Abstract
A poplar planted system resulted in the complete removal of at least 19 of the 29 potential polychlorinated biphenyl (PCB) congeners detected in trace amounts (37.9 ng g-1 in total) in a commercial garden soil, while the unplanted soil only had 2 congeners completely removed after 96 days. In addition, the most recalcitrant congener, PCB 52, only decreased by 0.1% in the unplanted reactors while declining by 22.3% in the planted system. There was also greater removal of a PCB 77 spike in the planted system when compared to the unplanted system, 17.2% in the planted system versus 2.8% in the unplanted system. The results suggest that phytoremediation may be an effective tool in cleaning commercially available garden soils that are lightly contaminated with PCBs.
Keywords: Garden Soil, PCB, Poplar, Rhizosphere
1.0 Introduction
The wide spread use of polychlorinated biphenyls (PCBs) in a variety of products from the 1930s – 1970's allowed for the ubiquitous presence of PCBs in various environmental matrices. Low, but measurable amounts can be found in most environments. Both urban and rural soils have been impacted by atmospheric transport of PCBs (Aichner et al., 2007, Wilcke and Zech, 1998). It has been reported that, urban garden soils had elevated level of PCBs, while agricultural soils had lower median concentrations than garden soil (Krauss and Wilcke, 2003). Although below the critical level of 200 ng g-1 for agricultural use, total PCBs ranging from 8.4 -59.5 ng g-1 were detected in rural soils (Wilcke and Zech, 1998). Measurable levels of PCBs in yard waste are not uncommon. This may be because of PCB partitioning in the bark of trees (Hermanson and Hites, 1990). Therefore, compost made from yard waste is also likely to contain measurable levels of PCBs. Elevated PCB levels have found in many municipal solid waste (MSW) and sewage sludge (SS) compost (Malloy et al 1993). In one study, it was shown that maternal gardening contributed to PCB levels in prepubescent boys' serum. Prepubescent Russian boys whose mothers gardened locally, had higher serum levels of PCBs, compared to those whose mothers didn't (Burns et al., 2009).
Given the global presence of PCBs, PCB degraders are found in many environments (Hiraishi, 2008, Bedard, et al. 2007, Bedard, et al. 2008, Magar et al., 2005, Pakdeesusuk et al., 2005, Tiedje et al., 1993). However, in situ microbial degradation of PCBs in the natural environment is extremely slow and this accounts for their persistence in the environment. Left alone, PCB compounds degrade slowly in soil so the risk of exposure is longer in unplanted soil. A five year simulation showed that even in planted systems, PCBs remained in the root zone and slowly degraded (Hsu et al., 1993).
The ability of plants to stimulate microbial activity in the rhizosphere is known (Mackova et al., 2007). Poplar is a model plant for phytoremediation, and hydroponics studies involving PCB and poplar have been undertaken (Liu et al. 2008, Liu et al., 2009). However, most field-scale phytoremediation interventions involve planting in a soil matrix which is more complex than hydroponics, yet few studies have been undertaken with real garden or commercial soil.
Given the ubiquity of PCBs, soil amendments and soil sold commercially may often be contaminated with PCBs. It is hypothesized that by planting PCB contaminated soil with poplar, the degradation of PCBs will be enhanced. The plants will provide a copious supply of electron donors for the microbes through the production of various exudates. Therefore, the objective of this experiment is to enhance PCB degradation and transformation by planting hybrid poplar trees in commercial potting soil contaminated with PCBs. If successful, such a technique could be recommended for “cleaning” garden soil. Spiked additions of PCB 77 were also utilized to understand the rhizosphere degradation of higher concentrations of a commonly detected and toxic congener.
2.0 Materials and Methods
Seven (7.0) kg of Scott's lawn soil from Menards (total nitrogen 0.08%, available phosphate 0.03%, soluble potash 0.02%) was thoroughly mixed with 4.9 mg of 3,3′,4,4′ tetrachlorobiphenyl (PCB 77) dissolved in hexane to achieve artificial contamination. PCB 77 is one of the most toxic PCB congeners and exhibits dioxin like toxicity. The targeted initial soil concentration was 700 ng g-1 PCB 77. The contaminated soil was placed in a fume hood and the solvent allowed to evaporate in a sealed container for 3 days. At the end of 3 days, 300 g portions of soil were transferred to 473 ml mason jars (22 in total), with a 1.9 cm diameter hole drilled in the covers. The hole was used for planting of trees and addition of water to the reactors. Sixteen (16) jars were planted with 22.9 cm hybrid poplar cuttings (P. deltoids × nigra DN34) obtained from Segal Ranch Hybrid Poplars Nursery (Grand View, WA), while six were unplanted. The poplar cuttings were inserted into the soil medium through the hole in the cover (Figure 1).
Figure 1.
Schematic of reactors. Photo shows addition of water to reactor with a syringe. Bottom diagram shows schematic of reactor.
Plants were grown under a 16 hr light/ 8 hr dark photoperiod with a light intensity of 200 μmol m-2s-1 and at a temperature of 25°C. After planting, the excess space around the trees was sealed with silicone to reduce losses by volatilization. The experimental set up consisted of poplar trees planted in 300 g contaminated soil, controls of trees planted in uncontaminated soil, unplanted uncontaminated soil and unplanted contaminated soil with native bacteria only.
