Abstract
Vasoactive intestinal peptide (VIP), a 28-amino acid neuropeptide/neurotransmitter, is widely distributed in both the central and peripheral nervous system. VIP is released by both neurons and immune cells. Various cell types, including immune cells, express VIP receptors. VIP has pleiotropic effects as a neurotransmitter, immune regulator, vasodilator and secretagogue. This review is focused on VIP production and effects on immune cells, VIP receptor signaling as related to immune functions, and the involvement of VIP in inflammatory and autoimmune disorders. The review addresses present clinical use of VIP and future therapeutic directions.
Keywords: Vasoactive intestinal peptide, Autoimmunity, Inflammation, Neuroinflammation, Neuropeptides/neurotransmitters
Introduction
The 28-amino acids vasoactive intestinal peptide (VIP) was initially isolated from the intestine (Said and Mutt 1970) and identified soon thereafter as a neuropeptide localized both in the central and peripheral nervous system (Said and Rosenberg 1976). VIP belongs to a family of structurally related neuropeptides and hormones that include secretin, glucagon, growth hormone releasing factor, glucagon-like peptide-1 and -2, helodermin, gastric inhibitory peptide and the closely related pituitary adenylate cyclase-activating polypeptide (PACAP) [reviewed in (Vaudry et al. 2009)]. Presumably, the peptide superfamily resulted from an extensively diverged common ancestral gene. VIP is expressed by neurons in various brain areas, and stored and released from nerve fibers innervating numerous organs, including heart, lung, thyroid, kidney, urogenital and gastrointestinal tracts, and immune organs such as spleen, thymus, bone marrow and lymph nodes (Henning and Sawmiller 2001). Although the neuronal source of VIP has been firmly established, immune and endocrine cells have been also reported to express and secrete VIP [reviewed in (Delgado et al. 2004b). VIP is synthesized as preproVIP which contains the VIP sequence and the related peptide called PHM (peptide with N-terminal histidine and C-terminal methionine amide) in humans, or PHI (peptide with N-terminal histidine and C-terminal isoleucine amide) in other species [reviewed in (Fahrenkrug 2010)]. The fact that VIP and PHI/PHM are not always found in the same cell suggests alternative RNA or differential protein processing. The secondary structure of mature VIP is characterized by two β-turns containing the initial N terminus eight-amino acid residues, followed by two helices (residues 7–15 and 19–27) connected by a region of undefined structure that presumably confers molecular flexibility (Fry et al. 1989).
Its wide distribution reflects VIP's pleiotropic effects as a neurotransmitter, immune regulator, vasodilator and secretagogue. In the CNS, VIP has been shown to affect learning and behavior and to have neurotrophic effects [reviewed in (Gozes et al. 2003; Hill 2007; Masmoudi-Kouki et al. 2007; Passemard et al. 2011a)]. VIP affects cardiac output, bronchodilation, smooth muscle contraction and gastrointestinal motility [reviewed in (Dickson and Finlayson 2009; Snoek et al. 2010; Moody et al. 2011)]. As a secretagogue, VIP induces release of prolactin, luteinizing hormone and growth hormone from the pituitary, and regulates the release of insulin and glucagon in the pancreas (Mazzocchi et al. 1998; Winzell and Ahren 2007). In addition, VIP regulates bone metabolism, circadian rhythms and embryonic development, and plays an important role as immune modulator [reviewed in (Piggins and Cutler 2003; Jones et al. 2004; Ganea et al. 2006; Gonzalez-Rey et al. 2007; Vosko et al. 2007; Smalley et al. 2009; Passemard et al. 2011b)]. Although VIP has multiple physiological functions, its translation to the clinic occurred only recently. To date, VIP has been successfully applied to treatment of erectile dysfunction, pulmonary hypertension and sarcoidosis. Invicorp (Plethora Solutions, UK), a combination of VIP and phentolamine mesylate with complementary effects on arterial blood flow and veno-occlusion, has been used for the management of moderate to severe erectile dysfunction with high success rate and low to negligible side effects (Dinsmore et al. 1999). In idiopathic pulmonary arterial hypertension, VIP inhalations resulted in the reduction of pulmonary arterial pressure, temporary pulmonary vasodilation, improved stroke volume and mixed venous oxygen saturation, and reduction in pulmonary vascular resistance (Petkov et al. 2003; Leuchte et al. 2008). Nebulized VIP also reduced inflammatory markers in bronchoalveolar lavage fluid of patients with chronic sarcoidosis in a phase II clinical trial (Prasse et al. 2010).
VIP receptors: characterization and signaling
General characterization
In agreement with their high degree of structural similarity, VIP and PACAP bind to a subset of G protein-coupled plasma membrane receptors, which share a common molecular architecture consisting of seven transmembrane domains linked through three extracellular (EC1, EC2, and EC3) and three intracellular (IC1, IC2, and IC3) loops, a long N-terminal extracellular domain and an intracellular C-terminal domain. To date, three types of VIP/PACAP receptors have been cloned and classified as VPAC1, VPAC2 and PAC1 (Harmar et al. 1998). VPAC1 and 2 bind VIP and PACAP with equal affinitiy (Kd ≈ 1 nM) and activate primarily the adenylate cyclase pathway. PAC1 is a PACAP-preferring receptor, with high affinity for PACAP (Kd ≈ 0.5 nM) and low affinity for VIP (Kd >500 nM). PAC1 activates both adenylate cyclase and phospholipase C and consists of eight variants that result from alternative splicing of a single transcript and inclusion or exclusion of the so-called hip and hop cassettes [reviewed in (Langer and Robberecht 2007; Dickson and Finlayson 2009; Vaudry et al. 2009)].
There are differences in the localization of the three VIP/PACAP receptors. VPAC1 is expressed in brain (cerebral cortex and hippocampus) and in peripheral tissues such as liver, lung and intestine, as well as immune cells (see below). VPAC2 is expressed in the CNS (primarily in thalamus and suprachiasmic nucleus, and at lower levels in hippocampus, brainstem, spinal cord and dorsal root ganglia) and in a number of peripheral tissues, including the pancreas, skeletal muscle, lung, heart, kidney, adipose tissue, testis, stomach, blood vessels, the GI and reproductive tract. PAC1 is present predominantly in brain (olfactory bulb, thalamus, hypothalamus, dentate gyrus of the hippocampus and cerebellum) and in the adrenal medulla. The wide distribution of these receptors indicates that VIP/PACAP affect many different targets, both in the CNS and in the periphery.
VIP receptors in the immune system
The identification of VIP receptors in immune cells was the first indication of specific, receptor-mediated effects of VIP on the immune response. The expression of fully functional VIP receptors in the immune system was first described in human peripheral blood lymphocytes through 125I-VIP ligand binding and measurements of cAMP induction (Guerrero et al. 1981; O'Dorisio et al. 1981). The binding studies indicated either a single class of high-affinity binding sites, or two classes, i.e., a low number of high-affinity and a high number of low-affinity binding sites, depending on cell type and activation stage. Following cloning of the VIP/PACAP receptors, studies focusing on the expression of the three types of receptors showed that VPAC1 was constitutively expressed in lymphocytes (including thymocytes, CD4 and CD8 T cells), macrophages, monocytes, dendritic cells (DCs), microglia and mast cells, and that VPAC2 was expressed at low levels in naïve or resting cells, but could be induced following stimulation [reviewed in (Delgado et al. 2004b)]. More recently, expression of VPAC2 receptors in macrophages was reported to be regulated by TLR2 and TLR4 ligands, but not by TLRs specific for bacterial or viral nucleic acids (Herrera et al. 2009) PAC1 was found to be expressed in cells of the macrophage/monocyte lineage, and had equal affinity for VIP and PACAP, in contrast to the PAC1 receptors found in the CNS. The presence of VIP receptors on neutrophils is still controversial. The VIP-induced cAMP production in human neutrophils (Palermo et al. 1996) might be mediated through non-receptor mechanisms (Pedrera et al. 1994).
