Abstract
Although most cells are thought to respond to interferons, there is limited information regarding specific cells that respond in vivo. Viperin is an interferon-induced antiviral protein and therefore is an excellent marker for interferon-responsive cells. Here we analyzed viperin expression in vivo during acute LCMV Armstrong infection, which induces high levels of Type I IFNs, and in persistently infected LCMV carrier mice, which contain low levels of Type I IFNs. Viperin was induced in lymphoid cells and DCs during acute infection and highly induced in neutrophils and macrophages. The expression kinetics in neutrophils, macrophages, T and B cells paralleled IFNα levels, but DCs expressed viperin with delayed kinetics. In carrier mice, viperin was expressed in neutrophils and macrophages, but not T and B cells or DCs. For both acutely infected and carrier mice, viperin expression was IFN-dependent, as treating Type I IFNR knockout mice with IFNγ neutralizing antibodies inhibited viperin expression. Viperin localized to the endoplasmic reticulum and lipid droplet-like vesicles in neutrophils. These findings delineate the kinetics and cells responding to interferons in vivo and suggest that the profile of interferon-responsive cells changes in chronic infections. Furthermore, these data suggest that viperin may contribute to the antimicrobial activity of neutrophils.
Introduction
Type I interferons (IFNs) are produced in the context of viral infections and induce a potent anti-viral response that activates innate immunity and leads to a heightened antiviral state. Virally infected cells produce and secrete Type I IFNs, notably IFNα and IFNβ, that activate neighboring cells and alert them to ongoing infection. Upon IFN stimulation, cells that express the Type I IFN receptor (IFNAR) undergo a complex signaling cascade that leads to the induction of hundreds of genes and limits viral infection. Although many of the functions of these gene products are still unknown, several of them have dramatic effects on cells, halting protein synthesis and inhibiting cellular proliferation (1, 2).
Although IFN production during many different viral infections has been well characterized, little is known about the ensuing cellular response. While most tissues and cell lines express the IFNAR transcript to varying degrees, there is increasing evidence that a number of positive and negative regulatory molecules can modulate both the intensity and kinetics of IFNAR signaling (3). Furthermore, although low levels of IFNs are thought to persist throughout chronic viral infections (4–6), the levels are generally below the limit of detection and are difficult to measure. Both the challenge of detecting IFNs in vivo and the lack of a good marker for IFN stimulation have made it difficult to evaluate the nature and extent of the IFN response during various infections.
Viperin is one of the most highly induced interferon effector proteins (7, 8). Similar to other well-characterized IFN-induced effector proteins, viperin is rapidly induced upon interferon stimulation or infection with various viruses. Viperin, also known as RSAD2, cig5 in humans, and vig1 in mice, was originally identified as a gene induced in fibroblasts upon human cytomegalovirus (HCMV) infection (7). Subsequent analyses have shown that viperin is induced in a variety of cell types by both Type I and Type II interferons, poly I:C, dsRNA, viral DNA, and LPS(9–13). In addition, infection with numerous RNA and DNA viruses, including Japanese encephalitis virus (JEV), Sindbis virus (SIN), rhinovirus, hepatitis C virus (HCV), dengue virus, Sendai virus (SV), vesicular stomatitis virus (VSV), pseudorabies virus (PrV), and HCMV, induces high levels of viperin (8, 9, 12, 14–17).
Although viperin is highly conserved across mammals and lower vertebrates (9), its precise mechanism of action is still largely undefined. Viperin has been shown to localize to the endoplasmic reticulum and lipid droplets and to inhibit replication of various DNA and RNA viruses (9, 18, 19). Over-expression of viperin inhibits HCMV, HCV, SIN, and influenza A virus, while siRNA-mediated knockdown of viperin enhances the replication of SV, SIN, and HIV-1 (9, 15, 17, 20). For HCMV, viperin over-expression was specifically shown to reduce the synthesis of late viral proteins, including pp65, glycoprotein B, and pp28, but the mechanism of reduction is not known (9). Over-expression of viperin inhibits the budding and release of influenza A virions from infected cells by altering lipid raft microdomains on the plasma membrane (18). More recent studies have shown that viperin expression reduces protein secretion and alters ER membrane morphology (21).
In this study, we examined viperin expression in vivo during acute LCMV Armstrong infection, which produces high levels of Type I IFNs, and in chronically infected LCMV carrier mice, which produce transiently detectable levels early in infection that decline to undetectable levels as the infection persists (4, 6, 22). We show that viperin is an excellent marker for IFN-responsive leukocytes as it is rapidly and highly expressed in various cell types with an expression pattern that follows IFNα kinetics during acute LCMV infection. Specifically, viperin is highly induced in neutrophils (NΦ) and macrophages (MΦ) in lymphoid organs in acutely infected mice and in carrier mice, while expression in T cells, B cells, and dendritic cells (DCs) is only seen in acute infection. IFNs are both necessary and sufficient to induce viperin expression in NΦ and MΦ as treating mice with Type I or II interferons induced viperin expression, while inhibiting Type I and II interferon stimulation in both acutely infected mice and in LCMV carrier mice blocked viperin expression. Finally, analysis of NΦ by immunoelectron microscopy showed that viperin localized to the endoplasmic reticulum and to the membrane of intracellular vesicles that are morphologically similar to lipid droplets and also contain the ER marker calreticulin.
Overall, these findings suggest that many cells respond to IFN stimulation during viral infection and that NΦ and MΦ are the predominant cell type that express viperin during both acute and persistent infections. These findings indicate that although LCMV carrier mice produce persistently low levels of IFN, the cells capable of responding to IFN are significantly changed, which may ultimately impact their ability to clear viral infection. Furthermore, because NΦ and MΦ are primarily associated with phagocytic killing of extracellular microbes, the results also suggest that viperin may play a central role in bacterial or parasitic infections and may protect these cell types from infection.