At the end of exposure (96 days), soil and plant material were analyzed for PCB content using GC/MS/MS triple quadropole mass spectrometry (Agilent Technologies 6890N GC with an Agilent 7683 series autosampler coupled to a Waters Micromass Quattro micro GC mass spectrometer (Milford, MA). PCBs 14, 65, and 166 were used as surrogate standards and PCB 204 was the internal standard. The experimental set up and analyses were carried out in triplicate.
2.1 Extraction and Analysis of PCB
Denaturation and extraction of PCB in soil and plant material was conducted by adding 3 milliliters per gram of a 1:1 hexane: acetone mixture to 5 grams of grounded homogenized soil and sonicating for 1 hour. Prior to sonification, the samples were spiked with 50 ng of PCB14 (3,5 dichlorobiphenyl), PCB65 (2,3,5,6 tetrachlorobiphenyl) and PCB166 (2,3,4,4′,5,6 hexachlorobiphenyl) (Cambridge Isotope Laboratories, Inc.), which were used as surrogate standards. These congeners are not normally found in environmental samples, are not degradation products of the congener tested and can be used as a surrogate for the whole suite of 209 PCB congeners based on their physico chemical properties. The sonicated material was centrifuged at 3000 rpm for 5 minutes, after which the supernatant was transferred to a fresh vial. A second extraction was performed and the supernatants combined. The combined supernatant was evaporated to dryness using rotary evaporation and the solvent changed to hexane. Any loss from evaporation was corrected using the surrogate recoveries, Surrogate recoveries ranged from 85.2±5.3 % to 98.6±3.8% for PCB 14, 90.6±2.9% to 99.8±5.2% for PCB 65 and 98.4±5.3% to 102.9±3.8% for PCB 166.
Removal of lipids and other polar substances was achieved by double extraction with concentrated sulfuric acid and hexane. This hexane extract was concentrated to approximately 0.5 ml and eluted with 10 ml of hexane through a filter consisting of 0.1g of silica, 0.1g of sodium sulfite and 0.9 g acidified silica gel. The eluent was concentrated and PCB204 (2,2′,3,4,4′,5,6,6′ octachlorobiphenyl) was added as an internal standard before analysis by GC/MS/MS triple quadropole mass spectrometry (Agilent Technologies 6890N GC with an Agilent 7683 series autosampler coupled to a Waters Micromass Quattro micro GC mass spectrometer (Milford, MA)). The gas chromatogram (GC) was fitted with a Supelco SBP-Octyl capillary column (30 m × 0.25 mm ID, 0.25 μm film thickness) with helium as carrier gas at a constant flow rate of 0.8 ml min− 1. The GC operating conditions were as follows: injector temperature 270 °C, interface temperature 290 °C, initial temperature 75 °C, initial time 2 min. The GC temperature program was 75 to 150 °C at 15 °C min− 1, 150 to 290 °C at 2.5 °C min− 1, and final time 1 min. Identification and quantification of PCB congeners in the samples was performed using a calibration standard consisting of all 209 congeners and is described elsewhere (Hu et al. 2010).
2.2 Statistical Analysis
Microsoft Excel Analysis Toolpak's ANOVA analysis and Students' t-test were used for statistical testing. The significance level of 0.05 was utilized to indicate whether the treatments were significantly different than the controls.
3.0 Results and Discussion
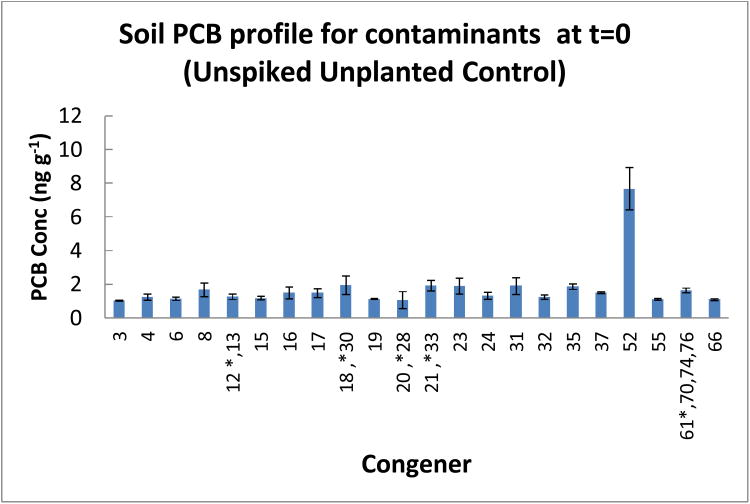
At the onset, it was expected that the only detectable amounts of PCB congeners in the soil would be the congener with which the soil was spiked. However, the analytical results indicated that there were clearly some other PCB congeners (here-after referred to as “contaminants”) in the soil at the beginning of the experiment. The “contaminants” are shown in Figure 2. They consisted of several congeners ranging from tetra chlorinated to mono chlorinated congeners. The predominant “contaminant” was the tetra chlorinated, PCB 52 (2,2′, 5,5′tetrachlorobiphenyl).
