/T2), which alters the image contrast in the vicinity of the nanoparticles. If the change in image intensity is similar to the endogenous intensity variation in the tissue of interest, then both a pre-contrast and a post-contrast image are required to locate contrast arising specifically due to nanoparticles. Co-registration of pre- and post-contrast images may be difficult if the subject is conscious and there is a delay between images. Although the background anatomy is depicted with good contrast and high resolution in MRI, it may interfere with the identification of magnetically-labeled cancer cells.
By contrast, SQUID detectors are directly sensitive to the relaxing magnetic fields of the nanoparticles themselves, and further, only bound nanoparticles have the appropriate relaxation time to be detected. Thus, the background is virtually zero, as there are no endogenous sources of magnetism in the body with the same temporal characteristics as bound magnetic nanoparticles. The lack of endogenous background and the lack of background from unbound particles are expected to greatly simplify the interpretation of detected signals, eliminating the need for a pre-contrast image.
Although SQUID-relaxometry cannot depict background anatomy, relaxometry may be readily integrated with SQUID-detected ultra-low-field MRI (ULF-MRI) (33,34) for accurate co-registry with anatomy. In an integrated system, the relaxometry and ULF-MRI could be acquired in one imaging session (i.e., one patient position) using the same SQUID sensor array for relaxometry and MRI, such that co-registration would be automatic. Such a system would be analogous to systems used in acquiring co-registered PET and CT images, where PET imaging provides specificity by showing the location of the uptake of a specific radioactive tracer, and CT is used to depict the background anatomy.
A significant advantage of magnetic relaxometry is the ability to quantify bound magnetic nanoparticles, because the detected moment is linear in the number of nanoparticles. In comparison, MRI contrast is non-linear and saturates at concentrations greater than ~1 mM[Fe]. Although T2-imaging (as opposed to T2-weighted imaging) is time-consuming, it would theoretically provide a more accurate measure of iron concentration; however, in clinical MRI it is not possible to make the echo time TE short enough (<< 5 ms) to enable accurate T2 imaging in voxels where the iron concentration exceeds ~1 mM[Fe]. On the other hand, the relaxometry signal is linear in the number of nanoparticles and does not depend on their concentration. Therefore, relaxometry may be better suited for therapeutic monitoring applications where fast and accurate measurement of a high concentration of magnetic nanoparticles is required. For example, in magnetic fluid hyperthermia therapy, the iron concentration of nanoparticle solution injected into the tumors can be as high as 2 M (35, 36) more than an order of magnitude greater than the concentration injected in the tumors in this study. In terms of guiding magnetic fluid hyperthermia, the current spatial resolution of our prototype SQUID system (spatial accuracy ~1 mm, resolution of distinct sources ~5 mm, for strong sources) is more than adequate to enable proper positioning of the coil used to generate the alternating magnetic fields used to heat the nanoparticles.
Although the sensitivity of the prototype SQUID system is currently lower than MRI for detecting the particles used here, the sensitivity of SQUID-detected relaxometry can be significantly increased through improvements in both the hardware and the nanoparticle properties. For example, the addition of RF shielding and vibration isolation is expected to reduce noise, due to RF interference and motion of the sensors in the Earth's field, by at least one order of magnitude at one Hz (37). Optimizing the average nanoparticle size and reducing the size polydispersity can theoretically increase the detected moment per kg of magnetic material by more than an order of magnitude (28). Furthermore, larger SQUID sensor arrays, already available commercially, will decrease the measurement time further while increasing localization accuracy.
4. Conclusions
In summary, MRI is currently more sensitive for detecting the Her2-conjugated magnetic nanoparticles used in this study; however, MRI is less specific, because non-uniform contrast may also arise due to endogenous factors. In addition to being more specific for detecting magnetic particles, SQUID-detected magnetic relaxometry enables accurate quantification over a wider range of nanoparticle concentrations compared to MRI. Relative to MRI, SQUID-detected magnetic relaxometry is faster to perform, and the costs associated with the hardware and siting are expected to be much lower. Magnetic relaxometry could be readily combined, on the same platform, with ULF-MRI for depicting anatomy, in a similar manner to combining PET and CT. We expect that realistically achievable improvements in the detection system and the nanoparticles will result in an increase in our sensitivity of two orders of magnitude or more, such that the sensitivity of SQUID-detected magnetic relaxometry will eventually exceed that of MRI for the detection of magnetic nanoparticles targeted to breast cancer and other diseases.
