Abstract
Introduction
Evidence has demonstrated that deficits in glutamate transmission impair neurocircuits involved in drug abuse or drug-seeking behaviour and affect many aspects of neuroplasticity associated with alcohol and drug addiction. Alcohol-seeking behaviour is promoted by increased glutamate transmission in key regions of the mesocorticolimbic reward circuit, including the nucleus accumbens and prefrontal cortex. Glutamate transmission or glutamate uptake is regulated by a number of glutamate transporters in the brain regions. Among these glutamate transporters, glutamate transporter 1 (GLT1; its human homolog is the excitatory amino acid transporter 2, EAAT2) regulates the removal of majority of the extracellular glutamate. The role of GLT1 has been tested in alcohol and other drugs of abuse models with dysfunction in glutamate transmission. We recently reported that treatment of alcohol-preferring rats with compounds ceftriaxone and GPI-1046, known to upregulate GLT1 levels, showed reduction in alcohol intake and attenuation of relapse-like ethanol-drinking behaviour. Furthermore, we demonstrated that upregulation of GLT1 was associated with attenuation of cue-induced cocaine relapse. Together, we suggest that GLT1 is considered as a potential therapeutic target for the treatment of drug dependence, including alcohol. The aim of this critical review was to discuss the potential therapeutic role of GLT1 for the treatment of alcohol dependence.
Conclusion
Dysfunction of glutamate transmission has been suggested to impair neurocircuits involved in alcohol dependence, which affect neuroplasticity that is associated with ethanol intake.
Introduction
Alcohol abuse and dependence continue to be significant public health problems. A better understanding of their neurobiology will facilitate the development of interventions targeting prevention and/or treatment of these major health issues. Evidence has suggested that several neurotransmitters are involved in the development of drug abuse and dependence, including alcohol.
While it is established that dopaminergic neurotransmission plays an important role in alcohol addiction, increasing evidence suggests that many aspects of neuroplasticity in drug addiction involve changes in glutamatergic neurotransmission as well. Neuroadaptations of the glutamatergic system play a key role in alcohol tolerance, dependence and withdrawal1. The selective effects of alcohol include inhibition of glutamatergic neurotransmission by alteration of N-methyl-D-aspartate (NMDA) receptors1. One of the effects of chronic alcohol exposure is the upregulation of NMDA receptors that are part of the compensatory mechanism, which results from chronic inhibition of glutamatergic neurotransmission2,3. In addition, the effects of alcohol withdrawal are associated with increased extracellular glutamate levels in the striatum of alcohol dependent rats4 and enhanced NMDA sensitivity in the nucleus accumbens (NAc)5. Importantly, drugs that have the potential to target NMDA receptors and metabotropic glutamate receptor subtype 5 (mGluR5) have indicated the important role of the glutamatergic system in alcohol dependence and seeking-behaviour6. In addition, a marked increase in the levels of extracellular glutamate was found in the NAc of animals exposed chronically to ethanol7.
Extracellular glutamate levels and glutamate neurotransmission are regulated by several glutamate transporters in the brain8,9. Among these glutamate transporters, glutamate transporter 1 (GLT1, termed also as excitatory amino acid transporter 2, EAAT2) is a key player in the removal of the majority of extracellular glutamate10,11. Similar to disease models in which there is dysfunction of the glutamatergic excitatory system, the role of GLT1 has been tested in drug abuse models that show dysfunction of glutamate transmission. It is noteworthy that the activation of GLT1 by MS-153, a cerebroprotective agent, was effective in reducing the induction of conditioned place preference to methamphetamine, cocaine and morphine12. Moreover, we have recently found that ceftriaxone, a β-lactam antibiotic, known to upregulate GLT1 levels13–15, attenuates cue-induced cocaine relapse in a dose-dependent manner in a rat model16. In accordance with this data, Knackstedt et al.17 have found similar effects with ceftriaxone in cocaine relapse-like behaviour. Focussing on the role of GLT1 in alcohol-drinking behaviour, we have previously reported that male alcohol-preferring (P) rats treated with ceftriaxone for five days, at different doses, showed a significant dose-dependent reduction in ethanol intake compared to saline-treated rats15. This reduction in ethanol intake was associated, in part, with the upregulation of GLT1 levels in the NAc and prefrontal cortex (PFC). Moreover, we have recently reported that chronic alcohol consumption can lead to downregulation of GLT1 levels in the NAc18. These findings suggest that GLT1 upregulation or activation has potential for the treatment of alcohol dependence and other drugs of abuse. In this critical review, we have discussed the neurocircuitry involving the glutamatergic system in alcohol dependence as well other drugs of abuse. We have further discussed the importance of upregulation of GLT1 in chronic ethanol consumption and relapse-like ethanol drinking behaviour.