Figure 2.
PCB congener profile of ‘contaminants’ present in unspiked garden soil at t=0. Error bars are 1 standard deviation.
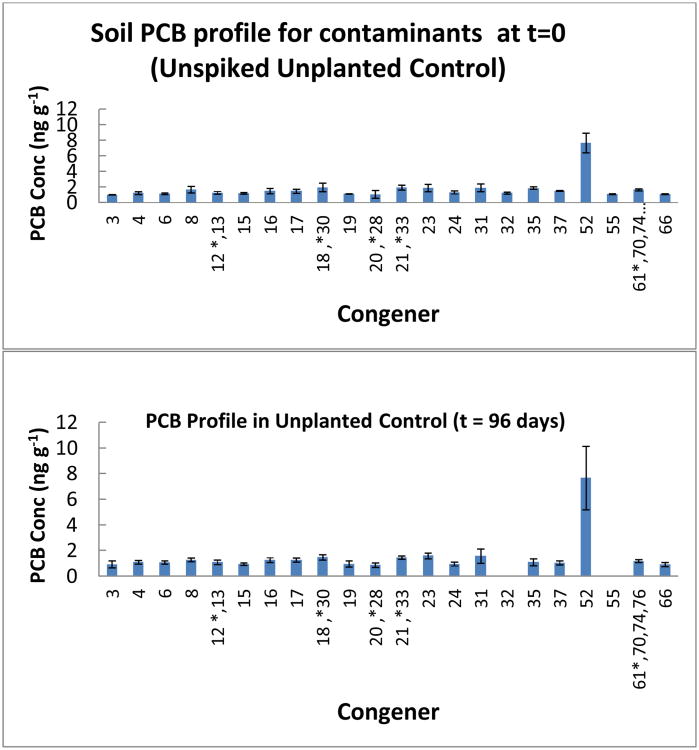
The congener profile of the unplanted control after 96 days is shown in Figure 3. The results show that the congener profile was similar to the “contaminant” profile of the unspiked soil at the start of the experiment. In the unplanted control only 2 congeners (PCB 32 and PCB 55) were completely removed (Table 1 and Figure 2). Degradation of the other “contaminants” in the unplanted control ranged from 0.1% (PCB 52) to 41.1% (PCB 35) (Table 1). However degradation or transformation was not sufficient enough to change the “contaminant” congener profile in the unplanted control after 96 days when compared to the congener profile at the start of the experiment.
Figure 3.
Contaminant congener profile in unplanted control at t = 96 days. This shows that the PCB congener profile in the unplanted control at t= 96 days was similar to the unspiked unplanted profile at the t=0 days. Error bars are 1 standard deviation.
Table 1.
Reduction in “contaminant” PCB congeners in unspiked garden soil after 96 days.
| IUPAC PCB Congener | Decrease in Unspiked Unplanted Control | Decrease in Unspiked Planted Control |
|---|---|---|
| 3 | 10.1% | 100.0% |
| 4 | 11.7% | 100.0% |
| 6 | 7.3% | 100.0% |
| 8 | 23.5% | 100.0% |
| 12*,13 | 13.6% | 100.0% |
| 15 | 19.7% | 100.0% |
| 16 | 14.9% | 100.0% |
| 17 | 13.7% | 100.0% |
| 18,*30 | 24.4% | 100.0% |
| 19 | 15.7% | 100.0% |
| 20,*28 | 18.1% | 100.0% |
| 21,*33 | 24.4% | 100.0% |
| 23 | 16.1% | 79.7% |
| 24 | 28.0% | 100.0% |
| 31 | 17.3% | 100.0% |
| 32 | 100.0% | 100.0% |
| 35 | 41.1% | 100.0% |
| 37 | 31.0% | 91.8% |
| 52 | 0.1% | 22.3% |
| 55 | 100.0% | 100.0% |
| 61*,70,74,76 | 27.6% | 100.0% |
| 66 | 15.8% | 100.0% |
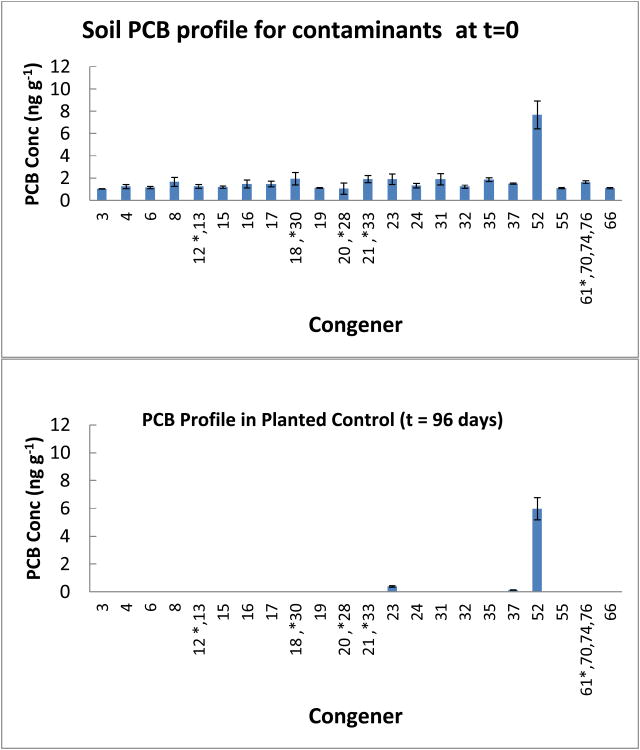
Figures 4 depicts the congener profile for the planted control after 96 days. It is evident that the congener profile of the planted control after 96 days is starkly different from the profile of the unspiked soil at the start of the experiment. After 96 days, the soil of the planted control was devoid of several congeners that were present at the beginning (Figure 4 and Table 1). PCB 52 showed some amount of recalcitrance and maintained a similar concentration to that at the beginning of the experiment, while recording a 22.3% decline (Table 1). The other two congeners that were not completely degraded in the planted control were PCB 23 and PCB 37. These decreased by 79.7% and 91.8%.