Materials and Methods
Reagents
The anti-Grp94 and anti-viperin (MaP.VIP) antibodies were previously described (21). Anti-calreticulin was purchased from Affinity BioReagents. Fluorescently-conjugated FACs antibodies were purchased from eBiosciences, including FITC-GR-1, PE-F4/80, PerCP-MHC II, Pacific Blue-CD11c, PeCy7-CD11b, PerCP-CD8, PE-CD4, and PE Texas Red-B220. Magnetic anti-rat IgG and IgM particles were obtained from Polysciences. LPS was purchased from Sigma, and the IFNγ neutralizing antibody (XMG1.2) was purchased from BioXCell. Mouse IFNα and IFNγ were purchased from R&D.
Mice, viral infections, and IFN and LPS treatments
The IFNAR1 mice were obtained from Warren Shlomchik (Yale University). All mice experiments were approved by the Yale Institutional Animal Care and Use Committee. Adult C57BL/6 mice were infected with 200,000 pfu of LCMV Armstrong intraperitoneally (i.p.) for acute infections or 200,000 pfu of LCMV Clone 13 intracranially into 1 day old neonates to create carrier mice [or i.p. for Clone 13 mice]. To neutralize IFNγ, mice were treated with 0.5mg of XMG1.2 i.p. on the noted days. For interferon and LPS treatment, C57BL/6 mice were treated 1.5 μg of IFNα or IFNγ or 500 μg of LPS i.p and then examined 16 hours post treatment. On the noted days, tissues and serum were collected.
Plaque assays
Tissues were homogenized using a PowerGen 700 (Fisher Scientific) in 1 ml of PBS containing protease inhibitors. Homogenates were serially diluted in DMEM containing 5% BCS and then added to Vero cell monolayers in 6-well plates. After a 1-hour incubation at 37°C, the inoculum was removed, and the cells were overlaid with media containing 1% SeaKem agarose (Lonza). On day three, an additional layer of media containing 1% agarose and 0.02% Neutral red (Sigma) was added to the cells and viral plaques were visualized the following day.
IFNα ELISA
96-well plates were coated with anti-IFNα capture antibody (Hycult Biotechnology; HM1001) in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) overnight at 4°C. After washing the plate in PBS-T (PBS with 0.5% Tween), the plates were blocked with 5% BCS in PBS-T. Homogenized tissues samples and a mouse IFNα standard (Hycult Biotechnology; HC1040) were diluted in blocking buffer, added to the plate, and then incubated overnight at 4°C. Plates were washed and incubated with the detection antibody (Cell Sciences; CP2012), followed by an HRP-conjugated anti-rabbit antibody, and HRP TMB Substrate (Pierce). The HRP reaction was stopped with 2N H2SO4 and analyzed at a 495 nm wavelength using a Wallac Victor 1420 (Perkin Elmer).
Tissue isolation and flow cytometry
Mouse tissues were harvested on the noted days and placed into RPMI containing 1% fetal bovine serum and 0.5% β-mercaptoethanol. Spleens, lungs, and livers were digested with in media containing 10 U/ml collagenase (Sigma) for 1 hr at 37°C. Tissues were manually homogenized and then filtered through a 100 μm nylon mesh filter (BD Falcon). Two million cells were surface stained in FACS buffer (PBS with 0.5% FBS) with the noted flow cytometry (FACS) antibodies and then fixed in 4% formaldehyde. Cells were permeabilized with 0.2% saponin in FACS buffer and then stained with FITC-or Alexa 647- conjugated MaP.VIP. All FACS samples were run on a LSR-II (Becton Dickinson) and analyzed using FlowJo software.
Immunofluorescence with tissue sections
For immunofluoresence of spleen sections, the tissues were snap-frozen in Cryo-OCT compound and then sectioned. Thin tissue sections were placed on glass slides and then fixed in 4% paraformaldehyde. Sections were permeabilized with 0.1% Triton X-100 in PBS, and stained with the indicated antibodies. All immunofluorescence samples were mounted with Prolong Gold AntiFade (Invitrogen) and then analyzed using a Leica TCS SP2 confocal microscope.
Western blot analysis of tissue homogenates
Homogenized tissue samples were brought to 1% Triton X-100 and then boiled in reducing sample buffer. Lysates were separated on 12% SDS-PAGE gels, transferred to PVDF membranes and then probed with the indicated antibodies.
Cell sorting and immunoelectron microscopy
Splenocytes from six LPS-treated mice were stained with rat anti-B220 (GK1.5), anti-CD4, and anti-CD8 (ATCC TIB-210) antibodies, washed extensively, and then incubated with magnetic anti-rat IgG and anti-rat IgM as previously described (23). After pulling down these cells with a magnet, the B-cell and T-cell-depleted splenocytes were stained with anti-GR-1 and anti-CD11b and then FACS sorted on a FACS Aria (Becton Dickinson). The sorted cells were collected into 2% paraformaldehyde in 0.25M HEPES, pH7.4 and then fixed in 4% paraformaldehyde containing 0.1% glutaraldehyde in 0.25M HEPES, pH 7.4 for 30 min on ice. Finally, the cells were washed and the incubated in 4% paraformaldehyde in 0.25M HEPES, pH 7.4 at 4°C overnight. The cells were prepared for immunocytochemistry as previously described (21) and stained with MaP.VIP and anti-calreticulin followed by 10nm and 5nm gold (Cell Microscopy Center, Utrecht University, The Netherlands), respectively. A Tecnai 12 Biotwein electron microscope and charge-coupled device camera (Morada, Olympus) were used to examine and capture images.
