Abstract
Phenomena in behavior and their underlying neural mechanisms are exquisitely complex problems. Infrequently do we reflect on our basic strategies of investigation and analysis, or formally confront the actual challenges of achieving an understanding of the phenomena that inspire research. Philip Teitelbaum is distinct in his elegant approaches to understanding behavioral phenomena and their associated neural processes. He also articulated his views on effective approaches to scientific analyses of brain and behavior, his vision of how behavior and the nervous system are patterned, and what constitutes basic understanding. His rubrics involve careful observation and description of behavior, simplification of the complexity, analysis of elements, and re-integration through different forms of synthesis. Research on the development of huddling behavior by individual and groups of rats is reviewed in a context of Teitelbaum’s rubrics of research, with the goal of appreciating his broad and positive influence on the scientific community.
Keywords: Behavior, Development, Rat, Mouse, Huddling, Social behavior, Social preference, Group behavior, Experience, Model
1. Introduction
Physiological psychology reigned supreme through the 1960s as a vibrant, visible and influential science. At that time, the University of Pennsylvania was probably the major hub of trend-setting research and innovative thinking for the discipline. One of the leaders there was Philip Teitelbaum. The science evolved, as did Phil Teitelbaum. Physiological psychology morphed into behavioral neuroscience, which has grown and branched and differentiated greatly. As a corpus of knowledge, it is a body that has grown asymmetrically; the neural/molecular side of this body has grown more than the behavioral side. Yet, it is the behaving organism that carries the brain, and it is the behaving organism on which evolution works its wonders. As a physiological psychologist and then as a behavioral neuroscientist, Phil Teitelbaum has been dedicated to promoting the behavioral side of the science. This is an important part of his important and broad contributions.
I never studied with Philip Teitelbaum; and I was not a student at Penn, or Illinois, or Florida. Yet over the years, I have been aware that my research has been affected by his ideas and his approach to science. There are a variety of paths on which I can trace his influence on me: As a graduate student at Princeton, I worked for a while in Bart Hoebel’s lab, and Bart was Phil’s first student. Byron Campbell, my PhD mentor, was an associate of Phil’s during their years at Harvard. While at McMaster University, I was influenced greatly by my mentor B.G. Galef, Jr., and by Edward Stricker, who were then both recent products of the Penn group, and they carried messages from Phil Teitelbaum, as did Elliott Blass, whose work influenced mine and who, as a former post-doc from Penn, is yet another vector of Phil’s science. It is a remarkable network. Most of these individuals helped solidify my love of studying behavior and I value Phil’s commitment to the behavioral side of behavioral neuroscience. Many of us especially appreciate Phil Teitelbaum’s awareness and explicit attention to the process of scientific, experimental analysis. Teitelbaum’s tidy, 1966 volume, Physiological Psychology, provides a guide to his artistry in scientific analysis. His approach remains timely; some of his ideas now seem prescient.
Phil never studied huddling or group behavior, yet I see his impact on the science I describe. I have selected some of the key ideas and have placed this partial review of my research on the development of huddling behavior in the context of Phil Teitelbaum’s rubrics for analyzing behavior.
“Typically, we start with a phenomenon in behavior that we wish to understand” [1,p.3].
Huddling, or contact behavior among rat pups, as well as between the pups and the mother is “a phenomenon in behavior” which I have endeavored to understand during much of my career. Initially, I recognized huddling as a species-typical behavior of Norway rats (Rattus norvegicus), a notorious “contact species”[2], and I study the ontogeny of that behavior. Huddling is a behavior that starts immediately after birth in rats, with the newborn pups living in a pile of bodies in the natal nest. It continues throughout the rest of their lives. Rats huddle with each other at every stage of life, while they change radically during development. The most obvious kinds of developmental changes include the infants’ sensory capabilities, and this led to studies of the sensory controls of huddling.
The major, initial question was: what sensory cues elicit and maintain contact behavior in rats during developmental stages when sensory function is undergoing its sequential development [3]? This question was answered with a series of experiments in which individual rat pups at 5-, 10-, 15-, or 20-days of age were placed in a chamber, scaled to the size and locomotor abilities of the infants [4]. The chamber contained an animate or inanimate object or two arranged to test a hypothesis about a sensory modality and the control of huddling behavior. For example, an anesthetized age-mate was placed in the observation chamber and rat pups generally huddled vigorously with it, i.e., for more than 3.5 h of a 4-h-long test. Perhaps the warmth of the anesthetized target animal was attractive to the subject. When we used room temperature stimulus animals (that had been previously euthanized), we determined that there were, indeed, non-thermal determinants of huddling as well. There followed a series of controlled experiments that helped define a variety of cues that attract and maintain contact behavior in the developing rat pup. Table 1 lists these cues.
Table 1.
Cues that attract and maintain contact behavior.
| Heat | Rat pups huddle with an anesthetized agemate, an anesthetized heterospecific, or a warm, cylinder. If the agemate is cool, huddling is diminished considerably. |
| Walls and corners | Pups tend to maintain contact with walls (thigmotaxis) and especially with corners; objects used as huddling targets are often more effective when they are located at a wall. |
| Attenuation of light | Pups prefer to contact wall areas beneath a dark, overhead shelf; pups display negative phototropic responses prior to eye–opening. |
| Olfactory stimuli | zinc sulfate–induced anosmia does not reduce contact behavior, but it can eliminate specificity. |
| Texture | Artificial furs of various types are attractive; the furry nap side of an artificial pelt is more attractive than the smooth back. |
| Surface contour | Convex surfaces create crevices that can alter orientation and facilitate probing and rooting. |
| Mass and inertia | A stuffed rat pelt attracts contact but if it lacks significant mass and inertia and does not resist pressure from a pup’s body, contact is not maintained. |
| Movement | Role of movement cues, such as respiratory movements, are not known for rodents, thought there is some evidence that human infants may prefer them. |
The cues sufficient for eliciting and maintaining contact behavior shown in Table 1 are listed without regard to the age of the pups. There are, in fact, age-related differences relevant to understanding some of the underlying developmental processes. Heat, however, is the dominant cue. Heat is also the physiological commodity that is exchanged and conserved with contact behavior. Pups approach and contact warm stimuli. Some of the other cues, such as the attenuation of light and the pups’ responses to furriness, do not strongly influence their huddling at early stages but affect the behavior after 10-days of age. But it is significant that for the infant rat, heat is both cue and commodity.
Olfactory cues become important around 2 weeks of age. Olfaction does not determine whether or how much huddling is expressed. Rather, it makes huddling selective [5]. Specifically, in our studies of the olfactory controls of huddling, postnatal day (PND) 15 is an age at which rat pups strongly and reliably respond to olfactory cues. Pups younger than 15-days of age have functional olfactory abilities [e.g., 3,6–8], so it is not the ontogeny of olfaction, per se, that limits the developmental role of olfaction in huddling.
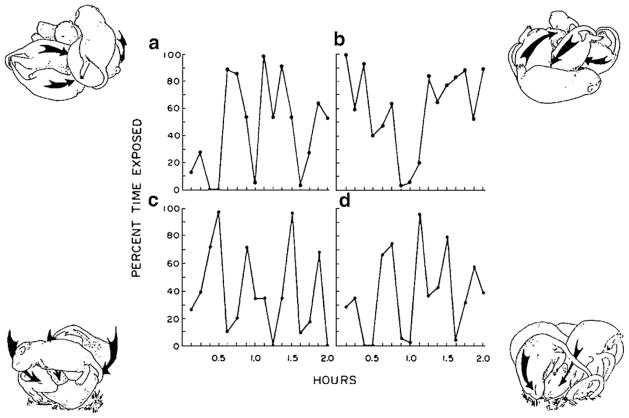
Fig. 1 shows the results of an early experiment in which rat pups at four different ages were presented simultaneously with an anesthetized gerbil at one end of a small chamber and same-sized anesthetized rat (thus having the same thermal characteristics as the gerbil) at the other end of the chamber. Five- and 10-day-old rats huddled indiscriminately with the rat and gerbil during the 4-h-long test. By summing the duration values for each age in Fig. 1, it can be seen that younger pups spent more than 3.5 h of the 4-h-long test huddling with these warm, furry targets. Their time was split quite evenly, so they show plenty of huddling, but no preference. Beginning on day 15, however, there is a strong species preference, which is maintained in the oldest, 20-day-old group. It is not that gerbils become aversive to rats. If there were only a gerbil present, then the 15- and 20-day rats huddle with it for most of the session. So what we see in Fig. 1 is the emergence of a species preference for huddling in rats.