Figure 4.
Congener profile of planted control at t = 96 days showing removal of some lower chlorinated congeners seen in the garden soil at t = 0 days. Error bars are 1 standard deviation.
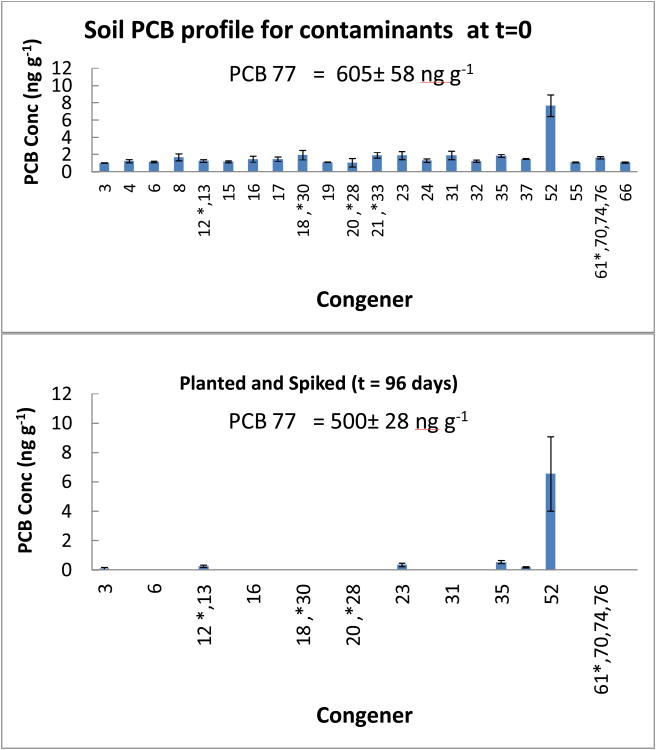
The ability of the plants to effect PCB removal in a spiked system was tested by spiking the garden soil with PCB 77. The “contaminants” accounted for 4 % of the total PCB mass content in the spiked system. Initially, it was thought that the PCB standard used to spike the soil could have been the source of the “contaminants” detected in the spiked soil at the start of the experiment. However, analysis of the PCB 77 standard revealed that impurities in the PCB 77 standard were not the source of contamination. This, coupled with the fact that the “contaminants” were also detected in the unspiked soil confirmed that the source of the “contaminants” was the garden soil. In terms of removal of the “contaminants” detected in the soil at the beginning of the experiment, a result similar to what was obtained with unspiked planted system was seen with the planted spiked system (Figure 5). Several of the congeners were removed, with only nine of the twenty eight contaminants detected at the start of the experiment remaining. Of the remaining congeners, PCB 52 had the least decrease in concentration registering a 14.8% decline (Table 2). The other PCB congeners remaining (PCB 3, PCB 12/13 which were co- eluted, PCB 21/33 co-eluted, PCB 23, PCB35 and PCB37) recorded declines of between 70.9% and 98.9% (Table 2).
Figure 5.
Congener profile of planted system spiked with PCB at t = 96 days showing removal of some lower chlorinated congeners seen in the garden soil at t = 0 days. The spiked PCB 77 concentration at t=0 and t = 96 days are shown on the top and bottom panels respectively. Error bars are 1 standard deviation.
Table 2.
Reduction in “contaminant” PCB congeners in spiked garden soil after 96 days.