Results
Viperin is expressed in most lymphoid cells during LCMV Armstrong infection
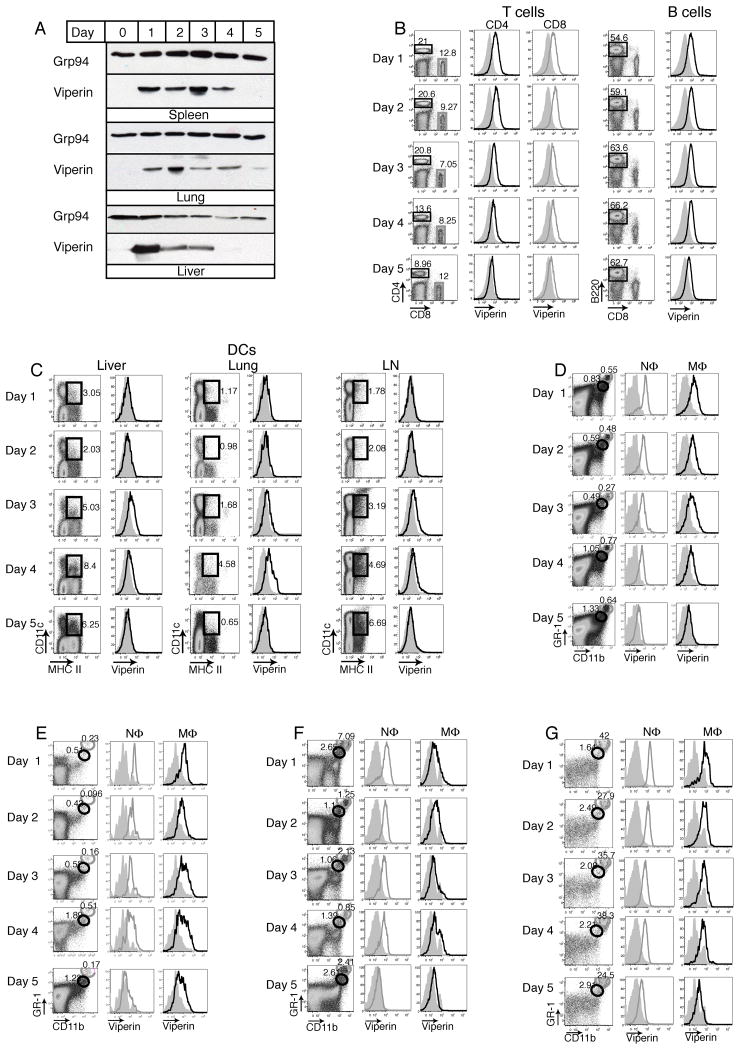
To establish that viperin is a good marker for IFN responsive cells during viral infections, we used LCMV Armstrong as a model of an acute, systemic viral infection that produces high levels of IFNαβ. C57BL/6 mice were infected with 200,000 pfu LCMV i.p. On days 1–5, spleens, lungs, and livers were harvested from infected and naïve mice and tissue homogenates were analyzed for viperin expression by Western blot. Viperin was detectable in these tissues with fairly rapid kinetics and expression declined around day 4 to 5 (Figure 1A). To determine the specific cell types within these tissues that expressed viperin, hematopoetic cells within the spleens, lungs, livers, and lymph nodes were analyzed for viperin expression by FACS analysis, while viral titers and IFNα levels in tissue homogenates and the serum were assayed by plaque assay and ELISA, respectively. The specificity of the anti-viperin monoclonal antibody, MaP.VIP, was confirmed using wild-type, heterozygous, and viperin knockout mice, which showed a dose-dependent decrease in viperin expression by FACS analysis (Supplemental Figure 2A). During infection, viperin was expressed in most cell types, including T cells, B cells, DCs, NΦ, MΦ, and NK cells (Figure 1B–G, Supplemental Figure 1). Both CD4 and CD8 T cells as well as B cells in the spleen expressed moderate levels of viperin with rapid kinetics (Figure 1B), while DCs also expressed moderate levels but with delayed kinetics. Viperin expression in DCs in the liver, lung, and lymph node (LN) was not readily detectable until day 3 and rapidly declined by days 4 and 5 (Figure 1C). Viperin expression was highest in NΦ and MΦ in the liver, spleen, lung, and lymph nodes (Figure 1D–G). These cell types were distinguished by their levels of GR-1 and CD11b and FACS sorting and electron microscopic analysis of the polynuclear morphology of GR-1 high, CD11b high cells confirmed that these cells were neutrophils(24). Furthermore, these cell types rapidly expressed viperin by day 1 post infection and maintained this level of expression until day 4 or 5.
Figure 1. Viperin is expressed in neutrophils, macrophages T cells, B cells, and dendritic cells during acute LCMV Armstrong infection.
C57BL/6 mice were infected with 200,000 pfu i.p. of LCMV Armstrong. (A) On days 1–5, the spleen, lung, and liver were harvested and examined by Western Blot for viperin and Grp94 as a loading control. (B) On days 1–5, the spleens were harvested from infected and naïve (grey, filled histograms) mice and analyzed by FACS staining for viperin expression in CD4+ T cells (black boxes and histograms), CD8+ T cells (grey boxes and histograms) and B cells (B220+, CD8−). (C–G) On days 1–5, viperin expression was examined by FACS analysis in dendritic cells (CD11c+ MHC II +) in the liver, lung, and lymph nodes (LN) (C) and in neutrophils (NΦ; CD11b high, GR-1 high; grey circles and histograms) and macrophages (MΦ; CD11b medium, GR-1 medium; black circles and histograms) in the spleen (D), lymph nodes (E), liver (F) and lung (G). Each figure is representative of four independent experiments for a total of four to six mice per day of infection.