Fig. 1.
Time spent huddling with an anesthetized juvenile rat and a same-sized anesthetized gerbil in a 4-h-long, two-choice preference test. Subjects were experimentally naïve rat pups, 5-, 10-, 15-, or 20-days of age (ns = 8 per age). Preferences can be gauged by comparing time spent with each of the stimuli.
From Alberts [4].
An age-related huddling preference such as the one seen in Fig. 1 could reflect the development of the ability to discriminate between two species, or to recognize the complex odors that represent conspecifics, but these were unlikely explanations for the phenomenon seen here. There is evidence of excellent olfactory acuity and odor discrimination by pups at this age and younger [7].
Studying the age-related sensory controls of huddling led us to recognize two major stages, defined in terms of the salience of the sensory cues that attracted and maintained the pups’ contact behavior. Huddling by pups up to at least 10-days of age is dominated by thermal cues. Heat is not the sole stimulus that affects huddling, but it is the most salient stimulus to the 5- and 10-day-old pups. Because heat is also the physiological resource that defines the function of huddling [9], we termed huddling by young pups as physiological huddling; for them, heat is the cue and the commodity. In contrast, huddling by pups 15-days of age and older is dominated by olfactory cues; olfaction is the cue and an odor that maintains huddling is a token of social affiliation. We termed this as filial huddling. Note that heat has not lost its efficacy, but olfactory stimuli have become more salient to the 15- and 20-day-old pups. From the developmental pattern, we identified a transition in the sensory controls of huddling, from physiological huddling filial huddling. Physiological huddling is dominated by heat cues, i.e., the physiological resource is the most salient cue. Filial huddling is dominated by olfactory cues, i.e., stimuli representing the pup’s affiliation with its species.
There were data indicating that the pups’ sense of smell and its associated neural structures developed substantially during the first postnatal weeks, but there was no evidence that the immaturity of the pups’ olfactory system limited the expression of an olfactory-guided huddling [6,7].
2. Induction of a perceptual preference by experience
In the absence of a ready explanation based on sensory development, it seemed possible that the emergence of the odor-guided species preference around day 15 was the result of experience. We pursued the question with manipulations that altered the odor of a rat dam, by anointing her fur each day with an artificial odor (initially we used a commercial “musk” cologne) and allowed the dam to raise her pups normally. Then, on day 15, we conducted a standardized huddling preference test in which pups chose freely between two anesthetized rats, one presenting the species-typical rat odors and the other an anointed “musk rat” odor. Pups raised by control dams showed the species preference on day 15 whereas pups raised by a musk mom huddled with a musk-scented target rat in preference to the normal species-typical stimulus [10]! The conclusion of these kinds of studies is that rats show social huddling preferences for species-typical odors because they are reared in species-typical environment. If the olfactory environment is varied, the olfactory preference varies accordingly.
By understanding that we were observing an experience-dependent development, the next set of questions concerned which experiences were the formative ones. In the embryological tradition proffered by Gottlieb [11,12], we sought to identify the experiences that induce the perceptual preference used by rat pups for filial huddling.
“In studying any phenomenon, dissect away everything that is not essential to it. In a word, simplify. Then study the simple system that remains, to discover the basic variables that produce the phenomenon” [1,p.4].
The challenge was to establish a paradigm that approximated the rich complexity of the mother–litter interactions during development, the kinds of interactions that lead to the formation of a filial huddling preference on postnatal day (PND) 15. The paradigm would have to be compatible with the kinds of controls, manipulations, and quantification that enable an experimental titration of experience. We knew that 2 weeks of experience with a scented mother induced a preference for her scent [10]. But of a pup’s myriad experiences with a scented dam, which experiences actively contribute to the formation of the filial preference, and which do not?
We sought a more tractable process by providing the experience of a scented mother to litters for 4 h each day. We did this various ways. For example, we separated litters from their own biological mother for 4 h each day and substituted a scented, lactating dam (matched to the lactational age of original dam), who served as a foster mother for 4 h/day. This was done each day from PND 1 to PND 14. Then, on PND 15 each pup was assessed in a two-choice huddling test, which involved spending 4 h in a round arena that had two rectangular openings located 180° apart through which a simulated furry “flank” was presented. The artificial fur on one of the targets was anointed with the odor of the pup’s foster mother and another, control odorant, was on the alternative flank. We video-recorded the entire 4-h test and quantified the duration of contact with each stimulus. As in the previous work, we obtained robust levels of huddling behavior. We also saw a profound preference for huddling with the target that bore the odor of the foster mother, as it was seen when the odor was on the pup’s own mother. We had a more tractable method for experimentally inducing a huddling preference.
It was possible that the preference associated with 4 h/day exposure to a scented foster dam, assessed in a forced choice test with a novel odor could be explained as the result of familiarity with one odor, and that an association with maternal behavior was not necessary for the preference. In fact, familiarity can be a potent, albeit general influence on behavior [13]. Familiarity can make stimuli attractive, especially in comparisons with novel stimuli that may have a negative valence. Thus, the potential roles of familiarity and novelty should not be ignored. In the imprinting literature, for example, some researchers recognize “mere exposure” or a process of “exposure learning” in the absence of a discernable reward as a potent mechanism for establishing an attraction to a stimulus [14,15].
We examined the possible role of familiarity as a factor in a rat pups’ huddling preferences by substituting daily 4-h bouts of mere exposure to an odor in the general atmosphere for the 4-h bouts with a scented foster dam. Fig. 2 depicts the 4/h/day paradigm and the outcomes of three conditions. The results were unambiguous. Pups from litters exposed daily from PND1 to PND14 to an ambient odor in the absence of a mother, displayed the same robust huddling preference for the exposure odor versus a novel scent, as did the pups exposed to the odor (Fig. 2, rows 1–3). A conservative conclusion is that under these conditions, the daily regimen of mere exposure is sufficient to induce a relative preference for the familiar scent.
Fig. 2.
Schematic representation of the paradigm and major results of three experiments in which daily experiences were paired with an odor and then the preference for that odor was assessed at 15-days of age. Pups were removed from their nest and biological mother for 4 h each day and housed with a scented foster dam (the stippled rat in the drawings) or placed in a plastic tub for “mere exposure” to an alternate scent. On postnatal day 15, pups were tested individually in a two-choice huddling test. Tests were conducted in a round arena that contained two scented, furry pelts as huddling targets. The results of each experiment are depicted in the bar graphs.
From Alberts and May [23].
But is there a difference between a maternally associated odor and an equally familiar odor that lacks an association with maternal care? We were able to answer this basic and important question by applying a modification of the 4 h/day exposure paradigm. Once again litters were removed from the home nest for 4 h each day and given a specific treatment. This time, however, we alternated the daily treatment so that over the first 14 postnatal days, pups experienced two odors (odor A and odor B, counterbalanced for order) seven times each. One odor was paired with a lactating foster dam and other odor was presented for mere exposure. The top row in Fig. 3 depicts this alternating daily regime. Using this within-subject design, pups had equal exposure to both odors (equal familiarity) but one odor was associated with “milky mother love” and other with “mere exposure”. In sense, we were now using the odors as tags with which we could label a kind of experience, much like we might use a radioactive marker to tag neurons while they undergo mitosis, or label cells that contain a specific transmitter. The outcome, seen in the uppermost histogram in Fig. 3, was clear: an odor associated with maternal care induces a huddling preference that is far stronger than that from mere exposure. Combined with the earlier results, we can conclude that while both maternal behavior and mere exposure are sufficient to induce a huddling preference for an associated odor, the relative strength of filial preference derived from maternal care is far greater.