5. Experimental
5.1. Nanoparticles and Anti-body Conjugation
SHP nanoparticles (Ocean Nanotech; Springdale, AR, USA) are single-core magnetite particles, coated with a thin (~3-4 nm thick) layer of polymer and functionalized with carboxyl groups to enable conjugation to antibodies. Ocean SHP-20 Lot 8-30A was used for all experiments. The iron concentration (mg[Fe]/ml) of the stock solution was determined to be 4.2 mg/ml by dissolving in acid, forming the phenanthroline/Fe2+ complex, and quantifying the concentration of a known dilution spectrophotometrically (38). The phenanthroline assay was also used to determine the iron content of nanoparticle-labeled cell preparations.
The carboxyl-functionalized nanoparticles were conjugated to anti-Her2/neu antibody by the carbodiimide method according to a protocol described previously (21) with the exception of centrifugation speed, which was increased to 7500 g to account for the use of smaller nanoparticles. Briefly, 10 mg[Fe] of nanoparticles were conjugated to 50 μg of Her-2/neu antibody (Bender MedSystems; Burlingame, CA, USA), then washed twice and resuspended in 800 μl double distilled water to which 200 μl phosphate buffered saline (PBS) (Gibco-BRL; Rockville, MD, USA) with 0.5% fetal bovine serum (FBS) (HyClone; Logan, UT, USA) was added. Conjugated nanoparticles were stored at 4 °C for up to 20 weeks with no loss of stability.
5.2. TEM and Image Analysis
The nanoparticles were imaged by transmission electron microscopy using a Tecnai G2 F30 at 300 kV (FEI Corporation; Hillsboro, Oregon, USA). Size distributions were determined from the TEM images using ImageJ (public domain software, National Institutes of Health; Bethesda, MD, USA). Briefly, the Feret diameter (defined as the maximum caliper diameter) was measured from a sample of 2100 particles selected from multiple TEM images. Particles in contact with the edge of an image were automatically excluded, and overlapping particles were manually excluded from the size analysis.
5.3. SQUID-susceptometry
DC and AC magnetic characterizations were performed using an MPMS-7 SQUID magnetometer system (Quantum Design; San Diego, CA, USA). DC Magnetization curves were acquired by equilibrating the sample at the measurement temperature in zero field, then incrementally increasing the field and pausing 100 s at each field before measurement. Five sequential measurements were taken at each field, a mean of those measurements calculated, and the three values with the lowest deviation from the mean were averaged and reported as the moment. Zero Field Cooled (ZFC) curves were determined by cooling the sample in the absence of a magnetic field to 5 K, then slowly warming in a 1 mT field. After thermally equilibrating at a target temperature, a series of five measurements was taken and the values processed as described to obtain the magnetization value. AC susceptibility curves were obtained by applying a 0.2 mT (159 A/m) AC magnetic field at various frequencies (f = 0.1 – 1000 Hz) to a thermally equilibrated sample. The in-phase (χ’) and out-of-phase (χ”) AC susceptibilities of the sample were digitized and recorded.