Viperin expression corresponds to IFNα levels in LCMV Armstrong-infected mice
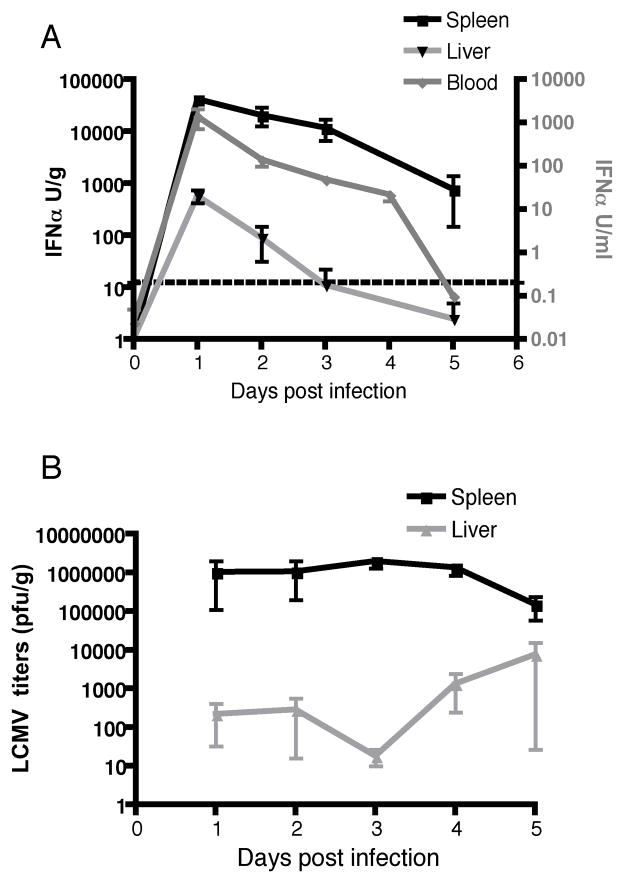
Next, we examined IFNα levels in LCMV Armstrong infected LCMV mice to determine if viperin expression correlated with IFNα levels. IFNα levels were detectable in the serum, liver, and spleen on day 1 and gradually declined to undetectable or lower levels at days 4 and 5 (Figure 2A). This decline in IFNα levels in the serum and target organs on day 4 or 5 in LCMV Armstrong-infected mice correlated with a reduction in viperin expression. IFNα was detected in the spleen and liver, which are the two organs predominantly associated with LCMV Armstrong infection, but not the lung or lymph nodes (25).
Figure 2. Viperin expression corresponds with IFNα levels and viral titers during acute LCMV Armstrong infection.
(A) C57BL/6 mice were infected with 200,000 pfu i.p. of LCMV Armstrong. On days 1–5, the spleen and liver were harvested and homogenized, and serum was collected. Tissue homogenates and serum were analyzed for IFNα levels by ELISA. The left and right y-axes express Units of IFNα per gram of tissue and Units of IFNα per milliliter for the serum, respectively. (B) Tissue homogenates in A were plaqued on Vero cell monolayers to determine the LCMV Armstrong titer. The graphs represent an average of three independent experiments for a total of five to fourteen mice per day of infection.
Consistent with the IFNα results, LCMV Armstrong viral titers were only detected in the spleen and liver, with higher levels in the spleen (Figure 2B). Furthermore, the decline in LCMV viral titers in the spleen correlated with IFNα levels and viperin expression in this organ. Consistent with previous findings, LCMV titers in the spleen decreased by one log on day 5, while in the liver, viral titers increased by approximately one log on days 4 and 5 (Figure 3B) (25).
Figure 3. Viperin is moderately expressed in neutrophils and macrophages in LCMV carrier mice.
Naïve C57BL/6 mice (grey, filled histograms), one-day-old C57BL/6 mice infected with 200,000 pfu of LCMV Clone 13 intracranially (black histograms), and 6-week-old C57BL/6 mice infected with 200,000 pfu of LCMV Clone 13 i.p. (grey histograms) were analyzed six months post infection. The spleens were harvested and examined by FACS staining for viperin expression in neutrophils (N√ grey boxes), macrophages (M√ black boxes) (A), B cells (B), and CD4+ T cells (black boxes) and CD8+ T cells (grey boxes) (C) as described in Figure 1. The data are representative of three independent experiments for a total of three to six mice per group.
Viperin is expressed in NΦ and MΦ in LCMV carrier mice
To further determine if viperin can be used as a marker of IFN-responsive cells in a persistent viral infection that produces low levels of IFNs, we examined viperin expression in LCMV carrier mice (6). One day after birth, C57BL/6 mice were infected with 200,000 pfu LCMV Clone 13 intracranially. Both uninfected C57BL/6 mice and C57BL/6 mice that were infected with Clone 13 at 2 months of age were used as controls. When the mice reached six months of age, we examined viperin expression in DCs, T cells, B cells, NΦ and MΦ in the spleen, liver, lymph nodes and lungs by FACS analyses. These analyses showed that viperin was moderately expressed in NΦ and MΦ, but not in T cells, B cells, or DCs (Figure 3, data not shown). The level of viperin in the carrier mice was not as high as that seen during acute viral infection. Consistent with previous reports, interferon levels in LCMV carrier mice were below the limit of detection in the serum, spleen, and kidney by ELISA (data not shown)(4).
IFNα- and IFNγ-treated mice express moderate levels of viperin in NΦ and MΦ
Because viperin expression closely paralleled the IFN kinetics in LCMV Armstrong-infected mice and viperin has been consistently shown to be upregulated upon IFN stimulation in vitro, we examined viperin expression in IFNα- or IFNγ-treated mice to confirm that IFNs are sufficient to induce viperin expression in vivo. C57BL/6 mice were treated with 1.5 μg IFNα and IFNγ. Sixteen hours post treatment, the lymphoid organs were harvested from both naïve and IFN-treated mice and examined for viperin expression. Viperin was moderately expressed in GR-1- and CD11b-positive cells, which could not be further delineated into NΦ and MΦ (Figure 4A). Viperin was also moderately expressed in DCs but was not detectable in CD4 or CD8 T cells or B cells (Figure 4B–C).
Figure 4. Viperin is expressed in neutrophils/macrophages and DCs after IFNα and IFNγ treatment.
(A–C) C57BL/6 mice were either left untreated (grey filled histograms) or treated with 1.5 μg IFN/mouse (black histograms). Sixteen hours post treatment, the spleens, lymph nodes (LN), liver, and lung were harvested and analyzed by FACS staining for viperin expression in GR1+ CD11b+ cells (A). Splenocytes in A were analyzed for viperin expression in DCs (MHC II+ CD11c+ cells) (B) or CD4+ T cells (black histograms) and CD8+ T cells (grey histograms) (C). (D–E) Spleens sections from IFN-treated mice were fixed, permeabilized, and stained for viperin and B220 or F4/80 as markers of the white and red pulp, respectively (D) or with GR-1 and CD11b (E). The data are representative of two independent experiments for a total of four to six mice per group.
