Abstract
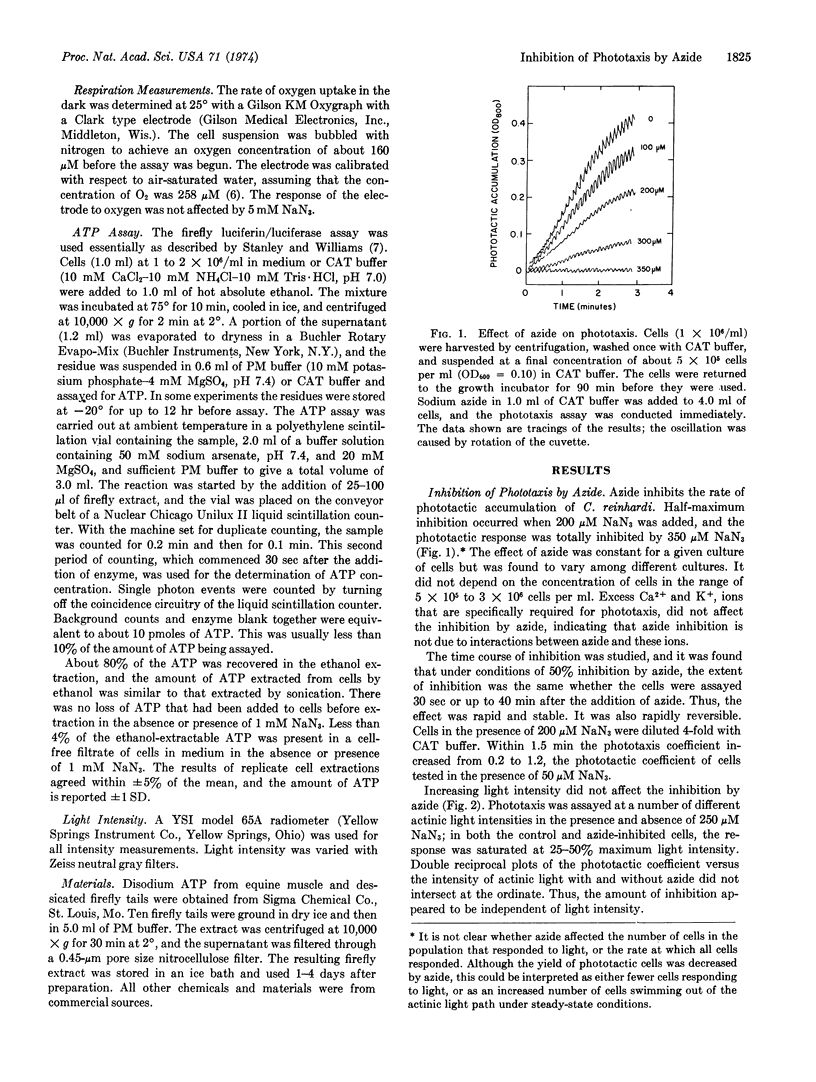
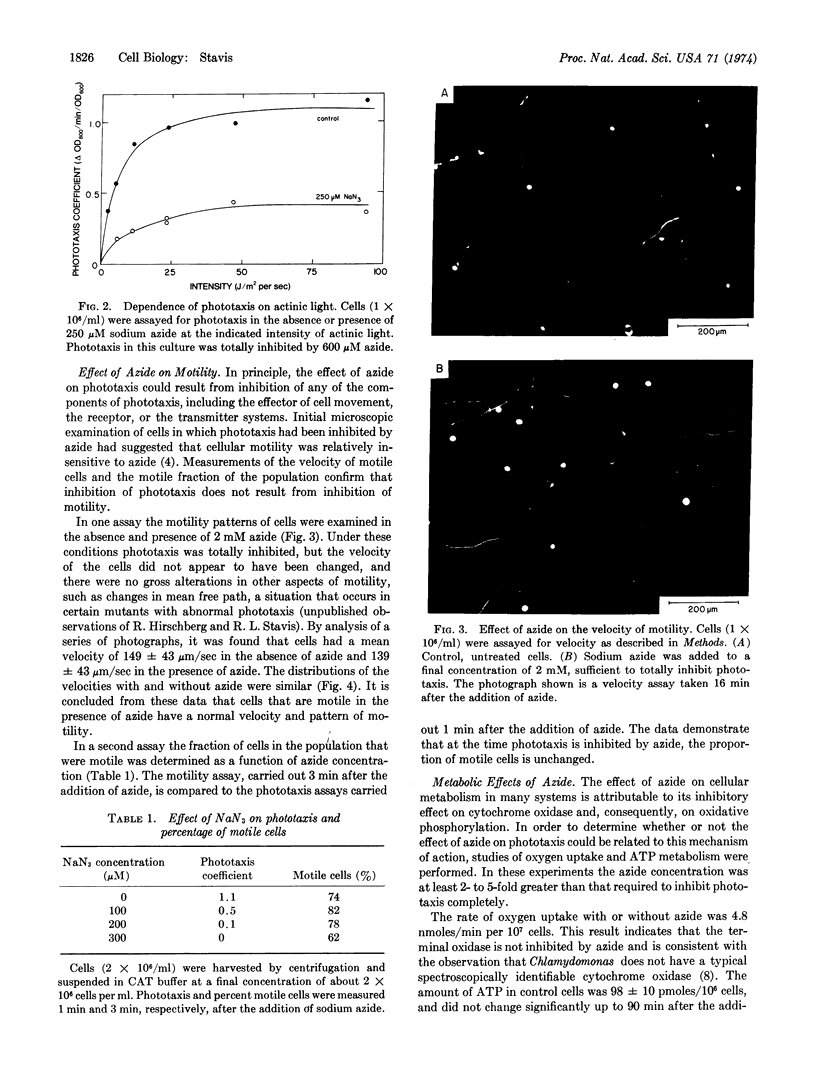
Phototaxis in Chlamydomonas reinhardi was specifically inhibited by azide. The effect of azide was rapid and reversible, and did not depend upon the intensity of actinic light. Under conditions of completely inhibited phototaxis, azide had no effect on the number of motile cells in the population or on the rate of motility. The effect was not related to changes in oxygen uptake or cellular ATP concentration. Apparently, a cellular component or process specifically involved in phototaxis is inactivated by azide.
Keywords: motility
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance B., Sager R. Oxygen and Light Induced Oxidations of Cytochrome, Flavoprotein, and Pyridine Nucleotide in a Chlamydomonas Mutant. Plant Physiol. 1957 Nov;32(6):548–561. doi: 10.1104/pp.32.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Kung C., Eckert R. Genetic modification of electric properties in an excitable membrane (paramecium-calcium conductance-electrophysiological measurements-membrane mutant). Proc Natl Acad Sci U S A. 1972 Jan;69(1):93–97. doi: 10.1073/pnas.69.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING G., GERARD R. W. The membrane potential and metabolism of muscle fibers. J Cell Physiol. 1949 Dec;34(3):413–438. doi: 10.1002/jcp.1030340307. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Stavis R. L., Hirschberg R. Phototaxis in Chlamydomonas reinhardtii. J Cell Biol. 1973 Nov;59(2 Pt 1):367–377. doi: 10.1083/jcb.59.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi H. Mitochondrial membrane potential: evidence from studies with a fluorescent probe. Proc Natl Acad Sci U S A. 1974 Feb;71(2):583–585. doi: 10.1073/pnas.71.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]