Discussion
The authors have referenced some of their own studies in this review. The protocols of these studies have been approved by the relevant ethics committees related to the institutions in which they were performed. Animal care was conducted in accordance with the institution guidelines.
Neurocircuitry of the glutamatergic system in alcohol dependence and other drugs of abuse
Although the neurocircuitry of the glutamatergic system in the modulation of alcohol dependence and drugs of abuse is not fully defined, it is suggested that this system within the PFC19 and the NAc20 plays a critical role in drug reinforcement. These brain regions receive substantial input from the midbrain dopaminergic neurons and all major drugs of abuse, including alcohol, and increase forebrain dopamine transmission21,22. It is important to note that the NAc is suggested as a gateway for limbic structures targeting the motor system for the development of drug dependency, including alcohol23.
Among the glutamatergic projections are those occurring from the PFC to the NAc and reciprocal glutamatergic connections occurring between the PFC and the amygdala22. Moreover, glutamatergic projections from the PFC to the NAc are critical in the expression of addictive behaviours22. In addition, activation of the glutamatergic neurons of the PFC, targeting the amygdala, was found to be directly involved in the development of drug addiction. It is noteworthy that both the PFC and NAc receive glutamatergic projections from the amygdala and hippocampus, and this neurocircuit was suggested to be implicated in the initiation of drug-seeking behaviour24. There are also glutamatergic projections from the PFC to dopaminergic neurons in the ventral tegmental area (VTA). The importance of the glutamatergic projections from the PFC to the NAc and the VTA have been observed in neuroimaging studies performed during craving periods in several different paradigms, for commonly abused drugs such as alcohol, cocaine, methamphetamine, heroin and nicotine19,20. Alternatively, the basal lateral amygdala is considered as a critical brain region for reinstatement to cue-drug-seeking behaviour25.
Role of GLT1 in alcohol-drinking behaviour in the chronic alcohol consumption paradigm
GLT1 is an excitatory amino acid transporter, such that its modulation depends on the electrochemical gradient of sodium and potassium ions. The transporter is a membrane-bound pump that regulates the extracellular glutamate levels26. Increased levels of extracellular glutamate were revealed in post mortem brains that suffered from amyotrophic lateral sclerosis (ALS)27. These increased levels of extracellular glutamate were associated, in part, with the downregulation of GLT1 in post mortem brains with ALS11. GLT1 activity is important in maintaining normal excitatory synaptic neurotransmission, and its dysfunction is implicated in different neurological disorders, including Huntington’s disease (HD), stroke, ALS, brain tumours and epilepsy11,28–31. Although there is no evidence related to any deficit in GLT1 levels in post mortem drug addicts, preclinical studies from our laboratory and other studies have shown downregulation of GLT1 and increased extracellular glutamate levels which were associated with alcohol dependence, relapse-like alcohol-drinking behaviour and relapse to cue-cocaine-seeking behaviour14–16,32. Thus, the importance of identifying and developing drugs that upregulate GLT1 would have therapeutic benefits for the treatment of drug dependency, including alcohol, and also for the treatment of certain neurodegenerative diseases.
Rothstein et al. screened 1,040 Food and Drug Administration-approved drugs and nutritionals and discovered that many β-lactam antibiotics are potent stimulators or upregulators of GLT1 levels, which might be mediated through increased transcription of the GLT1 gene13. In addition, a later study demonstrated that several β-lactam antibiotics, including ceftriaxone, were the most active in increasing GLT1 levels in the brain. Furthermore, ceftriaxone treatment delayed neuronal loss and increased animal survival13. This neuroprotective effect was associated, in part, with upregulation of GLT1 levels in the brain. We have recently shown that ceftriaxone treatment increased GLT1 levels in the striatum, which consequently decreased the extracellular levels of glutamate in a HD R6/2 mouse model28. In addition, we also demonstrated that ceftriaxone inversed the deficit in GLT1 levels observed in the PFC and striatum of R6/2 mice33. It is noteworthy that ceftriaxone-induced upregulation of GLT1 levels may have a direct action on glutamate homeostasis.