Fig. 3.
Schematic representation of the daily exposure conditions used to titrate some of types of experiences comprising the receipt of maternal care and evaluate their contribution to an olfactory-guided huddling preference on postnatal day 15. Pups received daily, 4-h exposures to one of two conditions, each paired with a distinct odor, indicated by the stippling or stripes. When pups were tested at 15-days of age, they were equally familiar with each of the odors, and had accumulated seven repetitions of a specific kind of experience with each. The pups’ preferences are represented by the histograms; the relative heights depict huddling preference.
From Alberts and May [23].
Clearly, we had to continue to titrate the pups’ early experiences with odors and their association with non-olfactory stimuli, particularly those contained in the mélange of cues that comprise the experience of mammalian mother love. The work of Phil Teitelbaum and many others had ingrained in us the power and potency of ingestion processes; it was natural to hypothesize, that some aspect of suckling and ingestion would be key to the rewards of maternal care. More specifically, we knew that rat pups are sensitive to the rewards of mother’s milk from suckling, of milk alone, and of non-nutritive sucking [e.g., 17–21]. Thus, the most obvious question to pursue was the potential role of milk reward in the induction of a filial huddling preference: is milk reward the element of maternal care that makes the foster mother such a potent inductor of a filial huddling preference?
We first attacked this question with appropriate applications of the alternating daily exposure regime. In one series, litters were paired with a scented foster dam that provided the full range of maternal behaviors, i.e., nesting, brooding, transport, licking, handling, nurturant posturing over the litter (see Fig. 4), but no milk transfer. This was accomplished by using dams that had been previously sensitized to pups and had become fully maternal, but did not lactate [16]. Pups were exposed on alternating days to a scented, non-lactating foster mother (odor A) and to an equal number of exposures of an equal duration to an odor B—simply for mere exposure. The outcome, shown in Fig. 3 (top row), was that non-nutritive maternal care was a potent inductive stimulus for filial huddling preference. The experiences provided by the non-lactating dam produced the same effects – potentiated above those of mere exposure – as seen with the lactating foster dam (cf., Figs. 3, 1A, and 1C). This was a stunning result.
Fig. 4.

Drawing of a rat mother in a nursing position above pups. Most of the pups in this drawing are ventrum-down and are not attached to the dam’s nipples, but the figure depicts how the mother can orient her body to the litter (and vice versa) so that there is abundant skin-to-skin contact, which insulates and supports heat exchange by conduction.
Adapted from Alberts and Cramer (1988).
Even if milk rewards are not necessary for the induction of filial huddling, perhaps they make an incremental contribution to the rewards of maternal care. Thus, we next conducted an experiment in which pups were exposed on alternating days to a scented, non-lactating foster mother (odor A) and for an equal number of exposures for an equal duration to an odor B that was borne on the fur of lactating foster mother. The outcome, shown in the 2nd row of Fig. 3, was that the two forms of maternal care had equivalent effects on the pups’ odor-guided huddling preference. In other words, mother’s milk and suckling cues did not add to the non-nutritive rewards of maternal care. Again, an unanticipated finding was encountered.
Naturally, we turned to the question of what kind of non-nutritive stimuli might induce a filial preference in the infants. Working in the context of thermal significance of huddling, we considered the possible role of thermotactile stimulation. Perhaps it is literally the warmth of the mother that induces the preference for an associated odor. Thus, we exposed pups on alternating days to a scented (odor A) inanimate cylinder about the size of mother’s flank, warmed to the temperature of a maternal body surface (~36 °C) with which they could huddle, and for an equal number of exposures and for equal durations to an odor B, for mere exposure. The outcome, seen in the third row of Fig. 3, was that non-nutritive maternal care was a potent inductive stimulus for filial huddling preference, showing the same potentiated effects above those of mere exposure, as seen with the lactating foster dam.
“In practice, we must test our understanding to see if it is valid. The test of every analysis is synthesis” [1,p.10].
I have come to think that there are different kinds of syntheses in experimental analyses; even at intermediate stages, there are combinatorial, synthesizing steps that can validate that a path of study is providing a coherent, integrative picture. Teitelbaum’s analyses emphasized and exemplified the use of synthesis. After titrating separate elements of experience, it was important to check on the coherence of the emerging developmental picture. Could it be that mother love (in rats) can be reduced to the thermotactile stimulation derived from a warm cylinder? (See Teitelbaum and Pellis [22] for a discussion of the difference between reduction and reductionism.) Indeed, for certain forms of learning, this may be the case. In the next experiment, pups were exposed on alternating days to the scented (odor A) warm, inanimate cylinder and for an equal number of exposures for an equal duration to scented, lactating foster dam (odor B). As always, odors and order was counter-balanced. The outcome of the huddling preference test on day 15 was that the pups showed equivalent huddling preferences for the two odors! We learned a great deal about the power of a localized source of warmth in a cool ambience [23]. This experiment was a kind of test-by-synthesis. Although we were focused on an odor-heat association, the analysis had stripped away some elements, such as perioral cues and milk transfer, and was combining heat, and tactile cues (the thermotactile stimulus), along with contour and inertia. This rarified, compound stimulus proved to compare favorably with the natural, maternal stimulus.
Much more had been learned about the range and kinds of stimuli that influence learning during early development. Tactile stimulation, perhaps the kind derived from maternal licking of pups, was found to be rewarding, especially at early ages [e.g., 20,21,24]. In my lab, we used conductive warmth from surface on which infants lay as a reinforcer for an operant response of directional head-turning [25], while Johanson and Hall [26] and Rudy and Hyson [27] demonstrated other forms of operant and Pavlovian learning via oral infusions of milk.
Recently, Sayuri Kojima and I reported yet another paradigm with which we could systematically manipulate maternal stimuli and other experiential variables during development, and assess their effects with a two-choice huddling test. The major change in the new paradigm came from systematic efforts to reduce the number and duration of experiences necessary for the acquisition of a filial huddling preference [28,29]. Eventually, we established that a filial huddling preference in PND 15 pups could be induced with a single, 2-h experience with a scented foster dam. This finding enabled a new series of studies that has deepened our understanding of the necessary and sufficient processes underlying the formation of filial attachments.
The findings with the truncated procedure were concordant with those of the earlier work: 14-day-old rat pups receiving one 2-h session of care from a scented foster dam subsequently spent more time huddling with a conditioned odor and less time huddling time with a novel odor than did pups that were merely exposed for 2 h to the odor, or than did pups exposed to no odor. We replicated the basic finding that a filial huddling preference can be induced without milk reward: milk transfer did not occur in the present study, as evidenced by the weight loss during the 2-h interaction with the foster dam. Similarly, we replicated the finding that a scented, inanimate warm object can induce a huddling preference. We did not, however, detect a familiarization effect [23] after the single session procedure used here, perhaps due to the greatly reduced level of familiarity.
There were numerous benefits to the single-session paradigm for filial huddling preference. The 2-h-long session made it feasible to make continuous, timelapse video recordings and then to quantify many of the details of the behavioral interactions between the mother and pups. In fact, we used litters of four individually marked pups so that interactions among pups and between mother and pups could be recorded for each individual. We were then able to relate variation in individual experiences to variation in the same individual’s preferences on the subsequent test day! In addition to the power of the individual analyses, the single 2 h session made feasible pharmacological interventions during the induction period. Finally, the single, discrete induction period allowed us to conduct brain and serum assays before, during, and after the experiences associated with the odor learning, again with the ability to relate data to quantified, individual data. In effect, we had created opportunities for additional, tests-by-synthesis.