| IUPAC PCB Congener | Decrease in Spiked Unplanted System | Decrease in Spiked Planted System |
|---|---|---|
| 3 | 22.2% | 90.2% |
| 4 | 5.4% | 100.0% |
| 6 | 6.6% | 100.0% |
| 8 | 12.5% | 100.0% |
| 12 *,13 | 23.6% | 80.2% |
| 15 | 38.8% | 100.0% |
| 16 | 19.7% | 100.0% |
| 17 | 11.3% | 100.0% |
| 18,*30 | 14.2% | 100.0% |
| 19 | 13.9% | 100.0% |
| 20,*28 | 27.5% | 100.0% |
| 21,*33 | 17.0% | 98.9% |
| 23 | 18.4% | 81.5% |
| 24 | 22.7% | 100.0% |
| 31 | 19.4% | 100.0% |
| 32 | 100.0% | 100.0% |
| 35 | 28.2% | 70.9% |
| 37 | 19.7% | 87.9% |
| 52 | 0.1% | 14.8% |
| 55 | 100.0% | 100.0% |
| 61*,70,74,76 | 33.5% | 100.0% |
| 66 | 6.7% | 100.0% |
| 77 (Spike) | 2.8% | 17.2% |
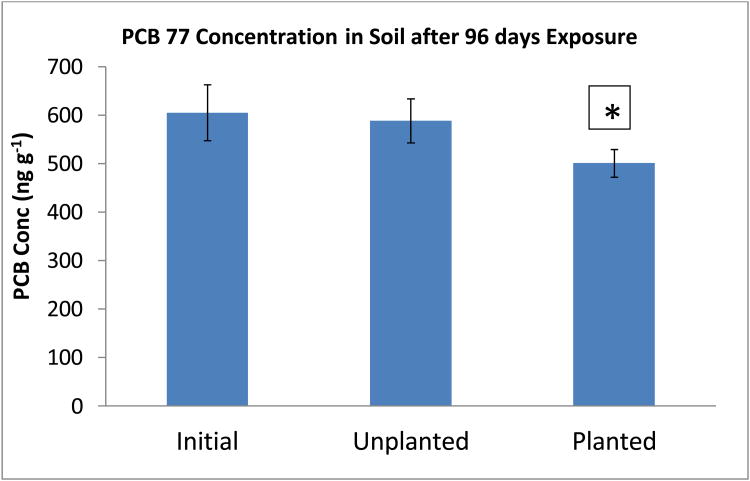
A comparison of the PCB 77 concentration in the soil of the planted and unplanted spiked systems at the end of exposure with the concentration at the beginning of incubation is shown in Figure 6. This illustrates that the unplanted spiked system did not have any significant reduction in PCB 77 at the end of exposure (p<0.05), only registering a 2.8% decrease. On the other hand, the planted system showed a decrease of 17.2%. So, the planted system was not only able to remove the trace “contaminants” present in the garden soil, but also record a modest (significant) decrease in the concentration of the PCB 77 spike (p<0.05).
Figure 6.
PCB 77 concentration in planted and unplanted soil after 96 days exposure. *= significant reduction in comparison to starting concentration (p<0.05). PCB 77 was not detected in the unspiked controls. Error bars are 1 standard deviation.
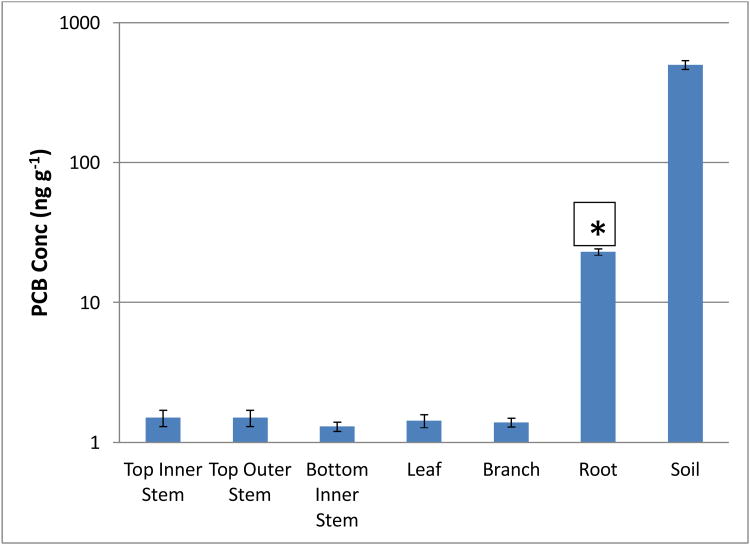
After exposure, most of the PCB remained in the soil with only a small portion (<2%) detected in the plant tissue (Figure 7). The root accounted for most of the PCB detected in plant material, with very little in the above ground material (Figure 7). Hexane was used to wash the roots to desorb any PCB that was reversibly sorbed, and explicity identify what was irreversibly bound or taken up by the plants. It was quite difficult, even after multiple rinses, to dislodge all the PCBs from the roots indicating that the remaining portion was plant bound. The stem material was separated in bottom (below ground) and upper (above ground) portions, to differentiate between effects caused by sorption versus translocation. Furthermore, the bottom and upper stems were divided between inner and outer sections, to definitively determine what was due translocation versus diffusion.
Figure 7.
Distribution of PCB in Soil and Plant material at t=96 days. * = significantly higher concentration than in other plant parts (p<0.05). Error bars are 1 standard deviation.
The trend observed in the planted and unplanted controls were also observed in the spiked systems. That is, a comparison of congener profile in the unplanted spiked system after 96 days, with the congener profile of the planted control at the start of the experiment, shows that the soil congener profile for the unplanted spiked system was similar to the congener profile of the planted control at the beginning of the exposure. This indicates that similar to the unplanted control, the removal of the contaminants in the unplanted spiked system after 96 days was not sufficient to alter the congener profile when compared to the congener profile at the start of the experiment (Table 2). Similar to the unspiked unplanted control PCB 52 was only removed by 0.1% in the unplanted spiked system (Table 2). The congeners remaining in the unplanted spiked treatment decreased by between 5.4% (PCB 4) and 38.8% (PCB15) (Table 2). The same two congeners (PCB 55 and PCB 32) that were completely removed in the unplanted unspiked system were completely removed in the unplanted spiked treatment (Table 2).