To further examine where viperin-expressing cells are located within the spleen after IFNα or IFNγ treatment, we also analyzed spleen sections by immunofluorescence. First, viperin-expressing cells were examined in the context of splenic architecture. B220 and F4/80 staining was used to delineate the white and red pulp, respectively. Staining with an anti-viperin antibody showed that viperin-expressing cells were predominantly located in the red pulp, which is consistent with the high level of viperin expression in macrophages (Figure 4D). These spleen sections were also stained with anti-GR-1, anti-CD11b, and anti-viperin antibodies. Consistent with the FACS analysis, viperin was highly expressed in GR-1- and CD11b-positive cells (Figure 4E). As expected, viperin was not expressed in the spleens of Type I and Type II IFNR knockout mice (data not shown).
Type I and II interferon signaling is necessary to induce viperin expression during both acute and chronic LCMV infections
The above data suggested that viperin was a marker of IFN-stimulated cells during infection in vivo and that IFN stimulation was sufficient to induce viperin expression in NΦ and MΦ. In addition, the kinetics of viperin expression paralleled IFNα levels in LCMV-infected mice, suggesting that IFNα may be the major contributor to viperin expression during LCMV infection and potentially other infections. To determine if viperin expression during LCMV infection was solely IFN-dependent, both C57BL/6 wild-type and IFNAR knockout mice were infected intraperitoneally with LCMV Armstrong (200,000 pfu) and treated with a neutralizing IFNγ antibody on day 0 and day 2 post infection. When viperin expression was examined on day 3 post infection by FACS, IFNAR mice had dramatically reduced viperin expression in splenic NΦ and MΦ (Figure 5A). Treating LCMV-infected IFNAR mice with the neutralizing IFNγ antibody completely abolished viperin expression. Analyses in LCMV carrier mice showed similar results. Viperin expression in NΦ and MΦ was reduced to baseline levels in IFNAR knockout mice and in IFNAR mice that were treated with neutralizing IFNγ antibodies (Figure 5B). Therefore, viperin expression in both LCMV Armstrong-infected and LCMV carrier mice is IFN-dependent.
Figure 5. Viperin expression during LCMV infection is interferon-dependent.
(A) Wild-type or IFNAR knockout C57BL/6 mice were infected with 200,000 pfu LCMV Armstrong. On days 0 and 2, the mice were treated with 0.5 mg anti-IFNγ antibody (XMG1.2) i.p. to neutralize IFNγ. On day 3, the spleen was harvested and examined for viperin expression in neutrophils and macrophages as described in Figure 1. (B) Naïve C57BL/6 mice (grey filled histograms) or one-day-old C57BL/6 wild-type or IFNAR knockout mice were infected with 200,000 pfu of LCMV Clone 13 intracranially. Seven weeks post infection, the mice were treated with 0.5 mg XMG1.2 every other day for 7 days. One day after the final XMG1.2 treatment, the spleens were harvested and examined by FACS staining for viperin expression in neutrophils (N√) and macrophages (M√). For both A and B, the gray open histograms and gray filled histograms represent naïve and LCMV Armstrong-infected (A) or carrier mice (B), respectively. The black histograms represent the mice and treatment described on the left. The data are representative of two independent experiments with a total of four to six mice per group.
Viperin localizes to the ER and lipid droplets in neutrophils
Next, we examined the intracellular localization of viperin within neutrophils. Because viperin has been reported to localize to lipid droplets and inflammatory reactions have been associated with lipid body formation in neutrophils, we examined neutrophils in LPS-treated mice (26). Mice were treated with 500 μg LPS intraperitoneally, which also induces IFN. Consistent with our previous findings, viperin was highly expressed in NΦ, MΦ, and DCs in the liver, LN, and spleen but not in T cells and B cells (Figure 6A, data not shown). To further examine viperin expression in NΦ, GR-1 high, CD11b high cells were FACS sorted (Supplemental Figure 3) and then examined my immunoelectron microscopy. Electron microscopy confirmed the purity of the NΦ population, as 100% of the cells were polynucleated and contained electron-dense granules (Figure 6B, panel 1). Furthermore, immunoelectron microscopy with an anti-viperin antibody showed that viperin not only localized to the ER, along with the ER marker calreticulin, but was also present at the limiting membranes of cytoplasmic vesicles that morphologically resembled lipid droplets (Figure 6B). These vesicles, which also co-stained for calreticulin, contained low-electron dense material, consistent with their definition as lipid droplets.
Figure 6. Viperin localizes to the ER and lipid droplets in neutrophils from LPS-treated mice.
(A) C57BL/6 wild-type mice were either left untreated or injected with 500 μg LPS i.p. Sixteen hours after treatment, the spleen, lymph nodes, liver, and lung were harvested and analyzed for viperin expression in neutrophils (N√ grey boxes and histograms) and macrophages (M√ black boxes and histograms) by FACS as described in Figure 1. (B) GR-1 and CD11b high cells were FACS sorted, fixed, and then examined for the intracellular localization of viperin (10 nm gold) and calreticulin (5 nM gold) by immunoelectron microscopy for viperin. Panel 1 shows an example of a GR-1- and CD11b-high sorted cell. Panels 2–3 and 4–6 show examples of viperin and calreticulin staining of ER and lipid droplet-like vesicles, respectively. Black and white arrows indicate viperin and calreticulin immunogold labeling, respectively. The data are representative of two independent experiments with a total of four mice per group.