With the new paradigm, our attention returned to the non-nutritive cues that are typical of maternal contact. We operationalized a definition of skin-to-skin contact to specify orientations of mother and a pup that afford conductive heat transfer from the dam’s body to the pup, as exemplified by the drawing in Fig. 4. Such skin-to-skin contact occurs when a mother hovers over pups or assumes a nursing posture with either low or high arched-back [c.f., 30,31], which also affords the mother ventral somatosensory stimulation from pups. When we related data from the individual interactions to the filial preferences of the same individuals in the huddling test, skin-to-skin contact was most associated with subsequent preferences in the pups [29].
We further titrated thermotactile stimulation from maternal contact. Pairs of pups were presented a scented, warm, fur-covered cylinder, this time for the single 2-h session. Again, such stimulation induced the filial preference, so it was relevant to ask whether general ambient warmth without the tactile component is an effective reinforcer, or if the pup’s experience of localized heat from a surface is needed for the learned association with an odor.
Additionally, a group of pups were exposed to stroking with a soft brush in the presence of an odor CS. This procedure was designed to replicate the methods of previous investigators [e.g., 20,21,32–35] who reported that stroking stimulation intended to mimic the mechanical effects of tactile stimulation from maternal licking can serve as a reinforcer for pups’ odor learning. Two control conditions were also included in the present experiment. One was an odor-only control group, used to control for possible effects of familiarization with an odor as the basis of a preference, especially in a two-choice test with another, novel odor [c.f., 23,36]. The other was a no-odor group that provided the necessary baseline condition for interpreting the impact of the various manipulations employed in the present experiment.
Fig. 5 summarizes the findings. Only thermotactile contact induced the odor-guided filial huddling preference. Again, the results indicate that specific experiences during interactions with a mother induce a filial huddling preference in preweanling rat pups. Nutritive reward is not necessary and mechanical stimulation mimicking maternal licking was not effective in this setting. The effects of maternal licking/grooming appear to be age-dependent. Stroking induces an odor preference in pups up to about 9-days of age, but becomes ineffective in older pups [24,35]. Interestingly, warmth, per se, and the metabolic consequences of warming are also not strong elements in the induction of the odor preference. Yet the thermotactile component of maternal care is vital. The present results add to our understanding of the active ingredients within a pup’s experience of thermotactile stimulation.
Fig. 5.
Huddling preferences for a conditioned odor following a single conditioning session in which an odor CS was paired with either a warm tube (tube), ambient warmth (warm-temp), stroking (stroke), or room temperature (odor-only); pups in the no-odor condition (no-odor) were placed in a chamber at room temperature without an odor CS. Huddling preference score captures differences in time spent huddling with the conditioned odor compared to the novel odor. Box plots contain data points show median differences in contact time between the conditioned odor and the novel odor, with quartiles depicted by the brackets. Scores significantly above or below 0 (no difference) reflect group differences in preference for the conditioned odor or for the novel odor, respectively. Lack of difference in contact time between test odors indicates no odor bias. The asterisk (*) indicates a statistically significant preference.
From Kojima and Alberts (2011).
It is pleasing and inspiring to consider these studies in a broader context. Thermal stimuli from maternal care are generally considered to be significant for infant development [37,38]. Effects of skin-to-skin contact with mother (e.g., “kangaroo care”) on infants have been examined in preterm human infants. In addition to positive effects on stress-induced responses, cognitive development, and emotional regulation [39–41], skin-to-skin contact with the mother facilitates maturation of autonomic systems, as measured heart rate (e.g., vagal tone) and state organization (e.g., active and quiet sleep and alert wakefulness) [41]. In rodent studies, however, skin-to-skin contact has received relatively little attention, and effects of thermotactile stimulation on pups are largely unexplored, with a few studies demonstrating that tactile stimulation (i.e., stroking with a paintbrush) has positive, physiological effects on pups [42–44]. The present research illustrates one method of studying effects of conductive warmth from skin-to-skin contact on rodent development and the importance of differentiating between thermotactile and mechanotactile stimulation from maternal contact and touch.
“If the physiological elements of behavior truly explain it, we should be able to combine the elements in different ways, in different amounts, to produce either new forms of behavior or phenomena we had not realized were determined by those elements” [1].
3. Glimpses into the brain
It has been suggested that maternal stimulation affects the offspring’s social behavior by affecting oxytocin (OT) systems in the offspring [45]. OT is a nonapeptide neurohormone that has become widely recognized for its associations with social behaviors and emotions [46], including social recognition [47–50], pair bonding [51,52], and maternal behavior [53–56] in rodents and ungulates, as well as a variety of affective processes in humans [e.g., 57–59]. The expression of OT receptors in adult rodents is associated with the maternal care that they received as infants [60,61]; disrupted maternal behavior alters levels of OT receptors in pups [62].
To date, OT mediation of infant behaviors, including early attachment and social behavior, has not been addressed. Likewise, little is known about OT’s roles in the developing brain, despite the fact that manipulation of OT concentrations in early life induces variations in social behaviors in adulthood, including parental behavior, partner preferences, and male–male aggression [63]. The few data available concerning OT effects on infants implicate it in early olfactory learning of social stimuli [64] and in aggregative behavior in rat pups [65;see, 66].
Again, with the efforts of Sayuri Kojima, we used the single-session paradigm to manipulate brain oxytocin in pups via intracerebroventricular (ICV) injection and investigate its role in the acquisition of a filial huddling preference. We also recorded the behavior of mother and pups during the 2-h odor conditioning session for subsequent analyses of how the OT manipulation affected their social interactions in the litter. We have learned that the time spent by a mother hovering over a pup is positively associated with the strength of the pup’s huddling preference for a maternally paired odor [28]. Similarly, we have learned that it is the thermotactile component of contact received via skin-to-skin contact with the mother that is critical for the acquisition of the pup’s preference [29]. Thus, it also seemed important to examine whether the OT manipulation modified social interactions among mother and pups and, if so, whether the modified interactions corresponded to the previous results.
The results with a basic OT manipulations were clear: pups treated intraventricularly with an oxytocin antagonist (OTA) failed to acquire a huddling preference following 2 h of interaction with a scented foster dam. In contrast, saline-treated siblings that experienced the same foster mother at the same time as the OTA-treated pups acquired a huddling preference for the dam-associated odor. When exogenous OT was administered, the filial preference was acquired, but was no greater than that displayed by saline-treated controls [67]. We were able to conclude that central OT is necessary for the formation of a filial huddling preference in rat pups.
A particularly exciting outcome of this research is a novel perspective on how centrally OT modulates both individual and group behavior of rat pups [28,29,65,66]. Recall that skin-to-skin contact between mother and pup correlates with the magnitude of the pups’ subsequent filial preferences [29]. Therefore, it was exciting to find that manipulation of central OT in the pups altered contact behavior between dam and pups in the 2-h induction session. As I describe these changes, keep in mind that the experiments involved a dam and four individually identified pups and that only two of the four pups received an OT manipulation.
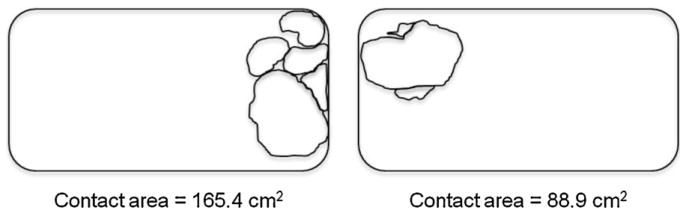
When mother–pup interactions under the influence of an OT manipulation during the 2-h sessions were analyzed, several forms of altered contact were discovered. We found that OT litters displayed more frequent dam-pup contacts during the conditioning session than did OTA litters. When we used a descriptive tool to catalog and quantify the combinations of bodies in contact within the family group, the aggregations or “contact patterns” remained surprisingly constant, despite the OT manipulations. Nevertheless, planimetric measures of the cohesion of the social contacts revealed that central OT mediated these interactions, and that these effects conformed to the consequences seen in the pups’ learned preferences. Fig. 6, for example, contains images of two rat families, drawn to represent the outline of the family “units”. The images were captured during a series systematic video samples and these were part of our analysis of family cohesion when all five individuals (mother + four pups) are in contact with one another. The figure on the left shows that a representative OTA family with area that subtends about twice that of the representative OT family in the right-hand panel. This difference in family cohesion between the OTA and OT litters appeared across the various mother–pup combinations that can occur during close interactions. The methods and the analyses for these studies are described in detail elsewhere [58,67], and a full discussion is beyond the scope of the present essay. It is significant, however, to recognize that these impressive effects on the entire family are related to OT manipulations of just two of the four pups. Those localized changes in OT alter the interactions and structure of the entire family unit.