5.4. In Vitro Cell Labeling
MCF7 cells overexpressing Her-2 antigen (Bender Medsystems, Burlingame, CA, USA), designated MCF7/Her2-18, were kindly provided by Mien-chi Hung (Benz, 1992). MCF7/Her2-18 cells were harvested and washed using sterile PBS (Gibco-BRL). Each sample contained 8 × 106 cells suspended in 200 μl of cold PBS to which 0.24 mg[Fe] of Her2 antibody-conjugated Ocean SHP-20 Lot 8-30A nanoparticles were added. Cells and Her2-nanoparticles were incubated on ice with SQUID-relaxometry measurements (described below) taken at 1, 3, 5, 7, 9, 11, 13 and 15 minutes. For light microscopy, cytospin slides were prepared by adding 200 μl of albumin solution, from bovine serum (Sigma, St. Louis, MO, USA) with 5 μl of cell/NP sample to a cytofunnel. The slides were then placed in the Shandon Cytospin 4 machine (Thermo Scientific, Waltham, MA) and centrifuged at 1100 × g for 7 minutes. Slides were stained with Prussian Blue stain, which reveals the presence of iron. For Prussian blue stain, slides were then fixed by dipping 5 times in 0.01% sodium azide in 1 g/l Xanthene Dye (Siemens, Newark, DE, USA). Potassium ferrocyanide solution was prepared fresh with a 1:1 solution of 20% of HCL (EMD Chemicals Inc., Gibbstown, NJ, USA) and 10% potassium ferrocyanide (Sigma, St. Louis, MO, USA). The potassium ferrocyanide solution was applied directly to the cell sample on the slide and incubated in the dark for 20 minutes. Brazilliant (Anatech Ltd, Battle Creek, MI, USA) was applied directly to the cell sample on the slide and incubated in the dark for 5 minutes. Following Prussian Blue or Diff-Quik (Siemens, Newark, DE, USA) staining, the slides were dipped in double distilled water three times and allowed to dry. The slides were then coverslipped with Cytoseal XYL (Richard-Allan Scientific, Kalamazoo, MI, USA).
After incubation with nanoparticles, the 8 million cells were washed with PBS (by centrifugation and removal of supernatant) to remove unbound particles, resuspended in PBS, and divided; 4 million cells were serially diluted (by factors of 2) with PBS to produce a series of 8 samples (each 50 μl) with cell numbers ranging from 2 million down to 15,600 cells. Each 50 μl cell sample was subsequently mixed with 100 μl 1% low melting temperature agarose gel (SeaPlaque Agarose, Cambrex, Rockland, ME, USA) with a final concentration of 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in a 5 mm NMR tube to produce a uniform concentration of labeled cells in a total volume of 150 μl for MR relaxivity and SQUID-detected magnetic relaxometry measurements.
5.5. SQUID-detected magnetic relaxometry
Detection of nanoparticles by relaxometry was performed using a BTi 2004 seven-channel low-temperature SQUID array (4D-Neuroimaging; San Diego, CA, USA) originally designed for magnetoencephalography. Second order gradiometers with a baseline of 4.0 cm are used to reject background magnetic fields due to distant sources, allowing the measurements to be performed without magnetic shielding. Due to RF interference, the sensitivity of the sensors is currently limited to ~10−12 T/√Hz. The seven gradiometer coils are located at the bottom of the liquid He dewar, arranged with six in a circle of 2.0 cm radius and one at the center. For in vitro measurements on small samples, the sample is located at a distance z ≈ 3 cm below the bottom of the center gradiometer. The samples are uniformly magnetized (parallel to the center gradiometer axis) using a pair of 49 cm diameter, 100 turn Helmholtz coils powered by a 5 kW current-regulated supply (Sorenson SGA 80/63). The current through the magnetizing coils is monitored by a Hall Effect transducer to assure constant magnetic fields. The decaying magnetization is sampled at a rate of 8 kHz (beginning 40 ms after switching off the magnetizing pulse), digitized using a National Instruments PXI8336 16-channel digitizer, and acquired using software written in LabWindows™/CVI (National Instruments, Austin, TX, USA). Acquired data is decimated by a factor of 8 (down to 1 kHz) to improve signal-to-noise. Our standard measurement protocol is to apply a 4.9 mT field for 0.75 s and then acquire data for 2.215 s, with 10 repetitions at each sample position, subsequently averaged. Magnetization curves (M vs. B) are obtained, using the same apparatus and timing, by varying the applied field between 0 and 5.8 mT.