Discussion
This analysis of viperin expression revealed several novel findings that elucidated the kinetics of IFN stimulation and identified IFN-responsive cells during both acute and persistent viral infections, and also indicated how viperin may function during infection. Because viperin is one of the most highly induced genes in response to IFNs, we used viperin as a marker for cells that responded to interferon stimulation. We showed that many hematopoietic cell types rapidly respond to interferon stimulation during an acute LCMV Armstrong infection but that NΦ and MΦ are the only examined cell type that express viperin in LCMV carrier mice. During acute LCMV infection, the induction of viperin closely paralleled the kinetics of IFNα production, and preventing stimulation by Type I and II IFNs in acutely infected and carrier mice returned viperin expression to baseline. Because viperin expression in an acute and chronic viral infection model was strictly IFN-dependent, these results indicate that viperin is an excellent marker for IFN-stimulated leukocytes. Although not examined in this study, viperin is likely to be a general marker of IFN-responsive cells, including non-hematopoetic cells. Previous studies have shown that viperin is induced in many cell types by Type I, II, and III IFNs (27). While elimination of the Type I and II response eliminated viperin expression in leukocytes (Figure 5), Type III IFNs could be important for induction in non-hematopoetic cell types.
Our findings also indicate that chronic LCMV carrier infection, and likely other infections, persistently induce IFN that activates downstream gene expression. Although previous studies have shown that low levels of IFN are produced during chronic infections (6, 22, 28), there is little information on how these low levels affect or alter downstream gene expression and the innate immune response. Here, we show that these interferon levels are sufficient to upregulate viperin expression in NΦ and MΦ, albeit to lower levels than in acutely infected mice. Interestingly, other cell types, notably T cells, B cells and DCs, did not express viperin during chronic LCMV infection. The absence of viperin expression in lymphoid cells of carrier mice could be a direct consequence of low interferon levels. A threshold of IFN stimulation may be required to upregulate viperin expression in certain cell types and this level may not be reached during chronic LCMV infection. Low IFN levels are thought to be present in LCMV carrier mice (6).
Previous studies have shown that LCMV encodes a nucleoprotein that inhibits IFNβ production by blocking the nuclear translocation of IRF-3. This protein is expressed at high levels in mice chronically infected with LCMV Clone 13 (29), which may reduce IFN production and limit cell types that respond to low IFN levels. Alternatively, chronic viral infection may suppress the IFN responsiveness of certain cells by an unknown mechanism. Studies with chronic HIV patients have shown that certain cell types, notably plasmacytoid DCs, have a dampened response to IFNα stimulation in vitro, despite high levels of circulating IFNα (30, 31). These findings suggest that constant IFN stimulation leads to unresponsiveness or even cellular exhaustion.
Regardless of the mechanism that suppresses IFN production and/or subsequent unresponsiveness, alterations in the types of cells that respond to IFN stimulation may ultimately affect the ability to clear the virus. During viral infections, IFNs play a critical role not only in inducing antiviral innate immune responses but also in activating various cells that are involved in the adaptive immune response and viral clearance. For example, IFN has been shown to play a central role in activating T, NK, and B cells during HIV infection (30). During LCMV infection, IFNα is necessary to induce the maximal expansion of antigen-specific CD8 T cells (32). Because T cells did not express viperin during chronic LCMV infection, their ability to sense and/or respond to IFN may be partially or fully compromised, and this may extend to their ability to expand and clear virus.
The profile of viperin expression not only indicates which cells respond to the low IFN level in LCMV carrier mice but also illustrates the kinetics of IFN stimulation during acute LCMV infection. Neutrophils, macrophages, T cells and B cells upregulated viperin expression within one day after infection. Viperin expression persisted for 24–48 hours and then began to decline around day 4 or 5. Furthermore, the expression of viperin in these cell types was consistent with the peak of IFN levels in the serum, spleen, and liver. These findings indicate that these cell types rapidly respond to IFN stimulation during infection. However, DCs expressed viperin with delayed kinetics, peaking on day 3. DCs become rapidly phenotypically activated by Type I IFNs during various infections, including LCMV (33, 34), indicating that they are IFN responsive during the initial phase of infection. The fact that viperin expression was delayed in DCs suggests that these cells may have different mechanisms that regulate downstream IFNAR signaling and delay upregulation of certain IFN-induced genes. This delay may correlate with the function of DCs during LCMV infection and potentially other infections. During LCMV infection, DCs are thought to be able to prime T cells only during a short window of infection with optimal T cell priming occurring within the first 24 hours of infection (33). Because viperin and other IFN-induced genes have been shown to significantly impact cellular functions (1, 35), DCs may delay the expression of these genes in order to successfully initiate the adaptive immune response. Specially, viperin has been shown to alter plasma membrane fluidity and inhibit protein secretion. Because the T cell-DC interaction largely depends on the rigidity of plasma membrane microdomains, viperin expression could affect the ability of DCs to efficiently prime T cells, and viperin expression may be delayed in DCs until after T cell priming.
Although expression of viperin has been reported previously in T cells, DCs, and MΦ, this has never been reported in NΦ (14, 18, 36). Furthermore, given that NΦ and MΦ are mostly associated with antibacterial and antiparasitic immunity and viperin has been primarily associated with antiviral immunity, the high levels of viperin in these populations are informative and surprising (37, 38). NΦ are primarily associated with phagocytic killing of extracellular microbes. During microbial infections, they are the first immune cells that are recruited from the circulation to the site of infection. At the site of infection, NΦ engulf bacteria, parasites, and fungi into phagosomes that then fuse with specialized lysosomes containing antimicrobial peptides, enzymes, and reactive oxygen species (38, 39). Although NΦ have a very short half-life of several hours, these cells are essential for bacterial and parasitic immunity, and defects in NΦ function, such as in chronic granulomatous disease (CGD), are associated with a severe susceptibility to bacterial and fungal infections (38, 40).
NΦ have also been associated with viral infections. Several research groups have detected HCMV transcripts in polymorphonuclear leukocytes where HCMV has been proposed to undergo abortive replication (41, 42). Because viperin has been shown to inhibit HCMV, viperin may be responsible for limiting its replication in NΦ. It is also conceivable that viperin is highly induced in NΦ during viral infection in order to protect these them from subsequent bacterial co-infections.