Fig. 6.
Examples of aggregon areas for unified contact pattern with all of the animals in contact with at least one other, thus forming a single aggregation. Although both families (mother and pups) are in the same contact pattern, the group with two, oxytocin-treated pups (on the right) has formed a measurably smaller aggregon area.
From Kojima and Alberts [28].
4. Bi-directional roles for oxytocin and behavior in the acquisition of the pups’ filial preference
When there is a dramatic cohesion observed in a rat family (measured as a reduction in the total area subtended by the bodies of the group), it is most often caused by the mother covering pup bodies (see Fig. 4). This arrangement is typical of “hovering” behavior, a maternal posture and activity often used to define maternal responsiveness [31,68–71]. Mothers that provide more of such contact (i.e., skin-to-skin contact) are providing more stimulation that modulates central OT concentrations in the pups [72], which, in turn, is associated with higher levels of filial preference for a dam-associated odor [29]. Thus, when we observe mothers with OT litters producing smaller aggregon areas per aggregon index, we are seeing the pups’ central OT affecting maternal behaviors that create conditions that enhance acquisition of a maternal odor preference—via the pups’ central OT system. Thus, the present findings suggest that OT mediates the acquisition of filial, huddling preference not only neuroendocrinologically, but also behaviorally.
Importantly, such dual action appears bi-directional. Mother–pup contact modulates OT concentrations in the pups [72], potentially affecting pup’s olfactory learning as well as altering the pup’s behavior, so that the pup makes more contact with the dam. Such active contact by pups can stimulate maternal responses that are expressed in forms (e.g., greater contact cohesion through maternal hovering) that potentiate skin-to-skin contact with pups [30,31]. Such maternal contact facilitates the pup’s preference learning [67] via the OT system, which, in turn, further augments the mother–pup interactions and the conditions for learning an odor-based social affiliation.
The causal pathways between oxytocin and behavior are truly multi-dimensional, for they work “horizontally” on the behavioral exchanges of the dam and pups and they work “vertically” by affecting behaviorally-relevant processes on different levels, including neural substrates of learning, proximity regulation and alterations in the sensory events that follow from that, as well as modulation of neuroendocrine responses. We are on the threshold of deciphering the co-actions of these pathways [77] and Teitelbaum’s sense of emergence and covert causality, seen in his principle of combining elements and witnessing novel outcomes will serve us well in many domains.
“Synthesis by Model:…We build into the model the elements we think are important and also our conception of the way these elements interact to produce the phenomenon. Such models can be purely theoretical, as in mathematical models…in which…we postulate the essential elements and processes involved, in such a way that they can be described quantitatively”[1,p. 11].
5. Synthesis and hypothesis-testing by computational modeling
We can begin to synthesize the diverse body of knowledge about rat pups by constructing models of group and individual behavior. Here we provide a glimpse of what may be possible with these new tools and techniques.
In a typical study, which simplifies many contextual variables, litters of eight rat pups are placed in the stalls of a starting corral, arranged in two rows of four pups each, and centered in an arena (8 in. × 12 in.) that is a well-controlled, warm environment (e.g., 34 °C floor; 34 °C air). When the corral is removed, the pups can move freely and aggregate. The upper photo in Fig. 7 depicts eight pups immediately after the corral was removed. Video images of pups’ movements and aggregations provide a surfeit of information, from which we sample to make comparisons with virtual or physical models. Image sampling every 5 s is sufficient to collect meaningful data concerning individual and group behavior [73–76].
Fig. 7.
Index of 22 possible aggregons created by eight pups. The upper photo illustrates aggregon #1, in which all pups are separate. The middle photo shows them aggregated in a 4,2,1 and 1 contact pattern (aggregon #12). In the lower photo, all eight pups are in contact (aggregon #22). Group organization changes constantly, and aggregon distribution/time is used to capture the dynamics of the group. To test computational models in comparison to pups, the positions of the real pups and agent-pups are scored every 5 s and the aggregon distributions are compared.
From Alberts and Schank [77].
Data on locomotion are obtained by encoding from the images pup positions as x–y coordinates; these data allow for analysis of activity, pup contact, wall contact, and aggregation [75,76]. As the pups aggregated, they formed combinations with other pups that we codified as aggregon patterns (i.e., the distribution of individuals in contact groups formed on the surface of the arena). There are 22 possible aggregon combinations of 8 pups, listed in the aggregon index in Fig. 7. The dynamics of aggregon frequencies provide a detailed and diagnostic description of group behavior.
To understand how 7-day-old rat pups, still blind and deaf, could form the aggregon patterns, we built an agent-based model [74–76]. Pup movement was simplified: with each time-step, a pup could move to one of eight adjacent locations in the virtual arena. The rules of behavior are simple: (a) if one or more pups are located in front or on the side of a pup’s head, then there is an increased probability that the pup moves towards that location. Similarly, we assumed that (b) pups moved toward the walls of the arena directly in front of them. The two other rules governed activity: (c) a pup remains active as a function of the number of active and inactive pups it contacts and (d) a pup remains inactive again as a function of the number of active and inactive pups contacted. Thus, where a pup moves and whether it is active is situation-dependent. The dynamics of pup huddling under the simple conditions used here were captured by the frequency of each aggregon pattern over time (the filled squares in Fig. 8).
Fig. 8.

Frequency of aggregons exhibited by 7-day-old litters (data) and litters of agent-based computational pups (model) during 15-min tests.
From Schank and Alberts [75].
To find a model that behaves like real pups involves determining specific values for the probabilities of movement toward pups or toward a wall. As illustrated in Fig. 8, the best fit model generates aggregon frequency distributions that mimic those of the real 7-day-old rat huddles using the movement and activity rules previously described. We explored the landscape of results derived from changing parameters of the model and have shown that this is a robust phenomenon [75].
The success of the model to emulate faithfully the complex pattern of aggregon dynamics was an exciting accomplishment. It is important to emphasize that the model contains no rules of group behavior, but only rules for individual movement and activity. Nonetheless, in the absence of group instructions, pups aggregate and age-specific patterns of aggregation emerge [74,76]. A new, lawful form of behavior–group behavior–emerged from the interactions of individuals, with no information about group organization provided in the model!
“Synthesis by Model:…We can also construct physical analogies…all such models are attempts to duplicate a behavioral phenomenon through the workings of a model which embodies its essential elements” [1,p.11].
6. Synthesis and hypothesis-testing by construction
Jeff Schank and his colleagues at the University of California, Davis built robots that behave like rat pups. This exercise proved to be instructive on many levels and dimensions, which we have reviewed briefly [77] and can be pursued in depth in their full reports [78]. Among the most striking discoveries made during the early phases of this work are the contrasting outcomes when the robotic pups are programmed with “thigmotaxic-reactive architecture” and “random reactive architecture”. Robotic pups that followed strict sensory rules repeatedly followed walls, circling the arena repeatedly, and manifested sequences resembling the “stereotypies” often observed in animals in zoos, as well as some of those described by Teitelbaum, in his studies of animals recovering from brain damage. In contrast, when the robots were programmed to move randomly, with no influence on their behavior from tactile sensors, the behavior of individuals and that of the groups they comprised displayed patterns that were remarkably faithful to the behavior of litters of real pups. Fig. 9 provides a sample of the remarkable group patterns that emerge in real pup groups and in the robotic groups.