Initial studies using specific VIP/PACAP receptor agonists and antagonists established VPAC1 as the major mediator for the immunomodulatory effects of VIP (Delgado et al. 2004b; Gonzalez-Rey and Delgado 2007). However, later studies using VPAC2- and PAC1-deficient mice showed increased susceptibility for inflammatory disorders, suggesting that these receptors also participate in immune regulation (Goetzl et al. 2001; Martinez et al. 2002; Lauenstein et al. 2010; Samarasinghe et al. 2011). A critical immunoregulatory role for VPAC1 has been reported in humans. Immune cells of patients with autoimmune/inflammatory diseases such as ankylosing spondylitis, rheumatoid arthritis and osteoarthritis were reported to express lower VPAC1 levels and to respond poorly to VIP (Sun et al. 2006; Delgado et al. 2008a; Juarranz et al. 2008; Paladini et al. 2008). Interestingly, reduced expression of VPAC1 in patients with rheumatoid arthritis is associated with a polymorphism in the 3′ UTR region of the VPAC1 gene (Delgado et al. 2008a; Paladini et al. 2008). A decrease in VPAC2 expression in Th1 cells of patients with multiple sclerosis has been also described, although no associations with genetic polymorphisms were reported (Sun et al. 2006). These findings suggest that defects in the VIP receptor/signaling system might be a predisposing factor in the development of autoimmune diseases.
General signaling pathways for VIP receptors in immune cells
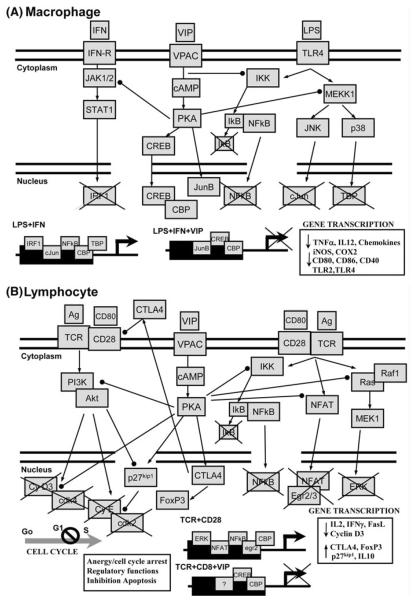
Specific signaling pathways mediating VIP effects on various types of immune cells are discussed in detail in the sections below. Here, we address only the general characteristics for VIP/PACAP signaling described in various biological systems. Similar to other tissues and cells, VPAC1, VPAC2 and PAC1 are coupled to adenylate cyclase activation and subsequent activation of the protein kinase A (PKA) in immune cells [reviewed in (Delgado et al. 2004b)]. PAC1 also activates phospholipase C and protein kinase C (PKC) in macrophages and monocytes. The cAMP/PKA pathway appears to be the major signaling pathway for the anti-inflammatory action of VIP in macrophages, monocytes, DCs and microglia (Fig. 1a), and in the regulation of the T lymphocyte response (Fig. 1b). However, a second, PKA-independent pathway also participates in the inhibitory effect of VIP on macrophages and monocytes, resulting in the inhibition of NFκB nuclear translocation (Fig. 1a) [reviewed in (Delgado et al. 2004b)]. In contrast to VPAC1/VPAC2 signaling, the involvement of PAC1 and PKC has been limited to VIP-induced stimulation of IL-6 production in resting macrophages (Martinez et al. 1998).
Fig. 1.
VIP tunes immune and inflammatory response by regulating multiple signaling pathways. a VIP signaling during inflammatory response (macrophage, microglia and DCs). Binding of VIP to its receptor induces cAMP, activates PKA and exerts several effects: (1) inhibition of IFNγ-induced Jak1/Jak2 phosphorylation, STAT1 activation and subsequent expression of IRF-1, which is critical for gene activation of various inflammatory genes (CD40, CXCL10, iNOS, COX2); (2) inhibition of various MAPK cascades, initiated by suppression of MEKK1/MEK4 and of MEKK1/MEK6 and subsequent inhibition of Jun-kinase (JNK) and p38 MAPK, respectively. This results in a change in the composition of the transcription factor AP-1 with c-Jun being replaced by JunB, and inhibition of phosphorylation/activation of TATA-box binding protein (TBP). Moreover, through a PKA-independent mechanism, VIP inhibits IκB-kinase (IKK) activity and suppresses nuclear translocation and activation of the transcription factor NFκB. AP1, TATA-box protein and NFκB act in concert to activate gene transcription of most of inflammatory cytokines and chemokines, as well as costimulatory molecules. Moreover, in a PKA-dependent manner, VIP stimulates cAMP-responsive element binding (CREB) factor to compete with NFκB for coactivators, such as p300-CBP, required for transcription of inflammatory genes. b VIP signaling in T lymphocytes. Binding of VIP to its receptors activates the cAMP/PKA pathway, which regulates cell cycle and activation of T lymphocytes at multiple levels. First, VIP downregulates the PI3K-Akt pathway and, consequently, the activity of cdk4-cyclin D complexes, which induce genes involved in DNA replication and progression through the S phase. VIP also inhibits the expression of cyclin D3. In parallel, VIP increases the levels of the cdk-inhibitor p27kip1 by inhibiting the Akt-mediated phosphorylation/degradation of p27kip1 and by inducing p27kip1 gene expression. Thus, VIP promotes the p27kip1-mediated inactivation of the cyclin E-cdk2 complexes that result in cell cycle arrest in the G1 phase. Second, VIP inhibits signaling through the Ras–Raf1–MEK1–ERK cascade by reducing Ras activity and by impairing Raf1–Ras interaction. This deactivates AP-1 and reduces its binding to the IL-2 promoter. Moreover, VIP decreases the nuclear translocation of NFκB and NFAT, also required for IL-2 transcription. As a critical growth factor for T cells, inhibition of IL-2 by VIP reduces T cell proliferation and induces anergy. Third, VIP increases the expression of both soluble and membrane forms of CTLA4, which are critically involved in the induction of Foxp3 expression and of suppressor activity in T cells. Arrows indicate activation. Dotted-end lines indicate inhibition
VIP effects on immune cells
VIP affects both innate and adaptive immune responses and acts as a major anti-inflammatory factor in animal models of inflammatory and autoimmune diseases, suggesting that the VIP/VIP receptor system could serve as a target for new therapeutic strategies in immune disorders. The in vitro studies were performed in general with native VIP at an effective dose range of 1–10 nM, which corresponds to the Kd of the VIP receptors expressed on immune cells. In the absence of other stimuli, VIP did not have significant effects, and the highest efficacy was obtained when VIP was administered together or immediately after stimuli. Most of the in vivo experiments were performed in mice with VIP doses of 1–5 nmol (2–15 μg/mouse) administered intraperitoneally (i.p.). Although they showed improved stability, specific VIP receptors agonists had efficacies similar to VIP, suggesting the involvement of multiple receptors.
Effects of VIP on the innate immune response
VIP as a “deactivator” of macrophages, microglia and dendritic cells (DCs)
To eliminate invading pathogens, the immune system mounts two interconnected responses, i.e., innate and adaptive immunity. The innate immune response, characterized by phagocytosis/endocytosis, release of oxygen and nitrogen radicals, and production of proinflammatory cytokines and chemokines, occurs following ligation of pattern-recognition receptors (PRRs) by foreign molecular patterns shared by groups of pathogens. The major cell types involved are neutrophils, macrophages and DCs in the periphery, and microglia in the CNS. Following interaction with pathogens, macrophages, microglia and especially DCs have the capacity to initiate an adaptive immune response, by presenting processed antigens to naïve T cells bearing the appropriate T cell receptors (TCR). An uncontrolled inflammatory response can lead to tissue damage, organ failure and death, and endogenous anti-inflammatory factors such as glucocorticoids, lipid mediators, anti-inflammatory cytokines and neuropeptides such as VIP are essential in controlling the inflammatory response.
Activated macrophages, DCs and microglia secrete proinflammatory cytokines and chemokines. VIP was shown to inhibit the production of TNF, IL-6 and IL-12, the induction of iNOS, and to stimulate the production of the anti-inflammatory cytokine IL-10 in LPS-stimulated macrophages and microglia primarily through VPAC1 (Fig. 2) [reviewed in (Delgado et al. 2004b)]. Recent reports indicate that VIP inhibits COX2 expression in activated macrophages, DCs and microglia, downregulates macrophage-derived high mobility group box-1 (HMGB1), a late-occurring cytokine involved in lethal endotoxemia and sepsis, and suppresses the inflammatory response of microglia exposed to beta-amyloid fibrils or to the neurotoxin MPTP in a model of Parkinson's disease (Delgado and Ganea 2003a, b, c; Chorny and Delgado 2008; Delgado et al. 2008c). Local i.c.v. administration of VIP prevents LPS-induced neurodegeneration and microglia activation in vivo in models of neuroinflammation (Kim et al. 2000; Delgado and Ganea 2003a, b, c).