On the other hand, the spiked treatments that were planted with poplar showed disappearance of several congeners with only 9 congeners remaining (Figure 5). Of the remaining congeners, PCB 35 (3,3′,4 trichlorobiphenyl), PCB 37 (3,3,4′ trichlorobiphenyl), PCB 12(3,4 dichlorobiphenyl), PCB 13(3,4′dichlorobiphenyl) and PCB 3 (4chlorobiphenyl) are potential degradation products of PCB 77 (3,3′4,4′tetrachlorobiphenyl). Volatilization as a source of the loss was ruled out because, at the end of exposure, the congener profile of the unplanted systems, were similar to the congener profile of the contaminants profile at the beginning. Volatilization would have occurred in both the unplanted and planted systems after 96 days if this was the source of the loss. A mass balance on the spiked reactors resulted in a 99.5% recovery in the unplanted reactors, and 88.1% of the PCBs were recovered in the planted reactors. This indicates that sorption to the reactor walls was not a major factor in the loss of PCB from the reactors. In the planted reactors, the lack of a complete mass balance could have been due to aerobic bio-oxidation.
An indication of PCB degradation is the accumulation of less chlorinated congeners, particularly mono chlorinated ones (Bedard et al., 1987), but this was not evident in the spiked treatment. One possible reason could be the occurrence of ring cleavage under the aerobic conditions resulting in the lack of accumulation of mono chlorinated congeners. Another possibility is that there could have been mineralization, but that is highly unlikely as typically organisms that degrade PCBs are only able to dechlorinate, but not mineralize the congeners that they reduce (Abraham et al., 2002). Mineralization generally requires a different consortium of organisms. The aerobic degraders preferentially dechlorinate the least chlorinated ring, and release the other ring as chlorobenzoic acids (Abraham et al., 2002). Since the chlorobenzoates require a different consortium of aerobic microorganisms to facilitate further reduction they are a potential bottleneck in the mineralization process.
The overarching conclusion here, as shown by the results, is that the presence of planted material had a positive impact on the degradation of PCBs and the cleaning of the garden soil except for PCB 52. Another conclusion is that this method (phytoremediation) can clean up lightly contaminated commercial soils in only 96 days for most congeners. The mechanism by which this was achieved was probably due to microbial activity which was stimulated by the presence of the plants. There are several instances in which microbial activity and numbers have been stimulated in the rhizophere (Chekol et al., 2004, Jordahl et al., 1997). For example, Jordahl et al. (1997) reported significantly higher concentration of denitrifiers, pseudomonads, monoaromatic petroleum degraders and atrazine degraders in soil samples from the rhizophere of poplar trees than in surrounding adjacent agricultural soil. They also found poplar secreted a substantial amount of organic carbon into the rhizophere with the poplar producing up 0.25% of their biomass as soluble exudates. They speculated that the significant increase in microbial numbers in the rhizophere was a direct result of the exudates because the BOD decay coefficient of the exudates suggest that they would be easily degraded, thereby making them good substrates for the microbes.
Similarly, changes in the bacterial community structure and dioxygenase activity, and bacterial number have been observed in the rhizosphere of willow planted deliberately, and willow growing naturally, in PCB contaminated soil when compared to controls and other tree species (Ionescu et al. 2009, de Carcer et al., 2007; Leigh et al. 2006, Slater et al. 2011). Burkholderia sp. LB 400, a well-known PCB degrader has been shown to exhibit the same growth rate when propagated on flavonoidal compounds derived from mulberry roots as the sole carbon source as with biphenyl as the carbon source, suggesting that these compounds have similar efficacy to biphenyl which is normally used to culture PCB degraders in the laboratory (Leigh et al., 2002).
The importance of plants to ensuring that contaminants were removed from the soil is illustrated by the observation that, even planted systems, in which the plants died after 75 days into the experiment, but continued to be incubated for 96 days, showed removal of the contaminants that were present in the potting soil at the beginning of the experiment. This suggests that dead roots can provide a source of substrate for PCB degrading bacteria as postulated by Leigh et al. (2002). In their research, which investigated the effect of root turnover on PCB degradation, they observed that the increase in phenolic compounds in the fine mulberry roots increased 2 fold during the latter parts of the growing season, when most roots were dying.
Donnelly and Fletcher (1994) in investigating the ability of different plant species to release phenolic compounds found that all 17 species released phenols in the root matrix. Not surprisingly, there was variation in production among the plant species. A comparison of the released concentration, with a known concentration that supported PCB degraders, revealed that while the concentration in the entire rhizosphere was lower than the known substrate level, the concentration at the root surface was 3-5 times higher than composite leachate throughout the rhizosphere. This, they suggested, may indicate that at the root surface there would be production of substrate level phenolic compounds for PCB degraders.