“…we should be able to combine the elements in different ways, in different amounts, to produce either new forms of behavior or phenomena we had not realized were determined by those elements” [1,p. 15].
Fig. 9.
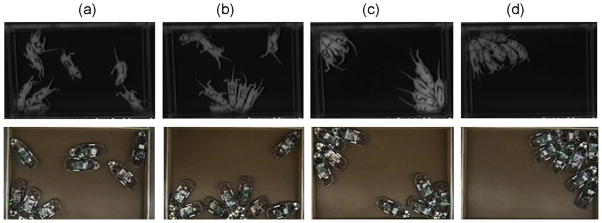
Examples of aggregons of PND10 pups (top row) and robots (bottom row) in arenas scaled to the size of the individuals. Column [a] illustrates the active pups and robots shortly after the trial began, at which time both sets of subjects were arranged similary, in two rows of four individuals. Pups and robots in column b are generally active and have begun to aggregate. The other images show the formation of two subgroups as well as aggregating in a single corner.
From Alberts and Schank [77].
7. Synthesis and the emergence of capabilities
The demonstrations of huddling of virtual rat pups, virtual huddles, robotic rat pups, and robotic huddles are mind-boggling. Yet, by design, they are unnaturally simple. Such simplicity is a virtue–if it is part of a greater effort to replace it in a more naturalistic and complex context, for we know that context matters. How it matters and whether and how it affects simplified process in context is at the heart of our search for methods that really work in the pursuit of understanding natural, complex processes.
Rat pups do not live in a perfectly flat, smooth, evenly illuminated, thermoneutral world. Their normal environments are decidedly three-dimensional, with varying ambient conditions. How are the rules of huddling expressed in more complex, changeable environments, and how are they expressed by a three-dimensional pile of pups? We have learned a bit about this, and thus far the results are intriguing.
Pups in nest-like environments reside in clumps. But these are not simply piles of bodies. They are moving, seething, masses. Viewed via timelapse video, the group is in almost constant motion. By marking pups and quantifying their movements, we have established that there exists a phenomenon of pup flow within huddles. The movements of pups in a naturalistic setting establish convection currents of bodies, depicted in Fig. 10, called “pup flow” [9]. The kinds of simple rules that govern aggregation on flat surfaces, expressed in a three-dimensional space, or some similar, additional rules seem to govern this behavior. But it is clear that there is much more to this aspect of individual and group behavior. Pup flow is typically downward, into the huddle. Is this because there is a rule to move in the direction of gravity? Is there a rule to push into crevices of bodies? In fact, it appears that the direction of flow is determined by thermal stimuli and that it reflects, at least in part, individual regulatory movements. When a nest is cool, flow is downward and if a nest is warmed artificially, the direction of flow is reversed, and the flow is upward. It seems likely that there exist rules for this, but they are thermal rules or regulatory rules. Recently, in a study of mouse (Mus musculus) litters, we have observed pup flow in an artificial nest and have studied it at different temperatures. Rate of pup flow, in addition to direction, is temperature-dependent, resembling aspects of convection currents in a fluid [79].
Fig. 10.
Figurative and quantitative depictions of “pup flow” within a huddle of rat pups. The arrows superimposed on the drawings of pups in a funnel-shaped nest depict their movements in a cool nest, one in which pups dive into its depths for warmth. The graphs depict the proportion of sequential 8-min intervals that a focal pup was visible on the surface of the group. The dramatic, saw-tooth pattern reflects the motion of the focal pup and littermates that obscure and reveal the focal animal.
From Alberts [9].
The temperature-dependence of pup movements brings us back to ideas about huddling that were noted at the beginning of the present discussion, but now, in the context of a higher-level synthesis, we are focusing on the level of the group. Pups live and behave in a group and as a group. As we learn to study their behavior on multiple levels, we can discover new dimensions of behavior, particularly new competencies. When huddling by rat pups first captured my attention, there was a small but ample literature documenting the physiological and ecological significance of contact behavior in a variety of adult animals. Adult animals huddle for warmth. Stated mechanistically, huddling reduces exposed surface area and thus decreases surface-to-mass ratio of each participant. If the surrounding temperature is cooler than body temperature, heat loss is reduced and metabolic expenditures necessary for body temperature maintenance are conserved. Contact with another warm or warmer body can augment body temperature by heat conductance. Hence, huddling is energetically efficient and can contribute importantly to a endotherm’s energy budget, often rendering it an ecologically significant behavioral strategy.
The situation for rat pups, however, was not so clear. Norway rats are altricial, meaning that they produce young that are grossly immature: small, furless, thin skinned, lacking subcutaneous fat, neuromuscularly incapable of shivering, blind, deaf…quite “helpless” and dependent on the mother for body heat. Experts in thermal physiology bluntly referred to such infant mammals as “cold-blooded” or ectothermic or poikilothermic. While these pejoratives would eventually be replaced with a more accurate characterization [c.f., 80], the infant is so thermally vulnerable that, in study after study, pups’ body temperatures quickly decreased toward ambient temperatures [9].
The thermally vulnerable infant mammal appeared to lack the ability to thermoregulate. This fundamental lack seemed to fit with other areas of incompetence. Infant rats were, it seemed, unable to respond to deprivation or to regulate milk intake [81]. The infant rat was viewed as an incomplete, incompetent, dependent, non-regulatory, reflex-dominated precursor of the successive stages during which it would become increasingly complete, competent, independent, regulatory, and volitional organism. Yet, pups in groups remain warmer than do the same pups in isolation [9]. Even more impressive was that infant rats in groups display the metabolic strategy a homeotherm, viz., oxygen consumption rate is greater in a cool surround and lower in a warm surround. Metabolically, the same individuals were known to display the metabolic strategy of an ectotherm, viz., their oxygen consumption rate was low in the cold and increased at higher temperatures [82]. In other words, individual infants displayed an ectothermic strategy whereas huddles of the same infants displayed the regulatory competence of endothermy.
How could it be that an assembly of incompetent individual pups, together, comprises a competent group? One explanation, admittedly a rather dull one, is that any pile of objects will lose heat more slowly than will the same objects not in a pile. Is a pile of pups just a pile of individuals or is it something different, something more? The way this question was answered was to challenge a huddle of pups with a range of temperatures and make measures of the pile, using tools that could indicate whether and how thermal adjustments are made. Fig. 11 illustrates the methods. Briefly, a huddle of individuals was viewed as an entity (ignoring contact boundaries), as depicted by the translation from the drawing on the left to the outline on the right. The size of a huddle was expressed in “pup units”, which was determined by the mean circumference of the individuals comprising the huddle, thus accounting for age-related size differences, camera distance, and the like. Litters of different ages were placed on a flat surface in a temperature-controlled compartment that was initially cool (20 °C). Images were taken every 10 min, as the ambient temperature gradually increased to about 40 °C and then back down to 20 °C. The entire cycle spanned about 2.5 h.
Fig. 11.
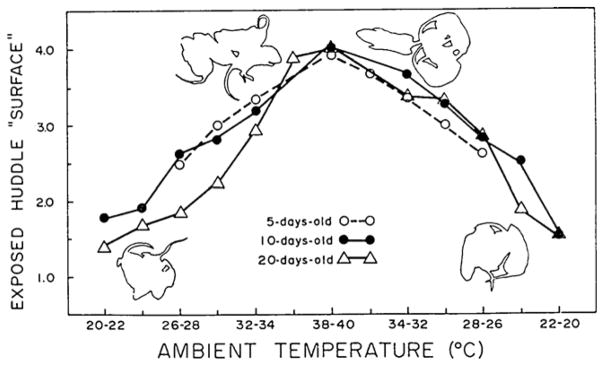
Group regulatory behavior by huddles of rat pups at 5-, 10-, and 15-days of age. This summary graph shows the surface area of huddles (expressed in size-corrected “pup units”). Huddles of pups exposed to a 2-h-long cycle of ambient temperatures that begin cool (20 °C), increase to as much as 40 °C, and then decrease to 20° C show a corresponding increase and decrease in exposed surface area.