The data are analyzed using the Multi-Source Analysis (MSA) program, a software package developed in our lab, also written in LabWindows™/CVI. After signal averaging and removal of 60 Hz line frequency contamination, background data (acquired when no magnetic source is present) is subtracted from the sample data. The seven relaxation curves are then fit with a logarithmic function (39), in order to determine and remove the DC offset. Next, the first 200 ms of the recorded signal decay is fit with an exponential function to obtain the magnetic field amplitude. Finally, we obtain the location (X, Y, Z) and magnetic moment (Mz) of each source by modeling the sample as one or more discrete magnetic dipoles. The magnetic inverse problem is solved using the Levenberg-Marquardt algorithm to perform a non-linear least-squares fit to the dipole model. Each magnetic dipole is assumed to be oriented along the z axis, i.e., in the direction of the magnetizing field. Thus, for a sample containing N magnetic sources, there are 4N parameters to be determined. This is accomplished by acquiring data at n different sample positions (equivalent to a sensor array with 7n elements) such that 7n ≥ 4N.[notdef]
To obtain the desired Néel relaxation, characteristic of bound nanoparticles (27) the particles must be immobilized. In the case of cell samples, the antibody-conjugated nanoparticles were immobilized by the binding of the antibodies attached to the nanoparticles to receptors on the cell surface. Alternatively, nanoparticles were immobilized by applying an aliquot of nanoparticle solution to a Q-tips cotton swab (Unilever, Trumball, CT, USA) and allowing it to dry in air.
5.6. In Vitro MRI
MRI of in vitro samples was performed on a Bruker Biospin 4.7 T/40 cm MRI system. T2-weighted images of the sample bundle were acquired using the multi-slice multi-echo (MSME) sequence with TR = 3500 ms and TE = 14, 28, and 42 ms. To measure the longitudinal relaxivity (r1), T1 imaging of the sample bundle was performed using an inversion recovery (IR)-prepared FISP sequence with eight IR values ranging from 100 – 856 ms. To measure the transverse relaxivity (r2), T2 imaging was performed using the MSME sequence with sixteen TE values ranging from 7.4 – 118.8 ms. The analysis of the resulting T1 and T2 image series was performed using built-in Bruker ParaVision software routines.
5.7. Imaging of Mice by MRI and Magnetic Relaxometry
Athymic nude mice were obtained from Harlan Laboratories (Frederick, MD, USA). Mice were handled in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of New Mexico. Four days prior to tumor cell injection, mice were implanted with a 17β-estradiol pellet (Innovative Research of America, Sarasota, FL, USA). Mice were injected with 2 × 106 MCF-7/Her2-18 cells in Matrigel (BD Biosciences, USA) in each hind limb flank. Mice were monitored every other day and tumor growth was measured weekly.
For post-mortem imaging studies, mice were euthanized by an overdose of isoflurane (Phoenix Pharmaceutical, Inc., St. Joseph, MO, USA) followed by cervical dislocation immediately prior to nanoparticle injection and imaging. An aliquot containing 0.09 mg[Fe] of Her2 antibody-conjugated Ocean SHP-20 Lot 8-30A nanoparticles was injected directly into each tumor. SQUID-detected magnetic relaxometry imaging was then performed as described above, using 2 acquisitions at each of 5 sample stage positions, resulting in a total acquisition time of 5 min. Immediately following magnetic relaxometry imaging, mice were frozen and stored at −80°C. Subsequently, MR images of the same mice (post-mortem, room temperature) were obtained at 4.7 T using an Oxford horizontal bore magnet (Oxford Instruments, Oxfordshire, UK) and a Varian console (Agilent Technologies, Santa Clara, CA, USA). The pulse sequence was a T2-weighted multi-slice Fast Spin Echo sequence (TR = 3000 ms, TEeff = 40 ms, NEX = 8, turbo factor = 8) requiring a total imaging time of 12.8 min. The in-plane resolution was 0.20 mm and the slice thickness was 2.0 mm.
For in vivo imaging studies, pre-contrast MRI was performed on a Bruker Biospin (Billerica, MA, USA) 4.7 T/40 cm MRI system. T2-weighted images of the mouse were acquired using a multi-slice RARE sequence (RARE factor = 8, NA = 8, TR = 3500 ms and TEeff = 47 ms), resulting in a total acquisition time of 15 minutes. The slice thickness was 1.0 mm and the in-plane resolution was 0.15 mm (FOV = 3.84 cm). Isoflurane anesthesia was maintained throughout the MRI procedures. An aliquot containing 0.09 mg[Fe] Her2-conjugated Ocean SHP-20 Lot 8-30A nanoparticles was then injected into each tumor, without removing the mouse from the MRI mouse holder. MR images were then obtained (15 minutes post-contrast) using the same sequence parameters and sample position. Immediately following the MRI procedures, the mouse was removed from anesthesia and transported to another facility for relaxometry imaging. Isoflurane anesthesia was re-established, and SQUID-detected magnetic relaxometry imaging was performed (75 minutes post-contrast) as described above, using 6 sample stage positions (6 min. imaging time). After the in vivo magnetic relaxometry imaging was completed, euthanasia was performed, and the tumors and liver were excised; their nanoparticle contents were then quantified individually by magnetic relaxometry.
