Attempts to identify an easily measured cell surface marker that would detect activation of T or B lymphocytes in a variety of species have been unsuccessful. Although insulin receptors have been identified for fat, liver, skeletal and cardiac muscle, fibroblasts and monocytes1–8, quiescent rat splenic T lymphocytes do not bear such receptors. However, insulin receptors do emerge on T lymphocytes after concanavalin A treatment or allogeneic skin grafting9,10. Following our work on characterising a lymphocyte insulin receptor which participates in the modulation of immune response11, we have examined and report here the possibility that the emergence of a membrane-bound receptor for the peptide hormone insulin may be a universal marker for activated T and B lymphocytes.
Rat splenic lymphocyte cell suspensions were established in RPMI-1640 media buffered with 5 mM HEPES and fortified with 5% sterile, heat-inactivated fetal calf serum (designated ‘enriched’ media). Leukocytes were collected from a Ficoll-Hypaque gradient. The cells were fractionated on nylon wool columns yielding T-enriched, nylon wool-nonadherent cells and B-enriched, nylon wool-adherent cells. Macrophages were removed from all final cell suspensions by removing the cells that phagocytose iron particles with magnetic attraction. Insulin binding assays were carried out in suspensions containing 107 cells per ml in Hank’s balanced salt solution enriched with 0.1% bovine serum albumin for analysis of receptor binding or the ‘enriched’ media for analysis of thymidine incorporation. Such purification techniques were evaluated using cell surface markers for the Fc receptor, the complement receptor, surface immunoglobulin, anti-θ determinants, and acridine orange and p-rosaniline staining characteristics.
The insulin receptor was measured by the use of a modification of the insulin binding assay described by Gammeltoft and Gliemann12 which has been extensively described10. In brief, the assay involves a competitive binding reaction between radiolabelled insulin and unlabelled monocomponent porcine insulin. By such competition, both total and nonspecific isotopic binding may be measured, and specific (receptor) binding can be calculated. Specific binding is 50–60% of the total binding and is similar to that of the fibroblast. Final separation of cell-bound from free insulin was accomplished over oil with virtually no contamination of the cell fraction by free insulin and recovery of 93% of cells for analysis. Lymphocyte cultures were also evaluated for the intracellular accumulation of tritiated thymidine after a four-hour pulse of 106 cells in flat-bottom microtitre plates (1 μ Ci per well).
Five separate experimental protocols which result in lymphocyte activation were tested (Table 1). In vivo activation was accomplished by: (1) skin transplantation with histoincompatible ((Lewis × Brown Norway) F1→ Lewis male rat) grafts; (2) graft-versus-host disease established by intraperitoneal injections of 2×l08 Lewis splenic lymphocytes weekly for four weeks into (Lewis × Brown Norway) F1 animals. In vitro activation was established in the following ways: (1) unidirectional mixed lymphocyte cultures were established using T-enriched Lewis splenic lymphocytes as responder cells and Brown Norway mitomycin-treated thymocytes as stimulating cells. Cells were collected after three days of culture. On termination of the cultures, stimulator cells were removed by specific antibody and complement treatment and the responder cells were assessed for the presence of insulin receptors; (2) varying lymphocyte populations were cultured with the plant lectin concanavalin A (con A), phytohaemagglutinin (PHA-P), or Escherichia coli lipopolysaccharide (LPS).
Table 1.
Appearance of specific insulin binding site on T or B lymphocytes after in vivo or in vitro stimulation
| Mode of activation | Receptor occurs on isolated | Half-maximal equilibrium concentration (nM) | |
|---|---|---|---|
| T | B | ||
| In vivo | |||
| Skin graft | + | ND* | 1.3 |
| Graft-versus-host disease | + | ND* | 1.5 |
| In vitro | |||
| Mixed lymphocyte culture | + | + | 1.0 |
| Mitogens | |||
| concanavalin A | + | − | 2.4 |
| phytohaemagglutinin | + | − | 2.0 |
| lipopolysaccharide | − | + | 1.5 |
Not determined.
Table 1 summarises our findings. After in vivo lymphocyte activation by either skin grafting or the graft-versus-host reaction, lymphocyte receptors with similar binding properties emerged. In vitro, T-enriched lymphocytes developed the insulin receptor after allogeneic stimulation in the unidirectional mixed lymphocyte culture or after mitogenic stimulation with con A or PHA but not LPS. B-enriched lymphocytes, but not T cells, developed the insulin receptor in vitro after specific T-dependent mitogen stimulation. When T lymphocytes and B lymphocytes were co-cultured with a T-cell mitogen (PHA-P), both cell populations developed the hormone receptor, probably secondary to T–B cellular interaction (data not shown). The specific binding site did not emerge after syngeneic skin grafting or co-culture with syngeneic cells. The insulin binding was saturable, specific, expressed high affinity for insulin, and was readily displaceable from the receptor. A representative binding isotherm and binding competition curve are shown in Fig. 1 for the experimental protocol using allogeneic skin engraftment.
Fig. 1.