Regardless of the evolutionary rationale for robust viperin expression in NΦ, the established functions of viperin, including alterations in ER morphology, protein secretion, and plasma membrane fluidity, could impact a number of these microbes at various stages of their life cycle. For example, both bacteria and parasites have been shown to alter lipid rafts in order to generate platforms that facilitate the internalization of these pathogens into cells (43, 44). In addition, several bacteria use the host secretory pathway to secrete soluble toxins from cells, while other bacteria, including Legionella, Brucella and Chlamydia, use ER membranes to generate a replication vacuole (45). Viperin could use these functions or other mechanisms to limit viral, bacterial, fungal, and parasitic replication not only in neutrophils and macrophages but also in other cell types.
Furthermore, we recently showed that viperin localizes to lipid droplets (19) and immunoelectron microscopy in this study suggested that viperin is also located on lipid droplet-like organelles in NΦ. Several bacterial pathogens have been reported to replicate on lipid droplets, and notably Chlamydia trachomatis has been shown to survive intracellularly within NΦ and to use lipid droplets for replication (40, 46). Therefore, viperin could play a role in protecting NΦ from pathogens that replicate on lipid droplets.
The range of inflammatory signals that induce viperin, including LPS, may indicate that viperin has much broader antimicrobial functions and is not limited to antiviral immunity. Based on these hypotheses and findings, viperin knockout mice may be more susceptible to bacterial and parasitic infections. To date, our studies on viperin knockout mice have not shown a statistically significant increase in susceptibility to several viruses, including LCMV, influenza A virus, and HSV (Supplemental Figure 2B, data not shown). Although expression of viperin inhibits several viruses in vitro, multiple interferon-induced genes are able to inhibit the replication of these viruses and this redundancy in the interferon response may mean that viperin plays a sufficient but not a necessary role in limiting viral replication. Alternatively, the antiviral functions of viperin could be more effective and essential to a group of viruses that has not yet been examined. It is equally plausible that while viperin has an antiviral function, it has a more essential role in limiting bacterial or parasitic infections.
Supplementary Material
C57BL/6 mice were infected with 200,000 pfu i.p. of LCMV Armstrong. On days 1, 2, and 4 post infection, the spleens were harvested from infected (black histograms) and naïve (grey, filled histograms) mice and analyzed by FACS staining for viperin expression in NK cells (DX5+, NK1.1+).
(A) C57BL/6 wild-type (gray filled histograms), heterozygous (gray histograms), or viperin knockout mice (black histograms) were infected with 200,000 pfu of acute LCMV Armstrong. On day 3 post infection, lymphoid organs were harvested and analyzed by FACS for viperin expression. (B) Wild-type and viperin knockout mice in A were examined by plaque assay for LCMV titers in the spleen.
(A) C57BL/6 mice were treated with 500 μg of LPS overnight. The spleens were harvested and analyzed for the frequency of GR-1- and CD11b-positive cells (Pre-sort). After depleting T cells with CD4 and CD8 antibodies, the cells were FACS sorted on the GR-1 and CD11b high population (Post-sort).
Acknowledgments
We thank Mrs. Nancy Dometios for manuscript preparation. This work was supported by the Ellison Foundation, the Howard Hughes Medical Institute and NIH grant R01AI074699 (S Kaech).
Abbreviations
- LCMV
Lymphocytic Choriomeningitis Virus
- MΦ
Macrophages
- MΦ
Neutrophils,
- IFNAR
Type I IFN receptor
References
- 1.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 2.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282:20053–20057. doi: 10.1074/jbc.R700006200. [DOI] [PubMed] [Google Scholar]
- 4.Lee LN, Burke S, Montoya M, Borrow P. Multiple mechanisms contribute to impairment of type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J Immunol. 2009;182:7178–7189. doi: 10.4049/jimmunol.0802526. [DOI] [PubMed] [Google Scholar]
- 5.Sklan EH, Charuworn P, Pang PS, Glenn JS. Mechanisms of HCV survival in the host. Nat Rev Gastroenterol Hepatol. 2009;6:217–227. doi: 10.1038/nrgastro.2009.32. [DOI] [PubMed] [Google Scholar]
- 6.Bukowski JF, Biron CA, Welsh RM. Elevated natural killer cell-mediated cytotoxicity, plasma interferon, and tumor cell rejection in mice persistently infected with lymphocytic choriomeningitis virus. J Immunol. 1983;131:991–996. [PubMed] [Google Scholar]
- 7.Zhu H, Cong JP, Shenk T. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc Natl Acad Sci USA. 1997;94:13985–13990. doi: 10.1073/pnas.94.25.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proud D, Turner RB, Winther B, Wiehler S, Tiesman JP, Reichling TD, Juhlin KD, Fulmer AW, Ho BY, Walanski AA, Poore CL, Mizoguchi H, Jump L, Moore ML, Zukowski CK, Clymer JW. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am J Respir Crit Care Med. 2008;178:962–968. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 9.Chin KC, Cresswell P. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc Natl Acad Sci USA. 2001;98:15125–15130. doi: 10.1073/pnas.011593298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 11.Rivieccio MA, Suh HS, Zhao Y, Zhao ML, Chin KC, Lee SC, Brosnan CF. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J Immunol. 2006;177:4735–4741. doi: 10.4049/jimmunol.177.7.4735. [DOI] [PubMed] [Google Scholar]
- 12.Severa M, Coccia EM, Fitzgerald KA. Toll-like receptor-dependent and -independent viperin gene expression and counter-regulation by PRDI-binding factor-1/BLIMP1. J Biol Chem. 2006;281:26188–26195. doi: 10.1074/jbc.M604516200. [DOI] [PubMed] [Google Scholar]
- 13.Suh HS, Zhao ML, Rivieccio M, Choi S, Connolly E, Zhao Y, Takikawa O, Brosnan CF, Lee SC. Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response. J Virol. 2007;81:9838–9850. doi: 10.1128/JVI.00792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudinot P, Riffault S, Salhi S, Carrat C, Sedlik C, Mahmoudi N, Charley B, Benmansour A. Vesicular stomatitis virus and pseudorabies virus induce a vig1/cig5 homologue in mouse dendritic cells via different pathways. J Gen Virol. 2000;81:2675–2682. doi: 10.1099/0022-1317-81-11-2675. [DOI] [PubMed] [Google Scholar]