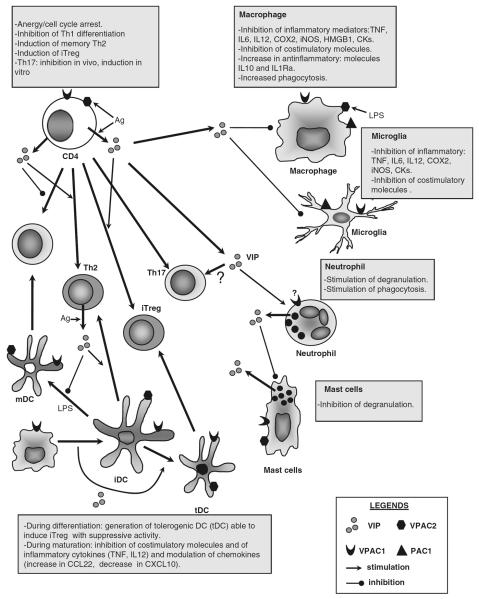
Fig. 2.
Effects of VIP on immune cells. VIP is released in the context of an immune response by CD4 and CD8 lymphocytes (especially, Th2 CD4 and Tc2 CD8 cells), and by mast cells and neutrophils. Various immune cells differentially express the three VIP receptors (VPAC1, VPAC2 and PAC1), whose expression is modulated by antigenic and inflammatory stimuli. VIP acts on macrophages and microglia inhibiting the production of inflammatory mediators, such as cytokines, chemokines (CKs), lipids (PGE2 by inhibiting cycloxygenase 2, COX2) and free radicals (nitric oxide by inhibiting the inducible form of NO synthase, iNOS), and by stimulating the production of anti-inflammatory cytokines (IL-10 and IL-1Ra). In addition, VIP reduces costimulatory molecule expression in macrophages and mature dendritic cells (mDCs), limiting the clonal expansion of Th1 cells under inflammatory conditions. VIP modulates the adaptive response in different ways. First, VIP inhibits the differentiation of Th1 cells and favors the expansion of Th2 cells through various non-excluding mechanisms that involve regulation of DC functions, Th1-differentiating factors, chemokines and apoptosis. Second, VIP induces the emergence of Treg cells (iTreg) through direct effects on naïve T cells and indirectly through the generation of tolerogenic DCs (tDCs). The effect on Th17 differentiation is controversial. VIP impairs mast cell degranulation, whereas the antimicrobial functions of neutrophils appear to be stimulated by VIP
Accumulation of immune cells at the inflammatory site is mediated primarily through chemokines. VIP inhibits the expression of several proinflammatory chemokines, i.e., CXCL1/KC, CXCL2, CCL2, CCL3, CCL4 and CCL5 in mouse macrophages and microglia, and IL-8 in human peripheral blood monocytes activated with LPS (Delgado and Ganea 2001a, b, c, 2003a, b, c; Delgado et al. 2002a). In a model of acute peritonitis, VIP reduced neutrophil, macrophage and lymphocyte recruitment to the peritoneal cavity (Delgado and Ganea 2001a, b, c).
In terms of signaling pathways, VIP affects the expression of pro- and anti-inflammatory factors in LPS-and LPS + IFNγ-stimulated macrophages and microglia by regulating the expression and/or transactivating activity of a number of transcription factors such as AP-1, NFκB, CREB and IRF-1 (Fig. 1) (Delgado et al. 1998; Delgado and Ganea 2000a, b, 2001a, b, c; Delgado 2002). In addition to the inhibition of these downstream signaling pathways, VIP also prevents the upregulation of toll-like receptors (TLRs), reducing the capacity of monocyte/macrophages to respond to endogenous and exogenous ligands. Daily administration of VIP in experimental colitis was reported to inhibit TLR-2 and TLR-4 expression in colonic extracts and on macrophages, DCs and lymphocytes from mesenteric lymph nodes (Gomariz et al. 2005; Arranz et al. 2008). In addition, VIP was shown to reduce LPS-induced upregulation of TRL-4 in human rheumatoid synovial fibroblasts through the inhibition of PU.1 (Gutierrez-Canas et al. 2006; Foster et al. 2007).
VIP induces tolerogenic DCs
Although DCs are essential for the stimulation of antigen-specific T cells, they also function to establish and maintain tolerance by inducing T cell anergy or generating regulatory T cells (Treg). The tolerance inducing DCs i.e., tolerogenic DCs (tDC), are rather heterogenous, including immature, semi-mature and in some cases even mature DCs (Maldonado and von Andrian 2010). tDC are of major therapeutic interest, since they can be induced with biological and pharmacological agents and manipulated to present specific autoantigens. Together with galectin 1, vitamin D3, IL-10 and TNF, VIP belongs to the group of biological agents that induce tDC (Maldonado and von Andrian 2010).
In our laboratory, differentiation of murine bone marrow-derived DC (BMDC) in the presence of VIP led to the development of DC with a tolerogenic phenotype, i.e., low expression of CD40/80/86, reduced production of TNF and IL-12, and increased the secretion of IL-10 following LPS stimulation (Fig. 2). In vitro and in vivo generated DCVIP induced IL-10-producing CD4+Foxp3+ Treg, which inhibited the proliferation of allogeneic or syngeneic Ag-specific T cells and were able to transfer tolerance to naïve recipients (Chorny et al. 2005; Delgado et al. 2005). More recently, BMDC transduced during differentiation with a lentiviral vector expressing VIP exhibited a tolerogenic phenotype (Toscano et al. 2010). Similar results were obtained with human blood monocyte-derived DC differentiated in the presence of VIP. VIP led to the generation of human tDC, which in turn induced IL-10 producing human CD4 and CD8 Treg. Both types of human Treg suppressed the proliferation/activation of antigen-specific Th1 cells (Gonzalez-Rey et al. 2006a).
Effects of VIP on T cells
VIP inhibits the capacity of antigen-presenting cells (APCs) to initiate adaptive immunity
The link between innate and adaptive immunity is built on the capacity of APCs, primarily DCs, to present processed antigen to naïve CD4 T cells and induce T cell proliferation and differentiation. In addition to TCR signaling, optimal T cell stimulation requires costimulatory signals, provided by CD40, CD80 and CD86 expressed and upregulated following APC stimulation. VIP prevents CD80 and CD86 upregulation in activated macrophages and DCs, reducing the stimulation of CD4 T cells in vivo and in vitro (Delgado et al. 1999b, 2004c). VIP also directly inhibits IL-2 gene expression and proliferation in activated CD4 T cells primarily through cAMP-dependent effects on NFAT and AP-1 (Wang et al. 2000) (Fig. 1).
VIP inhibits Th1 and promotes Th2 differentiation, survival and migration
Activated CD4 T cells differentiate into effector cells, which differ in their cytokine profile. The best characterized effectors are Th1, Th2 and the more recently described Th17 cells. Both in vivo and in vitro VIP inhibits Th1 and favors Th2 differentiation (Fig. 2) [reviewed in (Ganea et al. 2003)]. A similar Th2 preference was evident in vivo in transgenic mice overexpressing VPAC2 in CD4 T cells, whereas the Th1 response prevailed in VPAC2-deficient mice (Goetzl et al. 2001; Voice et al. 2003). These studies confirm that VIP affects the Th1/Th2 balance in vivo and indicate the prevalent role of VPAC2. A number of nonexcluding mechanisms contribute to the VIP-induced Th2 bias (Fig. 2). VIP affects Th1/Th2 generation indirectly by inhibiting IL-12 production in activated APCs, and directly by blocking IL-12 signaling through the inhibition of JAK2/STAT4 phosphorylation and by inducing c-Maf and JunB (Voice et al. 2004; Liu et al. 2007). VIP also supports Th2 survival in vivo and in vitro (Delgado et al. 2002b). The in vitro preferential survival of Th2 effectors was due to the VIP-induced inhibition of FasL and granzyme B expression, and correlated with higher levels of VPAC1/2 expression in Th2 cells (Sharma et al. 2006).
In addition, VIP also affects Th1/Th2 migration in a differential manner, by promoting Th2 and inhibiting Th1 migration under inflammatory and antigenic stimulation. This is due to the effect of VIP on promoting DC expression and release of CCL22, a Th2-attracting chemokine and inhibiting the release of the Th1-attracting chemokine CXCL10 (Jiang et al. 2002). In agreement with the in vitro studies, i.p. administration of VIP-treated DC led to the preferential accumulation of Th2 effectors in the peritoneal cavity (Delgado et al. 2004a).