From Alberts [9].
Fig. 11 summarizes the findings. At each age tested, huddles of pups interacted in a manner that regulated the size, i.e., the exposed surface, area of the group. As the temperature increased, as seen from the left side of the figure to about its middle, the area of the huddle unit increased. And then as the temperature decreased, the huddle itself decreased. In other words, the huddle displayed a form of group regulatory behavior whereby the group regulated its surface:mass ratio. As can be seen in Fig. 11, the huddles displayed robust group regulation. As a group, expressing their individual rules of behavior, new forms of behavior emerge [77]. Not only are these emergent, group behaviors novel and lawful, they are regulatory. Competence emerges.
There is more to this story but, hopefully, the moral is apparent and worth a pause for appreciation: by adhering to rules and guidelines articulated elegantly and demonstrated consistently by Phil Teitelbaum, we take pleasure in observing behavior, approach it with respect and simplify it for analysis, titrate it into fractions for study, reconstitute it by synthesis, use various methods to converge on synthesized truths, and revel in the pleasures of confirmed conjectures, relinquished ideas, and glimmers of understanding as we trace the beauty of behavior.
8. Reflections
It is telling, I think, that a research program spanning developmental processes of social affiliation, temperature regulation, olfactory learning, mother–infant relations, computational modeling, behavioral development, behavioral endocrinology, robotics, and group regulatory behavior can be united by a set of principles devised by a researcher engaged in other activities in another place at another time. It tells us that these are higher-order ideas. The principles presented so plainly by Teitelbaum can be powerfully effective in helping us see into complexity, organize our analytic activities, and step through phases of analysis. None of these principles are wholly original or unique, but Phil Teitelbaum arranged them, explained them, and set an example with his use of them. He provided a view of behavior, and created a map with landmarks and paths that can connect them. To use the map and follow those paths is to use a paradigm for which we can gratefully give credit.
Acknowledgments
Preparation of this paper and research described in it was supported, in part, by Grant MH-082019 to JRA.
References
- 1.Teitelbaum P. Physiological psychology. New Jersey: Prentice-Hall; 1966. [Google Scholar]
- 2.Barnett S. The rat: a study in behavior. London: Methuen; 1963. [Google Scholar]
- 3.Alberts JR. Huddling by rat pups: postures and body temperature during conductive heat exchange with warm and cool surfaces. unpublished observations. 1984 [Google Scholar]
- 4.Alberts JR. Huddling by rat pups: multisensory control of contact behavior. Journal of Comparative and Physiological Psychology. 1978;92:220–30. doi: 10.1037/h0077458. [DOI] [PubMed] [Google Scholar]
- 5.Alberts JR, Brunjes PC. Ontogeny of thermal and olfactory determinants of huddling in the rat. Journal of Comparative and Physiological Psychology. 1978;92:897–906. doi: 10.1037/h0077533. [DOI] [PubMed] [Google Scholar]
- 6.Alberts JR. Olfactory contributions to behavioral development in rodents. In: Doty RL, editor. Mammalian olfaction, reproductive processes, and behavior. New York: Academic Press; 1976. pp. 67–93. [Google Scholar]
- 7.Alberts J. Ontogeny of olfaction: reciprocal roles of sensation and behavior in the development of perception. New York: Academic Press; 1981. [Google Scholar]
- 8.Teicher MH, Blass EM. Suckling in newborn rats: eliminated by nipple lavage, reinstated by pup saliva. Science. 1976;193:422–5. doi: 10.1126/science.935878. [DOI] [PubMed] [Google Scholar]
- 9.Alberts JR. Huddling by rat pups: group behavioral mechanisms of temperature regulation and energy conservation. Journal of Comparative and Physiological Psychology. 1978;92:231–45. doi: 10.1037/h0077459. [DOI] [PubMed] [Google Scholar]
- 10.Brunjes PC, Alberts JR. Olfactory stimulation induces filial preferences for huddling in rat pups. Journal of Comparative and Physiological Psychology. 1979;93:548–55. doi: 10.1037/h0077571. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb G. Conceptions of prenatal development: behavioral embryology. Psychological Review. 1976:215–34. [PubMed] [Google Scholar]
- 12.Gottlieb G. The roles of experience in the development of behavior and the nervous system. In: Gottlieb G, editor. Neural and behavioral specificity. New York: Academic Press; 1976. pp. 25–54. [Google Scholar]
- 13.Leon M, Galef BG, Behse JH. Establishment of pheromonal bonds and diet choice in young-rats by odor pre-exposure. Physiology and Behavior. 1977;18:387–91. [Google Scholar]
- 14.Hoffman H. Laboratory investigations of imprinting. New York: Garland STPM; 1979. [Google Scholar]
- 15.Sluckin W. Imprinting and early learning. Chicago: Aldine; 1964. [Google Scholar]
- 16.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–4. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 17.Amsel A, Burdette DR, Letz R. Appetitive learning, patterned alternation, and extinction in 10-d-old rats with non-lactating suckling as reward. Nature. 1976;262:816–8. doi: 10.1038/262816b0. [DOI] [PubMed] [Google Scholar]
- 18.Brake SC. Suckling infant rats learn a preference for a novel olfactory stimulus paired with milk delivery. Science. 1981;211:506–8. doi: 10.1126/science.7192882. [DOI] [PubMed] [Google Scholar]
- 19.Kenny J, Blass E. Suckling as an incentive to instrumental conditioning in preweanling rats. Science. 1977;196:898–9. doi: 10.1126/science.860121. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. Journal of Experimental Psychology. 1982;8:329–41. [PubMed] [Google Scholar]
- 21.Sullivan RM, Hall WG. Reinforcers in infancy: classical conditioning using stroking or intraoral infusions of milk as USC. Developmental Psychobiology. 1988;21:215–24. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- 22.Teitelbaum P, Pellis S. Toward a synthetic physiological psychology. 1992 [Google Scholar]
- 23.Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Developmental Psychobiology. 1984;17:161–81. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- 24.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9:1004–6. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman CM, Flory GS, Alberts JR. Neonatal thermotaxis improves reversal of a thermally reinforced operant response. Developmental Psychobiology. 1999;34:87–99. doi: 10.1002/(sici)1098-2302(199903)34:2<87::aid-dev2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Johanson I, Hall W. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–21. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- 27.Rudy JW, Hyson RL. Consummatory response conditioning to an auditory stimulus in neonatal rats. Behavioral and Neural Biology. 1982;34:209–14. doi: 10.1016/s0163-1047(82)91598-9. [DOI] [PubMed] [Google Scholar]
- 28.Kojima S, Alberts J. Maternal care can rapidly induce an odor-guided huddling preference in rat pups. Developmental Psychobiology. 2009;51:95–105. doi: 10.1002/dev.20349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojima SA., Jr Warmth from skin-to-skin contact with mother is essential for the acquisition of filial huddling preference in preweanling rats. Developmental Psychobiology. 2011;53:813–29. doi: 10.1002/dev.20565. [DOI] [PubMed] [Google Scholar]
- 30.Stern J, Johnson SK. Premolar somatosensory determinants of nursing behavior in Norway rats (Rattus norvegicus) Journal of Comparative Psychology. 1989;103:269–80. doi: 10.1037/0735-7036.103.3.269. [DOI] [PubMed] [Google Scholar]
- 31.Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiology and Behavior. 1990;47:993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- 32.Roth T, Sullivan R. Examining the role of endogenous opioids in learned odor-stroke associations in infant rats. Developmental Psychobiology. 2006;7:1–8. doi: 10.1002/dev.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan RM, Brake SC, Hofer MA, Williams CL. Huddling and independent feeding of neonatal rats can be facilitated by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19:625–35. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19:615–23. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- 35.Woo C, Leon M. Sensitive period for neural and behavioral response development to learned odors. Developmental Brain Research. 1987;30:9–13. doi: 10.1016/0165-3806(87)90038-1. [DOI] [PubMed] [Google Scholar]