- 15.Chan YL, Chang TH, Liao CL, Lin YL. The cellular antiviral protein viperin is attenuated by proteasome-mediated protein degradation in Japanese encephalitis virus-infected cells. J Virol. 2008;82:10455–10464. doi: 10.1128/JVI.00438-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink J, Gu F, Ling L, Tolfvenstam T, Olfat F, Chin KC, Aw P, George J, Kuznetsov VA, Schreiber M, Vasudevan SG, Hibberd ML. Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl Trop Dis. 2007;1:e86. doi: 10.1371/journal.pntd.0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang D, Guo H, Xu C, Chang J, Gu B, Wang L, Block TM, Guo JT. Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus. J Virol. 2008;82:1665–1678. doi: 10.1128/JVI.02113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Hinson ER, Cresswell P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe. 2007;2:96–105. doi: 10.1016/j.chom.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Hinson ER, Cresswell P. The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic alpha-helix. Proc Natl Acad Sci USA. 2009;106:20452–20457. doi: 10.1073/pnas.0911679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Burke CW, Ryman KD, Klimstra WB. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J Virol. 2007;81:11246–11255. doi: 10.1128/JVI.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinson ER, Cresswell P. The N-terminal amphipathic alpha-helix of viperin mediates localization to the cytosolic face of the endoplasmic reticulum and inhibits protein secretion. J Biol Chem. 2009;284:4705–4712. doi: 10.1074/jbc.M807261200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong P, Heydari S, Garidou L, McGavern DB. Persistent viral infection elevates central nervous system MHC class I through chronic production of interferons. J Immunol. 2009;183:3895–3905. doi: 10.4049/jimmunol.0803085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagasse E, I, Weissman L. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 25.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller PF, Ryeom SW, Picard ST, Ackerman SJ, Dvorak AM. Cytoplasmic lipid bodies of neutrophils: formation induced by cis-unsaturated fatty acids and mediated by protein kinase C. J Cell Biol. 1991;113:137–146. doi: 10.1083/jcb.113.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saron MF, Riviere Y, Hovanessian AG, Guillon JC. Chronic production of interferon in carrier mice congenitally infected with lymphocytic choriomeningitis virus. Virology. 1982;117:253–256. doi: 10.1016/0042-6822(82)90524-4. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Sobrido L, Zuniga EI, Rosario D, Garcia-Sastre A, de la Torre JC. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2006;80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford ES, Puronen CE, Sereti I. Immunopathogenesis of asymptomatic chronic HIV Infection: the calm before the storm. Curr Opin HIV AIDS. 2009;4:206–214. doi: 10.1097/COH.0b013e328329c68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilton JC, Manion MM, Luskin MR, Johnson AJ, Patamawenu AA, Hallahan CW, Cogliano-Shutta NA, Mican JM, Davey RT, Jr, Kottilil S, Lifson JD, Metcalf JA, Lempicki RA, Connors M. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol. 2008;82:3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montoya M, Edwards MJ, Reid DM, Borrow P. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type 1 IFN. J Immunol. 2005;174:1851–1861. doi: 10.4049/jimmunol.174.4.1851. [DOI] [PubMed] [Google Scholar]
- 34.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar SN, Sen GC. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol Ther. 2004;103:245–259. doi: 10.1016/j.pharmthera.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Qiu LQ, Cresswell P, Chin KC. Viperin is required for optimal Th2 responses and T-cell receptor-mediated activation of NF-kappaB and AP-1. Blood. 2009;113:3520–3529. doi: 10.1182/blood-2008-07-171942. [DOI] [PubMed] [Google Scholar]
- 37.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 38.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 40.Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting factor. Immunobiology. 2008;213:183–191. doi: 10.1016/j.imbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Bitsch A, Kirchner H, Dupke R, Bein G. Cytomegalovirus transcripts in peripheral blood leukocytes of actively infected transplant patients detected by reverse transcription-polymerase chain reaction. J Infect Dis. 1993;167:740–743. doi: 10.1093/infdis/167.3.740. [DOI] [PubMed] [Google Scholar]
- 42.Gerna G, Percivalle E, Baldanti F, Sozzani S, Lanzarini P, Genini E, Lilleri D, Revello MG. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J Virol. 2000;74:5629–5638. doi: 10.1128/jvi.74.12.5629-5638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulbins E, Dreschers S, Wilker B, Grassme H. Ceramide, membrane rafts and infections. J Mol Med. 2004;82:357–363. doi: 10.1007/s00109-004-0539-y. [DOI] [PubMed] [Google Scholar]
- 44.Zaas DW, Duncan M, Rae Wright J, Abraham SN. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim Biophys Acta. 2005;1746:305–313. doi: 10.1016/j.bbamcr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Salcedo SP, Holden DW. Bacterial interactions with the eukaryotic secretory pathway. Curr Opin Microbiol. 2005;8:92–98. doi: 10.1016/j.mib.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci USA. 2008;105:9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C57BL/6 mice were infected with 200,000 pfu i.p. of LCMV Armstrong. On days 1, 2, and 4 post infection, the spleens were harvested from infected (black histograms) and naïve (grey, filled histograms) mice and analyzed by FACS staining for viperin expression in NK cells (DX5+, NK1.1+).
(A) C57BL/6 wild-type (gray filled histograms), heterozygous (gray histograms), or viperin knockout mice (black histograms) were infected with 200,000 pfu of acute LCMV Armstrong. On day 3 post infection, lymphoid organs were harvested and analyzed by FACS for viperin expression. (B) Wild-type and viperin knockout mice in A were examined by plaque assay for LCMV titers in the spleen.
(A) C57BL/6 mice were treated with 500 μg of LPS overnight. The spleens were harvested and analyzed for the frequency of GR-1- and CD11b-positive cells (Pre-sort). After depleting T cells with CD4 and CD8 antibodies, the cells were FACS sorted on the GR-1 and CD11b high population (Post-sort).