Effects of VIP on Th17 differentiation
The recently discovered Th17 cells play a major role in autoimmunity, dominating the inflammatory response in rheumatoid arthritis, multiple sclerosis, psoriasis and Crohn's disease (Fujino et al. 2003; Kebir et al. 2007; Bovenschen et al. 2011; Ferraccioli and Zizzo 2011). The effect of VIP on Th17 differentiation and function is controversial. In vitro, increased numbers of IL-17+ T cells were observed in the presence of TGF β and VIP. The TGF b/VIP-induced Th17 secrete IL-17A, but differ from classical Th17 generated in the presence of TGF β and IL-6 by not producing IL-21 (Yadav et al. 2008). The biological relevance of the VIP-induced Th17 is not clear at the present time. In contrast to the in vitro data, several in vivo reports indicate that VIP suppresses Th17 differentiation. In a model of type I diabetes, VIP administration to NOD mice resulted in delayed disease onset and reduced pancreatic expression of IL-17 and IL-22, suggesting an inhibitory effect on Th17 differentiation or function (Jimeno et al. 2010). Similar results were reported in a rat model of collagen-induced arthritis where VIP reduced the percentage of splenic IL-17+ T cells and the expression of STAT3 and ROR γ t (Deng et al. 2010). The in vivo effect of VIP on Th17 differentiation could be indirect, through the induction of antigen-specific Treg (see below). Indeed, VIP administration at the time of immunization with nitrated α-synuclein in a model of Parkinson's disease resulted in nitrostriatal protection through the induction of antigen-specific Treg, which in turn abrogated the development of the neurodegenerative Th17 response (Reynolds et al. 2010).
VIP induces Treg
Both natural and inducible regulatory T cells (Treg) play an essential role in maintaining tolerance and preventing immune attacks against self-antigens. Deficiencies in Treg were reported in several human autoimmune diseases and have been documented in experimental autoimmune models (Buckner 2010; Sakaguchi et al. 2010). VIP induces Treg through the generation of tDCs (Fig. 2). However, VIP also induced Treg directly in cultures of CD3/CD28-stimulated human CD4 T cells (Pozo et al. 2009; Anderson and Gonzalez-Rey 2010). Interestingly, VIP-treated human trophoblast cells co-cultured with maternal peripheral blood mononuclear cells also induced high numbers of Foxp3+CD4+CD25+ T cells, which might represent a direct effect of autocrine VIP on fetal survival (Fraccaroli et al. 2009). Recently,. it has been reported that nebulized VIP administered to patients with sarcoidosis resulted in increased numbers of CD4+FoxP3+CD127−CD25+ Treg in the bronchoalveolar lavage (Prasse et al. 2010).
VIP administration has been also reported to induce Treg in experimental models of collagen-induced arthritis, murine type I diabetes and experimental autoimmune encephalomyelitis (EAE) (Fernandez-Martin et al. 2006; Chen et al. 2008; Deng et al. 2010; Jimeno et al. 2010). Upon transfer into mice with established disease, the Treg from VIP-treated arthritic mice suppressed and ameliorated disease progression in the recipients (Gonzalez-Rey et al. 2006b, c). Along the same lines, in vivo delivery of a VIP-expressing lentiviral vector to arthritic mice at different phases of the disease resulted in significant disease amelioration, which correlated with the induction of CD4+CD25+Foxp3+ Treg in the draining lymph nodes (Delgado et al. 2008b).
An interesting difference between the role of VIP and PACAP in EAE became apparent recently in VIP- or PACAP-deficient mice. Initial studies using exogenous VIP administration established quite clearly that VIP has a beneficial effect in EAE associated, at least partially, with the generation of CD4+CD25+Foxp3+ Treg (Fernandez-Martin et al. 2006; Gonzalez-Rey et al. 2006b, c). Similar effects were reported with PACAP, a structurally related neuropeptide. The protective effect of PACAP in EAE was confirmed in PACAP-deficient mice, which developed a more severe disease associated with decreased expression of Foxp3 in the spinal cord, and lower numbers of CD4+CD25+Foxp3+ Treg in draining lymph nodes (Tan et al. 2009). In contrast, VIP-deficient mice were almost completely resistant to EAE. In agreement with the previously reported anti-inflammatory effect of exogenous VIP, the VIP-deficient mice developed a robust Th1/Th17 response. However, although CD4 T cells entered the meningeal and perivascular areas, their infiltration in spinal cord parenchyma was severely impaired (Abad et al. 2010), suggesting that VIP plays a dual role, affecting T cell differentiation as previously reported but also promoting immune cell infiltration in the central nervous system.
VIP expression in immune cells and the role of endogenous VIP
Two different sources of VIP have been described in lymphoid organs, i.e., the local innervations and immune cells themselves. VIPergic nerve fibers were identified in both central (thymus) and peripheral (spleen, lymph nodes and mucosal-associated lymphoid tissue) lymphoid organs establishing an anatomical link between the CNS and the immune system (Bellinger et al. 1997). However, autonomic denervation of thymus and spleen did not change the content of VIP in these organs (Bellinger et al. 1997), suggesting that resident immune cells function as the major source of VIP within the lymphoid organs. Indeed, immune cells and especially CD4 and CD8 T cells were shown to express VIP mRNA, to process preproVIP and to secrete the mature peptide (Fig. 2). The highest VIP producers are CD4 Th2 and CD8 T2 cells, responding to antigen stimulation (Delgado and Ganea 2001a, b, c). Interestingly, neutrophils and mast cells secrete VIP fragments (VIP6–28 and VIP10–28) that fail to signal through the VIP receptors, but have been found recently to exhibit antimicrobial activities (El Karim et al. 2008; Delgado et al. 2009).
The endogenous VIP generated by immune cells appears to play an important regulatory role. Early studies showed increased VIP levels in inflammatory and autoimmune conditions such as sepsis and rheumatoid arthritis, presumably as an attempt to downregulate the immune response (Brandtzaeg et al. 1989; Arnalich et al. 1994). In patients with lupus and autoimmune thyroiditis, there are high levels of VIPase autoantibodies and low levels of VIP, suggesting that the low VIP levels might promote or exacerbate disease (Bangale et al. 2003).
More definite answers regarding the role of endogenous VIP were provided recently by VIP-deficient mice. These mice were more susceptible to LPS-induced septic shock and more prone to develop bronchial asthma and pulmonary hypertension, had higher numbers of infiltrating immune cells, and higher levels of proinflammatory cytokines in bronchoalveolar lavage fluid (Hamidi et al. 2006; Szema et al. 2006). This is in agreement with the previous finding that exogenous VIP deactivates innate immune cells, particularly macrophages, reducing the production of proinflammatory cytokines and chemokines. A recent study using VIP-deficient mice showed improved survival after viral infections associated with a marked increase in MHCII and CD80, increased Th1/Tc1 cells and fewer IL-10 producing T cells (Li et al. 2011). This is also in agreement with reports showing that exogenous VIP inhibits MHCII and costimulatory molecule expression in DCs and shifts T cell differentiation from Th1 to Th2/Treg. The fact that VIP produced by immune cells is the major immunoregulatory factor is supported by two very interesting reports. In vitro experiments analyzing Th1/Th2 differentiation in purified T cell cultures established that T cell-derived VIP affected T cell differentiation (Voice et al. 2003). A similar conclusion was reached in vivo where increased anti-viral immunity was observed in both VIPKO and in wild-type (wt) radiation chimeras engrafted with VIP-KO hematopoietic cells (Li et al. 2011). Since neuronal VIP production was not affected in the bone-marrow chimeras, the increased anti-viral response was attributed to the lack of VIP production by hematopoietic cells. Moreover, T cells were identified as the source for the immunological active VIP using grafts consisting of wt T cells and VIP-KO DCs and hematopoietic stem cells (Li et al. 2011).