While poplar plays an important role in the apparent reduction of PCB 77 and the other contaminants detected at the beginning of the exposure period, there appears to be little if any uptake of PCBs. This is illlustrated in Figure 7, which shows that most of the PCB detected in the plant material resided in the roots, possibly as a result of sorption. This is consistent with the fact that most PCBs are hydrophobic with hydropobicity increasing with an increase in the degree of chlorination. Schnoor et al. (1995) posited that given their hydrophobicity PCB are unlikely to enter the transpiration stream. In addition, their octanol water (Kow) coefficient would suggest that they would be strongly adsorbed to organic material. However, it is possible that some lower chlorinated PCBs may be able to be taken up by plants given their greater solubilty compared to their higher chlorinated counterparts (Liu et al, 2008). Roots were washed with hexane to desorb any PCB that was reversibly sorbed, and explicity identify what was irreversibly bound or taken up by the plants. Even after multiple rinses, there was still some PCB bound to to the roots, but this was minimal (5.0%) compared to the mass that remained in the soil (83.1%).
4.0 Conclusion
The results have provided evidence of poplar assisted rhizosphere degradation of PCBs in contaminated commercially available garden soil. The importance of poplar in the overall scheme is highlighted by the fact, that even when the plants died, the planted system outperformed the unplanted system. The exact mechanism for the better performance of the planted systems could be a combination of factors, inclusive of increased microbial activity in the poplar rhizosphere, because of the secretion of exudates and secondary compounds by the plants. However, there was a clear demonstration that the systems planted with poplar resulted in the removal of the PCB contaminants detected in the garden soil at the beginning of exposure, while the unplanted sytems did not. This suggests that phytoremediation may be an important tool in cleaning commercial soils lightly contaminated with PCBs.
Acknowledgments
The National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program (SRP) Grant No. P42ES13661 provided funding to undertake this research. We also wish to recognize Dr. Hans Lehmler for providing PCB congeners. The authors would also like to acknowledge the expert analytical support provided by Dr. Dingfei Hu.
Contributor Information
Richard E. Meggo, Email: richard-meggo@uiowa.edu, Department of Civil and Environmental Engineering, 4105 Seamans Center, University of Iowa, IA, 52242, USA, Phone: 319-594-4263; Fax: 319-335-5660.
Jerald L. Schnoor, Email: jerald-schnoor@uiowa.edu, Department of Civil and Environmental Engineering, 4105 Seamans Center, University of Iowa, IA, 52242, USA.
References
- Abraham WR, Nogales B, Golyshin PN, Pieper DH, Timmis KN. Polychlorinated biphenyl-degrading microbial communities in soils and sediments. Current Opinion in Microbiology. 2002;5(3):246–253. doi: 10.1016/s1369-5274(02)00323-5. [DOI] [PubMed] [Google Scholar]
- Bedard DL, Ritalahti KA, Loffler FE. The Dehalococcoides population in sediment-free mixed cultures metabolically dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Applied and Environmental Microbiology. 2007;73(8):2513–2521. doi: 10.1128/AEM.02909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard DL, Wagner RE, Brennan MJ, Haberl ML, Brown JF. Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes-Eutrophus H850. Applied and Environmental Microbiology. 1987;53(5):1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Sergeyev O, Korrick S, Lee MM, Revich B, Altshul L, Patterson DG, Jr, Turner WE, Needham LL, Saharov I, Hauser R. Predictors of Serum Dioxins and PCBs among Peripubertal Russian Boys. Environmental Health Perspectives. 2009;117(10) doi: 10.1289/ehp.0800223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekol T, Vough LR, Chaney RL. Phytoremediation of polychlorinated biphenyl contaminated soils: the rhizosphere effect. Environment International. 2004;30(6):799–804. doi: 10.1016/j.envint.2004.01.008. [DOI] [PubMed] [Google Scholar]
- de Carcer DA, Martin M, Karlson U, Rivilla R. Changes in bacterial populations and in biphenyl dioxygenase gene diversity in a polychlorinated biphenylpolluted soil after introduction of willow trees for rhizoremediation. Applied and Environmental Microbiology. 2007;73(19):6224–6232. doi: 10.1128/AEM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly PK, Hegde RS, Fletcher JS. Growth of PCB degrading bacteria on compounds from photosynthetic plants. Chemosphere. 1994;28(5):981–988. [Google Scholar]
- Fava F, Gentilucci S, Zanaroli G. Anaerobic biodegradation of weathered polychlorinated biphenyls (PCBs) in contaminated sediments of Porto Marghera (Venice Lagoon, Italy) Chemosphere. 2003;53(2):101–109. doi: 10.1016/S0045-6535(03)00438-7. [DOI] [PubMed] [Google Scholar]
- Hermanson MH, Hites RA. Polychlorinated-biphenyls in tree bark. Environmental Science & Technology. 1990;24(5) [Google Scholar]