Co-registration of the magnetic relaxometry and MRI images was performed as follows. First, the geometric factors in the magnetic relaxometry data analysis software (constants specifying the positions and angles of the seven 2nd order gradiometers) were calibrated using phantoms containing small nanoparticle sources located at known positions in the x, y, and z directions. Next, the location of the origin of the centimeter grid affixed to the mouse platform was aligned with the origin (x=0, y=0) of the SQUID array using a small nanoparticle phantom (~1 mm sphere) affixed to the mouse platform. The mouse was then positioned so that a line bisecting the center of the tumors coincided with x=0 line on the grid, and the line coinciding with the mouse's spine coincided with the y=0 line on the grid, and a photograph was obtained. Thus the Cartesian coordinate system of the relaxometry image could be immediately co-registered with the photograph, which shows the centimeter grid. The MR images were scaled, using the known field-of-view in the x and y directions, to match the scale of the magnetic relaxometry image. Next, the line bisecting the tumors and the line coincident with the spine were manually marked on the MRI, and these axes were brought into coincidence with the x- and y-axes of the magnetic relaxometry image by rotation and translation of the MRI. The uncertainty in the co-registration is ± 1 mm in each direction.
Acknowledgements
NLA acknowledges helpful discussions with Mark Conradi and John Dixon. This work was supported by the National Institutes of Health under grants RAI066765B, RCA096154B, RCA105742B, and RCA123785B (to ERF) and by the Howard Hughes Medical Institute under a Medical Research Training Fellowship (JET). This work was performed, in part, at the Center for Integrated Nanotechnologies, a U.S. Department of Energy, Office of Basic Energy Sciences user facility. Sandia National Laboratories is a multi-program laboratory operated by Sandia Corporation, a Lockheed-Martin Company, for the U.S. Department of Energy under Contract No. DE-AC04-94AL85000. NLA has equity interests in ABQMR and nanoMR; neither company sponsored this work.
References
- 1.Hellquist BN, Duffy SW, Abdsaleh S, Björneld L, Bordás P, Tabár L, Viták B, Zackrisson S, Nyström L, Jonsson H. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2011;117(4):714–22. doi: 10.1002/cncr.25650. [DOI] [PubMed] [Google Scholar]
- 2.Kopans DB. Breast Imaging. Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 3.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 4.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257(1):246–53. doi: 10.1148/radiol.10100570. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein S, Rosen M. Breast MR imaging: current indications and advanced imaging techniques. Radiol Clin North Am. 2010;48(5):1013–42. doi: 10.1016/j.rcl.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–27. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Wu W, Wang X. Magnetic Iron Oxide Nanoparticles for Tumor-targeted Therapy. Curr Cancer Drug Targets. 2011;11(2):184–9. doi: 10.2174/156800911794328475. [DOI] [PubMed] [Google Scholar]
- 8.Gossuin Y, Gillis P, Hocq A, Vuong QL, Roch A. Magnetic resonance relaxation properties of superparamagnetic particles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(3):299–310. doi: 10.1002/wnan.36. [DOI] [PubMed] [Google Scholar]
- 9.Laurent S, Boutry S, Mahieu I, Vander Elst L, Muller RN. Iron oxide based MR contrast agents: from chemistry to cell labeling. Curr Med Chem. 2009;16(35):4712–27. doi: 10.2174/092986709789878256. [DOI] [PubMed] [Google Scholar]