Involvement of VIP in inflammatory and autoimmune diseases
The evidence reviewed in the previous sections indicates that endogenous VIP is an important player in limiting ongoing inflammatory and immune responses and promoting resolution of inflammation. Treatment with DCVIP pulsed with collagen II were administered to mice with established disease and shown to stop disease progression and T cell activation in an Ag-specific manner (Chorny et al. 2005). Inoculation of DCVIP also significantly ameliorated clinical TNBS-induced colitis and generated IL-10-secreting Treg, which suppressed autore-active T cells (Gonzalez-Rey and Delgado 2006). In a bone marrow transplantation model, DCVIP were shown to prevent graft-versus-host disease while maintaining the graft-versus tumor response through the generation of Treg (Chorny et al. 2006). More recently, lentiVIP-transduced DCs were shown to have a therapeutic effect on EAE and sepsis models (Toscano et al. 2010). LentiVIP-transduced DC have the double advantage of being tolerogenic, with the potential to induce Treg in vivo, and of secreting VIP locally avoiding problems associated with systemic VIP administration. Therefore, a promising future development for VIP therapy is the possibility to generate tolerogenic VIP-expressing human monocyte-derived DCs that could be loaded with relevant autoantigens and used in the treatment of chronic autoimmune diseases. exogenous VIP decreases symptom frequency and severity in various experimental models of sepsis, pancreatitis, hepatitis, respiratory inflammatory disorders, neurodegenerative disorders, rheumatoid arthritis, inflammatory bowel disease, type I diabetes, multiple sclerosis, Sjogren's syndrome, and autoimmune uveoretinitis (see Table 1 for references and details). In disorders characterized by an exacerbated inflammatory response, the beneficial effect of VIP appears to be exerted through the downregulation of a wide spectrum of inflammatory cytokines, chemokines and mediators of oxidative stress, at both systemic and local level (Fig. 3). VIP has been proven to be also effective in neurodegenerative diseases such as spinal cord injury (Dickinson et al. 1999; Kim et al. 2000), brain trauma (Delgado and Ganea 2003a, b, c; Favrais et al. 2007) and Parkinson's disease (Delgado and Ganea 2003a, b, c; Chorny et al. 2006; Korkmaz et al. 2010), mainly by inhibiting microglia activation.
Table 1.
Therapeutic effects of VIP in models of inflammatory and autoimmune diseases
| Immune disorder, experimental models | VIP dose/route | Mechanisms of action | References |
|---|---|---|---|
| Sepsis/endotoxemia | |||
| LPS injection E. coli injection | 1–5 nmol/i.p., once, within 4 h after sepsis | Inhibition of inflammatory cytokines (TNF, IL6, IL12, IFN), NO and chemokines | (Delgado et al. 1999a; Chorny and Delgado 2008); (Toscano et al. 2010) |
| CLP | 5 nmol/i.p., 4 times, initiated 12 h after sepsis | Main targets: macrophages/monocytes | |
| CLP | LentiVIP-DCs, i.p., once within 1 h after sepsis | Inhibition of late mediators: HMGB1 | |
| Cell targets: macrophages | |||
| Inhibition of inflammatory cytokines and chemokines | |||
| Rheumatoid arthritis | |||
| CIA | 1–5 nmol/i.p., 5 times, at onset and with established disease | Inhibition of inflammatory and autoreactive Th1/Th17 responses, induction of Th2 responses and antigen-specific Treg in DLNs and joints | (Delgado et al. 2001) (Chen et al. 2008) (Deng et al. 2010) (Chorny et al. 2005) (Delgado et al. 2008b) |
| CIA | VIP-tDCs/i.p., once, at onset | Inhibition of autoreactive Th1 responses, and induction of antigen-specific Treg in DLNs and joints | |
| CIA | Lenti-VIP/i.p., once, at onset and with established disease | Inhibition of inflammatory and autoreactive Th1 responses in DLNs and joints | |
| Inflammatory bowel disease | |||
| TNBS-colitis | 1–5 nmol/i.p., once, 12 h after TNBS | Inhibition of inflammatory and autoreactive Th1 responses and induction of Th2 responses in DLNs and colon | (Abad et al. 2003; Abad et al. 2005) (Gonzalez-Rey and Delgado 2006) |
| TNBS-colitis | >5 nmol/i.p., once | Caution: Increase of colitis and mortality | |
| TNBS-colitis DSS-colitis | VIP-tDCs/i.p., once, 12 h after TNBS | Inhibition of inflammatory and autoreactive Th1 responses and induction of Treg in DLNs and colon | |
| Multiple sclerosis | |||
| MOG-induced EAE PLP-induced EAE | 1–5 nmol/i.p., 5 times, at onset and with established disease | Inhibition of inflammatory and autoreactive Th1 responses, induction of Th2 responses and antigen-specific Treg in DLNs and CNS | (Fernandez-Martin et al. 2006; Gonzalez-Rey et al. 2006b) (Chorny et al. 2005; Toscano et al. 2010) |
| MOG-induced EAE PLP-induced EAE | VIP-tDCs/i.p., once at onset | Inhibition of inflammatory and autoreactive Th1 responses and induction of Treg in DLNs and CNS | |
| MOG-induced EAE | LentiVIP-DCs/i.v., once at onset | Inhibition of inflammatory responses on CNS | |
| Type 1 diabetes | |||
| STZ-diabetes CAD-NOD mice | 1 nmol/i.p., for 28 daysDNA-VIP/i.m., once at 2d before induction | Inhibition of oxidative stress and inflammation in pancreas | (Jimeno et al. 2010) (Yu et al. 2011) |
| NOD mice | 5 nmol/i.p., for 3 months, initiated 4 weeks before onset | Not determined | |
| Inhibition of inflammatory and autoreactive Th1/Th17 responses and induction of Th2 and Treg in pancreas and spleen | |||
| Uveoretinitis | |||
| hIRBP-EAU | 2 nmol/i.p., 10 times at onset and on established disease, also with VIP-treated macrophages | Inhibition of inflammatory and Th1 responses on eye and induction of Treg responses in spleen | (Keino et al. 2004; Camelo et al. 2009) |
| Sjogren's disease | |||
| NOD mice | AAV-VIP, in salivary glands, once before onset | Inhibition of inflammatory and Th1 responses in salivary glands | (Lodde et al. 2006) |
| Lung inflammation | |||
| Experimental COPD | 5 nmol/intratracheal, once, 1 h after smoke | Decrease of neutrophil infiltrarion in lung, inhibition of inflammatory cytokines | (Onoue et al. 2010; Prasse et al. 2010) |
| Human sarcoidosis | Inhalation 15 nmol/day, 28 days of established chronic disease | Decrease of inflammation cytokines on lung, inhibition of macrophage activation and induction of Treg | |
| Hepatitis/Pancreatitis | |||
| Cerulein/LPS-pancreatitis | 5 nmol/i.p., twice before and after LPS | Decrease of inflammatory cytokines in pancreas | (Kojima et al. 2005; Luo et al. 2009) |
| ConA-hepatitis | 5 nmol/i.p., twice before and after ConA | Decrease of inflammatory cytokines and induction of IL10 in live |
CIA collagen-induced arthritis, CLP cecal ligation and puncture, CAD cyclophosphamide accelerated diabetes, ConA concanavalin A, DLN draining lymph nodes, NO nitric oxide, HMGB1 high mobility group box-1, i.m. intramuscular injection, i.p. intraperitoneal injection, i.v. intravenous injection, VIP-tDCs VIP-induced tolerogenic DCs, lenti-VIP lentivirus vectors expressing VIP, LentiVIP-DCs DCs transduced with lentivirus expressing VIP, DLN draining lymph nodes, CNS central nervous system, EAE experimental autoimmune encephalomyelitis, NOD nonobese diabetic mice, STZ streptozotocin-induced diabetes, hIRBP-EAU human interphotoreceptor retinoid-binding protein-induced experimental autoimmune uveoretinitis, AAV-VIP adeno-associated virus expressing VIP, COPD chronic obstructive pulmonary disease
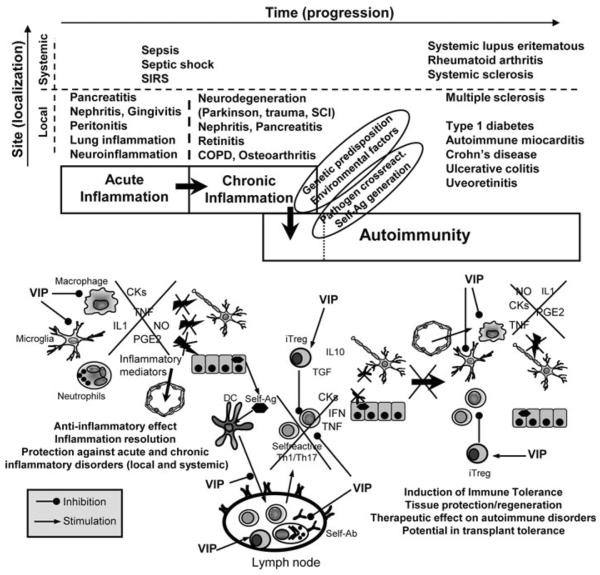
Fig. 3.