- 36.Galef BG. Acquisition and waning of exposure-induced attraction to a nonnatural odor in rat pups. Developmental Psychobiology. 1982;15:479–90. doi: 10.1002/dev.420150510. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblatt JS. Olfaction mediates developmental transition in the altricial newborn of selected species of mammals. Developmental Psychobiology. 1983;16:347–75. doi: 10.1002/dev.420160502. [DOI] [PubMed] [Google Scholar]
- 38.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–35. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 39.Feldman R, Eidelman AI, Sirota L, Weller A. Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics. 2002;110:16–26. doi: 10.1542/peds.110.1.16. [DOI] [PubMed] [Google Scholar]
- 40.Feldman R, Weller A, Sirota L, Eidelman AI. Skin-to-skin contact (kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Developmental Psychology. 2002;38:194–207. doi: 10.1037//0012-1649.38.2.194. [DOI] [PubMed] [Google Scholar]
- 41.Feldman R, Weller A, Sirota L, Eidelman AI. Testing a family intervention hypothesis: the contribution of mother–infant skin-to-skin contact (Kangaroo care) to family interaction, proximity, and touch. J Fam Psychol. 2003;17:94–107. [PubMed] [Google Scholar]
- 42.Evoniuk GE, Kuhn CM, Schanberg SM. Effect of tactile stimulation on serum growth-hormone and tissue ornithine decarboxylase activity during maternal-deprivation in rat pups. Communications in Psychopharmacology. 1979;3:363–70. [PubMed] [Google Scholar]
- 43.Pauk J, Kuhn CM, Field TM, Schanberg SM. Positive effects of tactile versus kinesthetic or vestibular stimulation on neuroendocrine and Odc activity in maternally-deprived rat pups. Life Sciences. 1986;39:2081–7. doi: 10.1016/0024-3205(86)90359-0. [DOI] [PubMed] [Google Scholar]
- 44.Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic–pituitary–adrenal axis in the infant rat—the roles of feeding and stroking. Developmental Brain Research. 1993;75:185–92. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- 45.Melo AI, Lovic V, Gonzalez A, Madden M, Sinopoli K, Fleming AS. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: tactile stimulation, nest odor, and social rearing prevent these effects. Developmental Psychobiology. 2006;48:209–19. doi: 10.1002/dev.20130. [DOI] [PubMed] [Google Scholar]
- 46.Insel T, Young L. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 47.Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behavioural Brain Research. 1998;90:89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. Journal of Neuroscience. 2001;21:8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25:284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 50.Popik P, van Ree JM. Neurohypophyseal peptides and social recognition in rats. Using eye movements as an experimental probe of brain function—a symposium in Honor of Jean Buttner-Ennever. 1998;119:415–36. doi: 10.1016/s0079-6123(08)61585-x. [DOI] [PubMed] [Google Scholar]
- 51.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 52.Young L, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 53.Fahrbach SE, Morrell JI, Pfaff DW. Oxytocin induction of short-latency maternal-behavior in nulliparous, estrogen-primed female rats. Hormones and Behavior. 1984;18:267–86. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- 54.Keverne E, Kendrick K. Oxytocin facilitation of maternal-behavior in sheep. Annals of the New York Academy of Sciences. 1992;652:83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen CA. Oxytocin control of maternal behavior—regulation by sex steroids and offspring stimuli. Annals of the New York Academy of Sciences. 1997;807:126–45. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiology and Behavior. 2003;80:233–41. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 59.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 61.Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. Journal of Neuroendocrinology. 2002;14:349–53. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- 62.Noonan LR, Caldwell JD, Walker CH, Pedersen CA, Mason GA, Li L. Neonatal stress transiently alters the development of hippocampal oxytocin receptors. Developmental Brain Research. 1994;80:115–20. doi: 10.1016/0165-3806(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 63.Carter CS. Developmental consequences of oxytocin. Physiology and Behavior. 2003;79:383–97. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 64.Nelson E, Panksepp J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behavioral Neuroscience. 1996;110:583–92. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- 65.Odya E, Sokoloff G, Alberts J. The effects of oxytocin on the aggregation of infant rats. Orlando, FL: Society for Neuroscience; 2002. [Google Scholar]
- 66.Alberts JR. Huddling by rat pups: ontogeny of individual and group behavior. Developmental Psychobiology. 2007;49:22–32. doi: 10.1002/dev.20190. [DOI] [PubMed] [Google Scholar]
- 67.Kojima SA., Jr Oxytocin mediates the acquisition of filial, odor guided huddling in preweanling rats. Hormones and Behavior. 2011;60:549–58. doi: 10.1016/j.yhbeh.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lonstein JS, Wagner CK, De Vries GJ. Comparison of the nursing and other parental behaviors of nulliparous and lactating female rats. Hormones and Behavior. 1999;36:242–51. doi: 10.1006/hbeh.1999.1544. [DOI] [PubMed] [Google Scholar]
- 69.Moffat SD, Suh EJ, Fleming AS. Noradrenergic involvement in the consolidation of maternal experience in postpartum rats. Physiology and Behavior. 1993;53:805–11. doi: 10.1016/0031-9384(93)90192-i. [DOI] [PubMed] [Google Scholar]
- 70.Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on maternal behavior in the postpartum rat. Hormones and Behavior. 2004;46:411–9. doi: 10.1016/j.yhbeh.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Seip KM, Morrell JI. Exposure to pups influences the strength of maternal motivation in virgin female rats. Physiology and Behavior. 2008;95:599–608. doi: 10.1016/j.physbeh.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kojima S, Stewart R, Demas G, Alberts J. Maternal contact differentially modulates central and peripheral oxytocin levels in rat pups during a brief regime of mother–pup interaction that induces a filial huddling preference. Journal of Neuroendocrinology. doi: 10.111/j.1365-2826.2012.02280.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.May CJ, Schank JC, Joshi S, Tran J, Taylor RJ, Scott IE. Rat pups and random robots generate similar self-organized and intentional behavior. Complexity. 2006;12:53–66. [Google Scholar]
- 74.Schank J. The development of locomotor kinematics in neonatal rats: an agent-based modeling analysis in group and individual contexts. Journal of Theoretical Biology. 2008;254:826–42. doi: 10.1016/j.jtbi.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 75.Schank J, Alberts J. Aggregation and the emergence of social behavior in rat pups modeled by simple rules of individual behavior. Nashua, NH: New England Complex Systems Institute; 1997. [Google Scholar]
- 76.Schank JC, Alberts JR. The developmental emergence of coupled activity as cooperative aggregation in rat pups. Proc Biol Sci. 2000;267:2307–15. doi: 10.1098/rspb.2000.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alberts JR, Schank JC. Multilevel development: ontogeny of group and individual behavior. In: Blumberg M, Freeman J, Robinson SR, editors. Handbook of behavioral neuroscience. New York: Oxford University Press; 2009. pp. 475–98. [Google Scholar]
- 78.Schank J, May C, Tran J, Joshi S. A biorobotic investigation of Norway rat pups (Rattus norvegicus) in an arena. Adapt Behav. 2004;12:161–73. [Google Scholar]
- 79.Shelton D, Alberts J. Development of temperature dependent pup flow in mouse huddles. Presentation at the meetings of the International Society for Developmental Psychobiology; 2011. [Google Scholar]
- 80.Blumberg M. The developmental context of thermal homeostasis. In: Blass EM, editor. The handbook of behavioral neurobiology. Vol. 13. New York: Plenum Press; 2001. pp. 199–228. [Google Scholar]
- 81.Friedman MI. Some determinants of milk ingestion in suckling rats. Journal of Comparative and Physiological Psychology. 1975;89:636–47. [Google Scholar]
- 82.Fairfield J. Effects of cold on infant rats; body temperatures, oxygen consumption, electrocardiograms. American Journal of Physiology. 1948;155:355–65. doi: 10.1152/ajplegacy.1948.155.3.355. [DOI] [PubMed] [Google Scholar]