- 10.Flynn ER, Bryant HC. A biomagnetic system for in vivo cancer imaging. Phys Med Biol. 2005;50(6):1273–93. doi: 10.1088/0031-9155/50/6/016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge S, Shi X, Baker JR, Jr, Banaszak Holl MM, Orr BG. Development of a remanence measurement-based SQUID system with in-depth resolution for nanoparticle imaging. Phys Med Biol. 2009;54(10):N177–88. doi: 10.1088/0031-9155/54/10/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter H, Kettering M, Wiekhorst F, Steinhoff U, Hilger I, Trahms L. Magnetorelaxometry for localization and quantification of magnetic nanoparticles for thermal ablation studies. Phys Med Biol. 2010;55(3):623–33. doi: 10.1088/0031-9155/55/3/005. [DOI] [PubMed] [Google Scholar]
- 13.Weizenecker J, Gleich B, Rahmer J, Dahnke H, Borgert J. Three-dimensional real-time in vivo magnetic particle imaging. Phys Med Biol. 2009;54(5):L1–L10. doi: 10.1088/0031-9155/54/5/L01. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson RM, Minard KR, Krishnan KM. Optimization of nanoparticle core size for magnetic particle imaging. J Magn Magn Mater. 2009;321(10):1548–51. doi: 10.1016/j.jmmm.2009.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radermacher KA, Beghein N, Boutry S, Laurent S, Vander Elst L, Muller RN, Jordan BF, Gallez B. In vivo detection of inflammation using pegylated iron oxide particles targeted at E-selectin: a multimodal approach using MR imaging and EPR spectroscopy. Invest Radiol. 2009 Jul;44(7):398–404. doi: 10.1097/rli.0b013e3181a49639. [DOI] [PubMed] [Google Scholar]
- 16.Radermacher KA, Boutry S, Laurent S, Elst LV, Mahieu I, Bouzin C, Magat J, Gregoire V, Feron O, Muller RN, Jordan BF, Gallez B. Iron oxide particles covered with hexapeptides targeted at phosphatidylserine as MR biomarkers of tumor cell death. Contrast Media Mol Imaging. 2010 Sep-Oct;5(5):258–67. doi: 10.1002/cmmi.382. [DOI] [PubMed] [Google Scholar]
- 17.Knappe S, Sander TH, Kosch O, Wiekhorst F, Kitching J, Trahms L. Cross-validation of microfabricated atomic magnetometers with superconducting quantum interference devices for biomagnetic applications. Appl Phys Lett. 2010;97(13):133703, 1–3. [Google Scholar]
- 18.Ludwig F, Heim E, Schilling M, Enpuku K. Characterization of superparamagnetic Fe3O4 nanoparticles by fluxgate magnetorelaxometry for use in biomedical applications. J Appl Phys. 2008;103(7):07A314, 1–3. [Google Scholar]
- 19.Heim E, Ludwig F, Schilling M. Binding assays with streptavidin-functionalized superparamagnetic nanoparticles and biotinylated analytes using fluxgate magnetorelaxometry. J Magn Magn Mater. 2009;321(10):1628–31. [Google Scholar]
- 20.Eberbeck D, Wiekhorst F, Steinhoff U, Schwarz K O, Kummrow A, Kammel M, Neukammer J, Trahms L. Specific binging of magnetic nanoparticle probes to platelets in whole blood detected by magnetorelaxometry. J Magn Magn Mater. 2009;321(10):1617–20. [Google Scholar]
- 21.Jaetao JE, Butler KS, Adolphi NL, Lovato DM, Bryant HC, Rabinowitz I, Winter SS, Tessier TE, Hathaway HJ, Bergemann C, Flynn ER, Larson RS. Enhanced leukemia cell detection using a novel magnetic needle and nanoparticles. Cancer Res. 2009;69(21):8310–6. doi: 10.1158/0008-5472.CAN-09-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tietze R, Jurgons R, Lyer S, Schreiber E, Wiekhorst F, Eberbeck D, Richter H, Steinhoff U, Trahms L, Alexiou C. Quantification of drug-loaded magnetic nanoparticles in rabbit liver and tumor after in vivo administration. J Magn Magn Mater. 2009;321(10):1465–8. [Google Scholar]