VIP reinstates tolerance in inflammatory and autoimmune disorders. In general, autoimmunity is initiated by aberrant immune responses against self antigens (self-Ag) and is the result of the progression from acute to chronic inflammation. Regulatory T cells (Treg) are key players in maintaining tolerance by their suppression of self-reactive Th1 and Th17 cells. VIP has the capacity to reinstate tolerance through various mechanisms. (1) VIP inhibits the production of a plethora of inflammatory cytokines, chemokines and free radicals by macrophages and resident cells (i.e., microglia), avoiding the perpetuation/dissemination of the inflammatory response and its cytotoxic effect against self-tissue components. (2) If autoimmune responses occur, VIP can decrease Th1/Th17 cell functions through direct actions on differentiating T cells, or indirectly by regulating DC functions in the peripheral lymphoid organs and locally in the affected tissue. As a consequence, infiltration/activation of neutrophils and macrophages is reduced and deposition of immune complexes (self-Ab) is avoided. (3) VIP induces the generation of peripheral iTreg that suppress activation of self-reactive T cells, both directly and through the generation of tDCs. COPD, chronic obstructive pulmonary disease; SCI, spinal cord injury; SIRS, systemic inflammatory response syndrome
In autoimmune disorders, the therapeutic effect of VIP is associated with a downregulation of early inflammatory events that initiate autoimmunity and of later events associated with a chronically activated inflammatory response. In vivo, VIP impairs the development of self-reactive Th1 and Th17 cells, their entry into the target organs, the release of pro-inflammatory cytokines and chemokines, and the recruitment and activation of macrophages and neutrophils, and induces the generation of iTreg (Fig. 3). The effects of VIP on T cell differentiation and function are both direct and indirect through immunomodulation of DCs.
Despite the effectiveness of VIP in experimental models of inflammation and autoimmunity, only a few clinical trials have been initiated. A major obstacle for the translation of VIP-based treatments into viable clinic therapies is related to its sensitivity to peptidases. Attempts to increase VIP stability involve chemical alterations of the native molecule, use of stable analogs or VIP receptor agonists, VIP administration in conjunction with peptidase inhibitor, or insertion of VIP in micelles or nanoparticles [reviewed in (Gonzalez-Rey et al. 2010). In addition, cellular approaches such as use of Treg or tDC generated ex vivo in the presence of VIP or of VIP-expressing DCs represent interesting options to be considered for future clinical use, especially in autoimmune diseases and transplantation.
The potential use of DCVIP in cellular therapy is aimed at the in vivo generation of Ag-specific Treg and was described for the first time in 2005 (Delgado et al. 2005). The Treg generated in vivo were specific for the Ag carried by the DCVIP and were able to transfer Ag-specific toler ance to naïve recipients (Delgado et al. 2005). Cellular therapy with DCVIP was then tested in models of inflammatory/autoimmune diseases. In collagen-induced arthritis, DCVIP pulsed with collagen II were administered to mice with established disease and shown to stop disease progression and T cell activation in an Ag-specific manner (Chorny et al. 2005). Inoculation of DCVIP also significantly ameliorated clinical TNBS-induced colitis and generated IL-10-secreting Treg, which suppressed autoreactive T cells (Gonzalez-Rey and Delgado 2006). In a bone marrow transplantation model, DCVIP were shown to prevent graft-versus-host disease while maintaining the graftversus tumor response through the generation of Treg (Chorny et al. 2006). More recently, lentiVIP-transduced DCs were shown to have a therapeutic effect on EAE and sepsis models (Toscano et al. 2010). LentiVIP-transduced DC have the double advantage of being tolerogenic, with the potential to induce Treg in vivo, and of secreting VIP locally avoiding problems associated with systemic VIP administration. Therefore, a promising future development for VIP therapy is the possibility to generate tolerogenic VIP-expressing human monocyte-derived DCs that could be loaded with relevant autoantigens and used in the treatment of chronic autoimmune diseases.
Acknowledgments
This work was supported by the following grants: NIH/NIAID RO1AI47325 (DG) and Spanish Ministry Health (MD).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- Abad C, Martinez C, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124(4):961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- Abad C, Juarranz Y, et al. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm Bowel Dis. 2005;11(7):674–684. doi: 10.1097/01.mib.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- Abad C, Tan YV, et al. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107(45):19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Gonzalez-Rey E. Vasoactive intestinal peptide induces cell cycle arrest and regulatory functions in human T cells at multiple levels. Mol Cell Biol. 2010;30(10):2537–2551. doi: 10.1128/MCB.01282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnalich F, de Miguel E, et al. Neuropeptides and interleukin-6 in human joint inflammation relationship between intraarticular substance P and interleukin-6 concentrations. Neurosci Lett. 1994;170(2):251–254. doi: 10.1016/0304-3940(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Arranz A, Juarranz Y, et al. VIP balances innate and adaptive immune responses induced by specific stimulation of TLR2 and TLR4. Peptides. 2008;29(6):948–956. doi: 10.1016/j.peptides.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Bangale Y, Karle S, et al. VIPase autoantibodies in Fas-defective mice and patients with autoimmune disease. FASEB J. 2003;17(6):628–635. doi: 10.1096/fj.02-0475com. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, et al. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides. 1997;18(8):1139–1149. doi: 10.1016/s0196-9781(97)00075-2. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, van de Kerkhof PC, et al. Foxp3+ regulatory T Cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Oktedalen O, et al. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24(1):37–44. doi: 10.1016/0167-0115(89)90209-7. [DOI] [PubMed] [Google Scholar]
- Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelo S, Lajavardi L, et al. Protective effect of intravitreal injection of vasoactive intestinal peptide-loaded liposomes on experimental autoimmune uveoretinitis. J Ocul Pharmacol Ther. 2009;25(1):9–21. doi: 10.1089/jop.2008.0074. [DOI] [PubMed] [Google Scholar]
- Chen G, Hao J, et al. The therapeutic effect of vasoactive intestinal peptide on experimental arthritis is associated with CD4+ CD25+ T regulatory cells. Scand J Immunol. 2008;68(6):572–578. doi: 10.1111/j.1365-3083.2008.02178.x. [DOI] [PubMed] [Google Scholar]
- Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172(5):1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, et al. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA. 2005;102(38):13562–13567. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, et al. Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood. 2006;107(9):3787–3794. doi: 10.1182/blood-2005-11-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the MEKK1/MEK4/JNK signaling pathway in endotoxin-activated microglia. Biochem Biophys Res Commun. 2002;293(2):771–776. doi: 10.1016/S0006-291X(02)00283-8. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of IFN-gamma-induced janus kinase-1-STAT1 activation in macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J Immunol. 2000a;165(6):3051–3057. doi: 10.4049/jimmunol.165.6.3051. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide inhibit the MEKK1/MEK4/JNK signaling pathway in LPS-stimulated macrophages. J Neuroimmunol. 2000b;110(1–2):97–105. doi: 10.1016/s0165-5728(00)00359-3. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol. 2001a;166(5):2907–2912. doi: 10.4049/jimmunol.166.5.2907. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol. 2001b;167(2):966–975. doi: 10.4049/jimmunol.167.2.966. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-kappa B-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem. 2001c;276(1):369–380. doi: 10.1074/jbc.M006923200. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson's disease by blocking microglial activation. FASEB J. 2003a;17(8):944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide inhibits IL-8 production in human monocytes. Biochem Biophys Res Commun. 2003b;301(4):825–832. doi: 10.1016/s0006-291x(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J. 2003c;17(13):1922–1924. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J Biol Chem. 1998;273(47):31427–31436. doi: 10.1074/jbc.273.47.31427. [DOI] [PubMed] [Google Scholar]
- Delgado M, Martinez C, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-alpha and IL-6. J Immunol. 1999a;162(2):1200–1205. [PubMed] [Google Scholar]
- Delgado M, Sun W, et al. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999b;163(8):4213–4223. [PubMed] [Google Scholar]