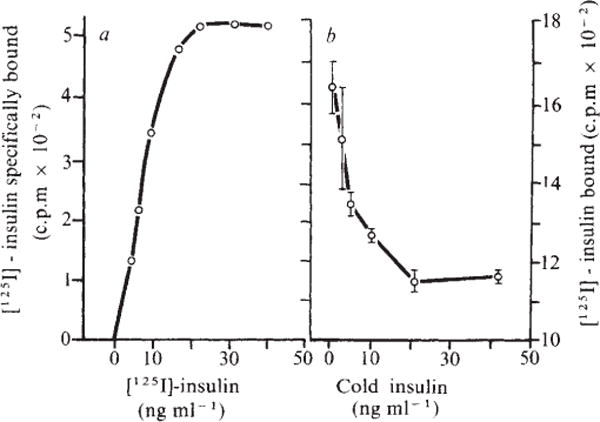
A representative binding isotherm (a) and binding competition curve (b) for insulin on lymphocytes from Lewis rats 7 d after receiving (Lewis × Brown Norway) F1 skin allografts. The half maximal equilibrium constant, calculated from the concentration of insulin at which binding is half-maximal, was 1.3 nM. The affinity of unlabelled insulin for its binding site is estimated from the concentration dependence of the binding inhibition curve20 and was 0.2 nM. Both values permit binding at physiological concentrations of insulin.
Most polypeptide hormones convey their signals to target cells through interaction with membrane recognition units, receptors13. The criteria which established binding sites on cell membranes as specific hormone receptors include a demonstration of ligand specificity, reversibility, saturation kinetics, an affinity for hormone consistent with ligand–receptor complex formation in vivo, and a biological function. The T lymphoctye insulin binding site satisfies the first four conditions after activation by in vivo or in vitro stimuli. The high affinity of binding potentially allows the lymphocyte to be functionally modulated by circulating concentrations of insulin. Furthermore, binding of insulin to its putative receptor augments cytotoxic effector lymphocyte function10,11. Thus, the specific binding site for insulin on the lymphocyte is a biological receptor.
Previous attempts at identification of cell surface markers that identify the active state of lymphocytes have been either unsuccessful or restrictive. The Fc receptor on the T cell14, once thought to identify the active state, has been found in several resting lymphocytes15,16. Most recently, an antigen, Ala-1, has been described in mouse, occurring on lymphocytes exclusively as a result of cellular activation17, 18. This antigen, however, is species specific and genetically restricted with at least several alleles. In contrast, the insulin receptor on lymphocytes is a marker of cellular activation which can be widely and generally applied. It is not clone specific, may be applied to activated B as well as T lymphocytes, and is not genetically or species restricted but occurs in all strains in rat and mice so far tested after manoeuvres causing cellular activation. As T-cell and B-cell mitogens selectively stimulate the appearance of T-cell or B-cell insulin receptors, these data suggest that only the subpopulation of cells that has been activated develops a receptor for insulin. Preliminary evidence from our laboratory demonstrates that the receptor also marks the active state in man19. Thus, the lymphocyte insulin receptor may be a universal marker of lymphocyte activation.
Acknowledgments
This work was supported in part by NIH grant 1-R01-AM21094-01 IMR.
References
- 1.Freychet P, Roth J, Neville DM., Jr Proc natn Acad Sci USA. 1971;68:1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuatrecasas P. J biol Chem. 1971;246:6532–6542. [PubMed] [Google Scholar]
- 3.Cuatrecasas P. Proc natn Acad Sci USA. 1972;69:1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olefsky J, Bacon VS, Baur S. Metabolism. 1976;25:179–191. doi: 10.1016/0026-0495(76)90048-2. [DOI] [PubMed] [Google Scholar]
- 5.Forgue ME, Freychet P. Diabetes. 1975;24:715–723. doi: 10.2337/diab.24.8.715. [DOI] [PubMed] [Google Scholar]
- 6.Gavin JR, III, Roth J, Jen P, Freychet P. Proc natn Acad Sci USA. 1972;69:747–751. doi: 10.1073/pnas.69.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollenberg MD, Cuatrecasas P. J biol Chem. 1975;250:3845–3853. [PubMed] [Google Scholar]
- 8.Schwartz RH, Bianco AR, Handwerger BS, Kahn CR. Proc natn Acad Sei USA. 1975;72:474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krug U, Krug F, Cuatrecasas P. Proc natn Acad Sci USA. 1972;69:2604–2608. doi: 10.1073/pnas.69.9.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helderman JH, Strom TB. J clin Invest. 1977;59:334–338. doi: 10.1172/JCI108646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strom TB, Bear RA, Carpenter CB. Science. 1975;187:1206–1208. doi: 10.1126/science.163492. [DOI] [PubMed] [Google Scholar]
- 12.Gammeltoft S, Gliemann J. Biochim biophys Acta. 1973;320:16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- 13.Cuatrecasas P. Proc nutn Acad Sci USA. 1969;63:450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida TP, Anderson B. Scand J Immun. 1972;1:401. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown G, Greaves MJ. Eur J Immun. 1974;4:302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- 16.Moretta L, Webb SR, Grossi CE, Lydyard PM, Cooper M. J exp Med. doi: 10.1084/jem.146.1.184. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feeney AJ, Hämmcrling U. Immunogenetics. 1976;3:369–377. [Google Scholar]
- 18.Feeney AJ, Hämmerling U. J Immun. 1977;118:1488–1494. [PubMed] [Google Scholar]
- 19.Helderman JH, Strom TB. Clin Res. 1977;25:484A. [Google Scholar]
- 20.Cheng Y, Prusoff WH. Biochem Pharmac. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]