- Hernandez BS, Koh SC, Chial M, Focht DD. Terpene-utilizing isolates and their relevance to enhanced biotransformation of polychlorinated biphenyls in soil. Biodegradation. 1997;8(3):153–158. [Google Scholar]
- Hiraishi A. Biodiversity of dehalorespiring bacteria with special emphasis on polychlorinated biphenyl/dioxin dechlorinators. Microbes and Environments. 2008;23(1):1–12. doi: 10.1264/jsme2.23.1. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Schnoor JL, Lichtand LA, St Clair MA. Fate and transport of organic compounds in municipal solid compost. Compost Science and Utilization. 1993;1(4):36. [Google Scholar]
- Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmospheric Environment. 2010;44(12) doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu M, Kochankova KL, Demnerova K, Macek T, Mackova M. Rhizosphere biodegradation studies on long-term PCB contaminated soil; isolation and characterization of different rhizosphere microbial communities from PCBs soil. 2007:S236–S237. [Google Scholar]
- Ionescu M, Beranova K, Dudkova V, Kochankova L, Demnerova K, Macek T, Mackova M. Isolation and characterization of different plant associated bacteria and their potential to degrade polychlorinated biphenyls. International Biodeterioration & Biodegradation. 2009;63(6):667–672. [Google Scholar]
- Jordahl JL, Foster L, Schnoor JL, Alvarez PJJ. Effect of hybrid poplar trees on microbial populations important to hazardous waste bioremediation. Environmental Toxicology and Chemistry. 1997;16(6):1318–1321. [Google Scholar]
- Krauss M, Wilcke W. Polychlorinated naphthalenes in urban soils: analysis, concentrations, and relation to other persistent organic pollutants. Environmental Pollution. 2003;122(1) doi: 10.1016/s0269-7491(02)00285-3. [DOI] [PubMed] [Google Scholar]
- Kuo C, Liu S, Liu C. Biodegradation of Coplanar Polychlorinated Biphenyl by Anaerobic Microorganisms from Estuarine Sediments. Chemosphere. 1999;39(9):1445–1458. doi: 10.1016/s0045-6535(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Leigh MB, Fletcher JS, Fu XO, Schmitz FJ. Root turnover: An important source of microbial substrates in rhizosphere remediation of recalcitrant contaminants. Environmental Science & Technology. 2002;36(7):1579–1583. doi: 10.1021/es015702i. [DOI] [PubMed] [Google Scholar]
- Leigh MB, Prouzova P, Mackova M, Macek T, Nagle DP, Fletcher JS. Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Applied and Environmental Microbiology. 2006;72(4):2331–2342. doi: 10.1128/AEM.72.4.2331-2342.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Hu DF, Jiang GB, Schnoor JL. In Vivo Biotransformation of 3,3 ′,4,4 ′-Tetrachlorobiphenyl by Whole Plants-Poplars and Switchgrass. Environmental Science &Technology. 2009;43(19):7503–7509. doi: 10.1021/es901244h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Schnoor JL. Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure. Chemosphere. 2008;73(10):1608–1616. doi: 10.1016/j.chemosphere.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magar VS, Brenner RC, Johnson GW, Quensen JF. Long-term recovery of PCB-contaminated sediments at the Lake Hartwell superfund site: PCB dechlorination. 2. Rates and extent. Environmental Science & Technology. 2005;39(10):3548–3554. doi: 10.1021/es0486216. [DOI] [PubMed] [Google Scholar]
- Malloy TA, Goldfarb TD, Surico MTJ. PCDDS, PCDFS, PCBS, chlorophenols (CPS) and chlorobenzenes (CBZS) in samples from various types of composting facilities in the United States. Chemosphere. 1993;27(1-3):325–334. [Google Scholar]
- Pakdeesusuk U, Lee CM, Coates JT, Freedman DL. Assessment of natural attenuation via in situ reductive dechlorination of polychlorinated biphenyls in sediments of the twelve mile creek arm of Lake Hartwell, SC. Environmental Science & Technology. 2005;39(4):945–952. doi: 10.1021/es0491228. [DOI] [PubMed] [Google Scholar]
- Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH. Phytoremediation of organic and nutrient contaminants. Environmental Science & Technology. 1995;29(7):A318–A323. doi: 10.1021/es00007a747. [DOI] [PubMed] [Google Scholar]
- Slater H, Gouin T, Leigh MB. Assessing the potential for rhizoremediation of PCB contaminated soils in northern regions using native tree species. Chemosphere. 2011;84(2):199–206. doi: 10.1016/j.chemosphere.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje JM, Quensen JF, 3rd, Chee-Sanford J, Schimel JP, Boyd SA. Microbial reductive dechlorination of PCBs. Biodegradation. 1993;4(4):231–40. doi: 10.1007/BF00695971. [DOI] [PubMed] [Google Scholar]
- Wilcke W, Zech W. Polychlorinated Biphenyls (PCBs) in bulk soil and particle size separates of soils in a rural community. Zeitschrift Fur Pflanzenernahrung Und Bodenkunde. 1998;161(3) [Google Scholar]
- Zanaroli G, Perez-Jimenez JR, Young LY, Marchetti L, Fava F. microbial reductive dechlorination of weathered and exogenous co-planar polychlorinated biphenyls (PCBs) in an anaerobic sediment of Venice Lagoon. 2006:19–27. doi: 10.1007/s10532-005-3752-7. [DOI] [PubMed] [Google Scholar]