- Delgado M, Abad C, et al. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7(5):563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Delgado M, Jonakait GM, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit chemokine production in activated microglia. Glia. 2002a;39(2):148–161. doi: 10.1002/glia.10098. [DOI] [PubMed] [Google Scholar]
- Delgado M, Leceta J, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide promote in vivo generation of memory Th2 cells. FASEB J. 2002b;16(13):1844–1846. doi: 10.1096/fj.02-0248fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, et al. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. Faseb J. 2004a;18(12):1453–1455. doi: 10.1096/fj.04-1548fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, et al. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004b;56(2):249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Reduta A, et al. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004c;75(6):1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, et al. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175(11):7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- Delgado M, Robledo G, et al. Genetic association of vasoactive intestinal peptide receptor with rheumatoid arthritis: altered expression and signal in immune cells. Arthr Rheum. 2008a;58(4):1010–1019. doi: 10.1002/art.23482. [DOI] [PubMed] [Google Scholar]
- Delgado M, Toscano MG, et al. In vivo delivery of lentiviral vectors expressing vasoactive intestinal peptide complementary DNA as gene therapy for collagen-induced arthritis. Arthr Rheum. 2008b;58(4):1026–1037. doi: 10.1002/art.23283. [DOI] [PubMed] [Google Scholar]
- Delgado M, Varela N, et al. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008c;56(10):1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- Delgado M, Anderson P, et al. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Differ. 2009;16(3):406–416. doi: 10.1038/cdd.2008.161. [DOI] [PubMed] [Google Scholar]
- Deng S, Xi Y, et al. Regulatory effect of vasoactive intestinal peptide on the balance of Treg and Th17 in collagen-induced arthritis. Cell Immunol. 2010;265(2):105–110. doi: 10.1016/j.cellimm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Mitchell R, et al. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology. 1999;38(1):167–180. doi: 10.1016/s0028-3908(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121(3):294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Dinsmore WW, Gingell C, et al. Treating men with predominantly nonpsychogenic erectile dysfunction with intracavernosal vasoactive intestinal polypeptide and phentolamine mesylate in a novel auto-injector system: a multicentre double-blind placebo-controlled study. BJU Int. 1999;83(3):274–279. doi: 10.1046/j.1464-410x.1999.00935.x. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, et al. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200(1–2):11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J. VIP and PACAP. Results Probl Cell Differ. 2010;50:221–234. doi: 10.1007/400_2009_24. [DOI] [PubMed] [Google Scholar]
- Favrais G, Couvineau A, et al. Involvement of VIP and PACAP in neonatal brain lesions generated by a combined excitotoxic/inflammatory challenge. Peptides. 2007;28(9):1727–1737. doi: 10.1016/j.peptides.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martin A, Gonzalez-Rey E, et al. VIP prevents experimental multiple sclerosis by downregulating both inflammatory and autoimmune components of the disease. Ann NY Acad Sci. 2006;1070:276–281. doi: 10.1196/annals.1317.026. [DOI] [PubMed] [Google Scholar]
- Ferraccioli G, Zizzo G. The potential role of Th17 in mediating the transition from acute to chronic autoimmune inflammation: rheumatoid arthritis as a model. Discov Med. 2011;11(60):413–424. [PubMed] [Google Scholar]
- Foster N, Lea SR, et al. Pivotal advance: vasoactive intestinal peptide inhibits up-regulation of human monocyte TLR2 and TLR4 by LPS and differentiation of monocytes to macrophages. J Leukoc Biol. 2007;81(4):893–903. doi: 10.1189/jlb.0206086. [DOI] [PubMed] [Google Scholar]
- Fraccaroli L, Alfieri J, et al. VIP modulates the pro-inflammatory maternal response, inducing tolerance to trophoblast cells. Br J Pharmacol. 2009;156(1):116–126. doi: 10.1111/j.1476-5381.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DC, Madison VS, et al. Solution structure of an analogue of vasoactive intestinal peptide as determined by two-dimensional NMR and circular dichroism spectroscopies and constrained molecular dynamics. Biochemistry. 1989;28(6):2399–2409. doi: 10.1021/bi00432a010. [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganea D, Rodriguez R, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: players in innate and adaptive immunity. Cell Mol Biol (Noisy-le-grand) 2003;49(2):127–142. [PubMed] [Google Scholar]
- Ganea D, Gonzalez-Rey E, et al. A novel mechanism for immunosuppression: from neuropeptides to regulatory T cells. J Neuroimmune Pharmacol. 2006;1(4):400–409. doi: 10.1007/s11481-006-9044-0. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Voice JK, et al. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC(2) receptor for vasoactive intestinal peptide. Proc Natl Acad Sci USA. 2001;98(24):13854–13859. doi: 10.1073/pnas.241503798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomariz RP, Arranz A, et al. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol. 2005;78(2):491–502. doi: 10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology. 2006;131(6):1799–1811. doi: 10.1053/j.gastro.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Delgado M. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007;28(9):482–491. doi: 10.1016/j.tips.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, et al. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006a;107(9):3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, et al. Vasoactive intestinal peptide induces CD4+, CD25+ T regulatory cells with therapeutic effect in collagen-induced arthritis. Arthr Rheum. 2006b;54(3):864–876. doi: 10.1002/art.21652. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, et al. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006c;168(4):1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, et al. Emerging roles of vasoactive intestinal peptide: a new approach for autoimmune therapy. Ann Rheum Dis. 2007;66(Suppl 3):iii70–iii76. doi: 10.1136/ard.2007.078519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Ganea D, et al. Neuropeptides: keeping the balance between pathogen immunity and immune tolerance. Curr Opin Pharmacol. 2010;10(4):473–481. doi: 10.1016/j.coph.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Divinsky I, et al. From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J Mol Neurosci. 2003;20(3):315–322. doi: 10.1385/JMN:20:3:315. [DOI] [PubMed] [Google Scholar]
- Guerrero J, Prieto J, et al. Interaction of vasoactive intestinal peptide with human blood mononuclear cells. Mol Cell Endocrinol. 1981;21(2):151–160. doi: 10.1016/0303-7207(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Canas I, Juarranz Y, et al. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology (Oxford) 2006;45(5):527–532. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- Hamidi SA, Szema AM, et al. Clues to VIP function from knockout mice. Ann NY Acad Sci. 2006;1070:5–9. doi: 10.1196/annals.1317.035. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, et al. International union of pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50(2):265–270. [PMC free article] [PubMed] [Google Scholar]
- Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49(1):27–37. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- Herrera JL, Gonzalez-Rey E, et al. Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in macrophages. J Cell Mol Med. 2009;13(9B):3209–3217. doi: 10.1111/j.1582-4934.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM. Vasoactive intestinal peptide in neurodevelopmental disorders: therapeutic potential. Curr Pharm Des. 2007;13(11):1079–1089. doi: 10.2174/138161207780618975. [DOI] [PubMed] [Google Scholar]
- Jiang X, Jing H, et al. VIP and PACAP down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J Neuroimmunol. 2002;133(1–2):81–94. doi: 10.1016/s0165-5728(02)00365-x. [DOI] [PubMed] [Google Scholar]
- Jimeno R, Gomariz RP, et al. New insights into the role of VIP on the ratio of T-cell subsets during the development of autoimmune diabetes. Immunol Cell Biol. 2010;88(7):734–745. doi: 10.1038/icb.2010.29. [DOI] [PubMed] [Google Scholar]
- Jones KB, Mollano AV, et al. Bone and brain: a review of neural, hormonal, and musculoskeletal connections. Iowa Orthop J. 2004;24:123–132. [PMC free article] [PubMed] [Google Scholar]
- Juarranz Y, Gutierrez-Canas I, et al. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthr Rheum. 2008;58(4):1086–1095. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino H, Kezuka T, et al. Prevention of experimental autoimmune uveoretinitis by vasoactive intestinal peptide. Arch Ophthalmol. 2004;122(8):1179–1184. doi: 10.1001/archopht.122.8.1179. [DOI] [PubMed] [Google Scholar]
- Kim WK, Kan Y, et al. Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci. 2000;20(10):3622–3630. doi: 10.1523/JNEUROSCI.20-10-03622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Ito T, et al. VIP attenuation of the severity of experimental pancreatitis is due to VPAC1 receptor-mediated inhibition of cytokine production. Pancreas. 2005;30(1):62–70. [PubMed] [Google Scholar]