- 23.Adolphi NL, Huber DL, Jaetao JE, Bryant HC, Lovato DM, Fegan DL, Venturini EL, Monson TC, Tessier TE, Hathaway HJ, Bergemann C, Larson RS, Flynn ER. Characterization of magnetite nanoparticles for SQUID-relaxometry and magnetic needle biopsy. J Magn Magn Mater. 2009;321(10):1459–64. doi: 10.1016/j.jmmm.2009.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hathaway HJ, Butler KS, Adolphi NL, Lovato DM, Belfon R, Fegan D, Monson TC, Trujillo JE, Tessier TE, Bryant HC, Huber DL, Larson RS, Flynn ER. Detection of Breast Cancer Cells using Targeted Magnetic Nanoparticles and Ultra-Sensitive Magnetic Field Sensors. Breast Cancer Research. doi: 10.1186/bcr3050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemla YR, Grossman HL, Poon Y, McDermott R, Stevens R, Alper MD, Clarke J. Ultrasensitive magnetic biosensor for homogeneous immunoassay. Proc Natl Acad Sci. 2000;97(26):14268–72. doi: 10.1073/pnas.97.26.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enpuku K, Tanaka T, Matsuda T, Dang F, Enomoto N, Hojo J, Yoshinaga K, Ludwig F, Ghaffari F, Heim E, Schilling M. Properties of magnetic nanoparticles in the Brownian relaxation range for liquid phase immunoassays. J Appl Phys. 2007;102(5):054901, 1–7. [Google Scholar]
- 27.Néel L. Some theoretical aspects of rock-magnetism. Adv Phys. 1955;4(14):191–243. [Google Scholar]
- 28.Adolphi NL, Huber DL, Bryant HC, et al. Characterization of single-core magnetite nanoparticles for magnetic imaging by SQUID relaxometry. Phys Med Biol. 2010;55(19):5985–6003. doi: 10.1088/0031-9155/55/19/023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown WF. Thermal Fluctuations of a Single-Domain Particle. Phys Rev. 1963;130(5):1677–86. [Google Scholar]
- 30.Bjørnerud A, Johansson LO, Briley-Saebø K, Ahlström HK. Assessment of T1 and T2* effects in vivo and ex vivo using iron oxide nanoparticles in steady state-dependence on blood volume and water exchange. Magn Reson Med. 2002;47(3):461–71. doi: 10.1002/mrm.10066. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Wängler B, Morgenstern B, Zentgraf H, Eisenhut M, Untenecker H, Krüger R, Huss R, Seliger C, Semmler W, Kiessling F. Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging. Langmuir. 2007;23(3):1427–34. doi: 10.1021/la061879k. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita T, Kusakabe Y, Fujii H, Murase K, Yamazaki Y, Murase K. Inflammatory imaging with ultrasmall superparamagnetic iron oxide. Magn Reson Imaging. 2011;29(2):173–8. doi: 10.1016/j.mri.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Zotev VS, Matlachov AN, Volegov PL, Sandin HJ, Espy MA, Mosher JC, Urbaitis AV, Newman SG, Kraus RH., Jr Multi-channel SQUID system for MEG and ultra-low-field MRI. IEEE Trans Appl Supercond. 2007;17(2):839–842. [Google Scholar]
- 34.Zotev VS, Volegov PL, Matlashov AN, Espy MA, Mosher JC, Kraus RH., Jr Parallel MRI at microtesla fields. J Magn Reson. 2008;192(2):197–208. doi: 10.1016/j.jmr.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johannsen M, Gneveckow U, Thiesen B, et al. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur Urol. 2007;52(6):1653–61. doi: 10.1016/j.eururo.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 36.van Landeghem FK, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30(1):52–7. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 37.Koch RH, Foglietti V, Rozen JR, et al. Effects of radio frequency radiation on the dc SQUID. Appl Phys Lett. 1994;65(1):100–2. [Google Scholar]
- 38.ASTM E394-00, Standard Test Method for Iron in Trace Quantities Using the 1,10-henanthroline Method. 2000.
- 39.Street R, Woolley JC. A study of magnetic viscosity. Proc Phys Soc A. 1949;62(9):562–72. [Google Scholar]