- Korkmaz OT, Tuncel N, et al. Vasoactive intestinal peptide (VIP) treatment of Parkinsonian rats increases thalamic gamma-aminobutyric acid (GABA) levels and alters the release of nerve growth factor (NGF) by mast cells. J Mol Neurosci. 2010;41(2):278–287. doi: 10.1007/s12031-009-9307-3. [DOI] [PubMed] [Google Scholar]
- Langer I, Robberecht P. Molecular mechanisms involved in vasoactive intestinal peptide receptor activation and regulation: current knowledge, similarities to and differences from the A family of G-protein-coupled receptors. Biochem Soc Trans. 2007;35(Pt 4):724–728. doi: 10.1042/BST0350724. [DOI] [PubMed] [Google Scholar]
- Lauenstein HD, Quarcoo D, et al. Pituitary adenylate cyclase-activating peptide receptor 1 mediates anti-inflammatory effects in allergic airway inflammation in mice. Clin Exp Allergy. 2010;41(4):592–601. doi: 10.1111/j.1365-2222.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchte HH, Baezner C, et al. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J. 2008;32(5):1289–1294. doi: 10.1183/09031936.00050008. [DOI] [PubMed] [Google Scholar]
- Li JM, Southerland L, et al. Absence of vasoactive intestinal peptide expression in hematopoietic cells enhances Th1 polarization and antiviral immunity in mice. J Immunol. 2011;187(2):1057–1065. doi: 10.4049/jimmunol.1100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yen JH, et al. A novel VIP signaling pathway in T cells cAMP–> protein tyrosine phosphatase (SHP-2?)–< JAK2/STAT4–> Th1 differentiation. Peptides. 2007;28(9):1814–1824. doi: 10.1016/j.peptides.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodde BM, Mineshiba F, et al. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren's syndrome. Ann Rheum Dis. 2006;65(2):195–200. doi: 10.1136/ard.2005.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Wang Y, et al. Vasoactive intestinal peptide attenuates concanavalin A-mediated liver injury. Eur J Pharmacol. 2009;607(1–3):226–233. doi: 10.1016/j.ejphar.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Delgado M, et al. VIP and PACAP enhance IL-6 release and mRNA levels in resting peritoneal macrophages: in vitro and in vivo studies. J Neuroimmunol. 1998;85(2):155–167. doi: 10.1016/s0165-5728(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Martinez C, Abad C, et al. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci USA. 2002;99(2):1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi-Kouki O, Gandolfo P, et al. Role of PACAP and VIP in astroglial functions. Peptides. 2007;28(9):1753–1760. doi: 10.1016/j.peptides.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Rebuffat P, et al. Vasoactive intestinal peptide stimulates rat adrenal glucocorticoid secretion, through an ACTH receptor-dependent activation of the adenylate cyclase signaling pathway. Horm Metab Res. 1998;30(5):241–243. doi: 10.1055/s-2007-978874. [DOI] [PubMed] [Google Scholar]
- Moody TW, Ito T, et al. VIP and PACAP: recent insights into their functions/roles in physiology and disease from molecular and genetic studies. Curr Opin Endocrinol Diabetes Obes. 2011;18(1):61–67. doi: 10.1097/MED.0b013e328342568a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dorisio MS, Hermina NS, et al. Vasoactive intestinal polypeptide modulation of lymphocyte adenylate cyclase. J Immunol. 1981;127(6):2551–2554. [PubMed] [Google Scholar]
- Onoue S, Misaka S, et al. Inhalable powder formulation of vasoactive intestinal peptide derivative, [R15, 20, 21, L17]-VIP-GRR, attenuated neutrophilic airway inflammation in cigarette smoke-exposed rats. Eur J Pharm Sci. 2010;41(3–4):508–514. doi: 10.1016/j.ejps.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Paladini F, Cocco E, et al. A functional polymorphism of the vasoactive intestinal peptide receptor 1 gene correlates with the presence of HLA-B*2705 in Sardinia. Genes Immun. 2008;9(8):659–667. doi: 10.1038/gene.2008.60. [DOI] [PubMed] [Google Scholar]
- Palermo MS, Vermeulen ME, et al. Human antibody-dependent cellular cytotoxicity mediated by interferon gamma-activated neutrophils is impaired by vasoactive intestinal peptide. J Neuroimmunol. 1996;69(1–2):123–128. doi: 10.1016/0165-5728(96)00078-1. [DOI] [PubMed] [Google Scholar]
- Passemard S, El Ghouzzi V, et al. VIP blockade leads to microcephaly in mice via disruption of Mcph1-Chk1 signaling. J Clin Invest. 2011a doi: 10.1172/JCI43824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passemard S, Sokolowska P, et al. VIP-induced neuroprotection of the developing brain. Curr Pharm Des. 2011b;17(10):1036–1039. doi: 10.2174/138161211795589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrera C, Lucas M, et al. Receptor-independent mechanisms are involved in the priming of neutrophil's oxidase by vasoactive intestinal peptide. Regul Pept. 1994;54(2–3):505–511. doi: 10.1016/0167-0115(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Petkov V, Mosgoeller W, et al. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest. 2003;111(9):1339–1346. doi: 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Cutler DJ. The roles of vasoactive intestinal polypeptide in the mammalian circadian clock. J Endocrinol. 2003;177(1):7–15. doi: 10.1677/joe.0.1770007. [DOI] [PubMed] [Google Scholar]
- Pozo D, Anderson P, et al. Induction of alloantigen-specific human T regulatory cells by vasoactive intestinal peptide. J Immunol. 2009;183(7):4346–4359. doi: 10.4049/jimmunol.0900400. [DOI] [PubMed] [Google Scholar]
- Prasse A, Zissel G, et al. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182(4):540–548. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, et al. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson's disease. J Immunol. 2010;184(5):2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169(951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Said SI, Rosenberg RN. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976;192(4242):907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Samarasinghe AE, Hoselton SA, et al. The absence of VPAC2 leads to aberrant antibody production in Aspergillus fumigatus sensitized and challenged mice. Peptides. 2011;32(1):131–137. doi: 10.1016/j.peptides.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Delgado M, et al. Granzyme B, a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol. 2006;176(1):97–110. doi: 10.4049/jimmunol.176.1.97. [DOI] [PubMed] [Google Scholar]
- Smalley SG, Barrow PA, et al. Immunomodulation of innate immune responses by vasoactive intestinal peptide (VIP): its therapeutic potential in inflammatory disease. Clin Exp Immunol. 2009;157(2):225–234. doi: 10.1111/j.1365-2249.2009.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek SA, Borensztajn KS, et al. Neuropeptide receptors in intestinal disease: physiology and therapeutic potential. Curr Pharm Des. 2010;16(9):1091–1105. doi: 10.2174/138161210790963814. [DOI] [PubMed] [Google Scholar]
- Sun W, Hong J, et al. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int Immunol. 2006;18(12):1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- Szema AM, Hamidi SA, et al. Mice lacking the VIP gene show airway hyperresponsiveness and airway inflammation, partially reversible by VIP. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L880–L886. doi: 10.1152/ajplung.00499.2005. [DOI] [PubMed] [Google Scholar]
- Tan YV, Abad C, et al. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2009;106(6):2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MG, Delgado M, et al. Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP-secreting tolerogenic-like DCs. Mol Ther. 2010;18(5):1035–1045. doi: 10.1038/mt.2009.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, et al. Pituitary adenylate cyclaseactivating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61(3):283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Voice JK, Grinninger C, et al. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170(1):308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- Voice J, Donnelly S, et al. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172(12):7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, et al. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152(2–3):165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Jiang XM, et al. The neuropeptides VIP and PACAP inhibit IL-2 transcription by decreasing c-Jun and increasing JunB expression in T cells. J Neuroimmunol. 2000;104(1):68–78. doi: 10.1016/s0165-5728(99)00244-1. [DOI] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. Role of VIP and PACAP in islet function. Peptides. 2007;28(9):1805–1813. doi: 10.1016/j.peptides.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Yadav M, Rosenbaum J, et al. Cutting edge: vasoactive intestinal peptide (VIP) induces differentiation of Th17 cells with a distinctive cytokine profile. J Immunol. 2008;180(5):2772–2776. doi: 10.4049/jimmunol.180.5.2772. [DOI] [PubMed] [Google Scholar]
- Yu R, Zhang H, et al. Anti-hyperglycemic, antioxidant and anti-inflammatory effects of VIP and a VPAC1 agonist on streptozotocin-induced diabetic mice. Peptides. 2011;32(2):216–222. doi: 10.1016/j.peptides.2010.11.017. [DOI] [PubMed] [Google Scholar]