Studies focused on the effects of upregulation or activation of GLT1 in animal models of drugs of abuse, including alcohol, have shown promising findings with the implication of this transporter for potential therapeutic targets in the treatment of these disorders23. We hypothesised that if an increase in extracellular glutamate is a key player in alcohol dependence, then upregulation of GLT1 levels would increase glutamate uptake, thus reducing ethanol intake. We tested this hypothesis in our established animal model for alcoholism, P rats, and found that ceftriaxone-induced GLT1 upregulation in the PFC and NAc reduced, in part, the ethanol intake15. Ceftriaxone was found to have a long lasting effect on ethanol intake at higher doses. Moreover, we have also recently examined the effects of neuroimmunophilin GPI-1046 (3-(3-pyridyl)-1-propyl (2S)-1-(3,3-dimethyl-1,2-dioxopentyl)-2-pyrrolidinecarboxylate), in our established P rats18. GPI-1046 is a derived compound from the FK506 immunophilin ligand (tacrolimus). We reported that GPI-1046 treatment reduced ethanol intake in a dose-dependent manner in male P rats. This attenuation of ethanol was associated, in part, with upregulation of GLT1 levels in the PFC and NAc. We postulated here that elevation of GLT1 levels in these brain regions may counteract the increase in the levels of extracellular glutamate caused by chronic ethanol intake.
Recent studies from our laboratory revealed that GLT1 levels were significantly downregulated in the NAc core but not in the PFC of male P rats that consumed ethanol chronically18. In accordance with these results, findings by Knackstedt et al.17 also revealed a significant reduction in GLT1 levels in the NAc but not the PFC in rats self-administered with cocaine17. As we have reviewed recently, it is not clear as to how both ethanol and cocaine had similar effects with regard to the GLT1 levels in the PFC and NAc18. It is important to note that cocaine and ethanol may act mechanistically different, but both may share the same neurocircuitry. The PFC receives and projects glutamatergic inputs and outputs, respectively to and from other brain regions, including the central reward brain regions10. Alternatively, the NAc receives only glutamatergic inputs from the PFC and amygdala, which may explain the differences in the levels of GLT1 in the NAc in chronic alcohol exposure and cocaine self-administration animal models.
Role of GLT1 in the relapse-like alcohol-drinking behaviour paradigm
Relapse behaviour is a persistent problem for individuals recovering from alcoholism. Although there are a number of criteria for relapse-like behaviour, our criteria are based on the definition that a return to levels of consumed alcohol equal to or greater than those observed prior to abstinence is considered relapse-like to ethanol-drinking behaviour. With regard to selectively bred high alcohol-consuming lines of rats including P rats, the alcohol deprivation effect (ADE) has been considered the primary model for assessing relapse-like behaviour. The ADE has been used to assess relapse-like behaviour in P rats34,35. Thus, we have used P rats as a model for relapse-like ethanol-drinking behaviour. We found that ceftriaxone treatment attenuated relapse-like ethanol-drinking behaviour in P rats32. This attenuation of relapse-like behaviour was associated with upregulation of GLT1 levels in the PFC and NAc core. In our recent study, we focused on the NAc core since findings have demonstrated that the increase in glutamate release was more pronounced in the NAc core than in the NAc shell in rats developing behavioural sensitisation36. Furthermore, glutamate release was increased in the PFC-NAc pathways, mainly in the NAc core; this increase in glutamate transmission was observed with reinstatement of cocaine-seeking behaviour36. Moreover, we have examined the effect of upregulation of GLT1 levels with ceftriaxone using the cocaine-seeking behaviour paradigm. We found that ceftriaxone treatment attenuated cue-induced relapse to cocaine self-administration, which was associated with upregulation of GLT1 levels in the PFC and NAc16. In accordance with these results, studies found similar findings showing that ceftriaxone treatment attenuated cue to relapse to cocaine-seeking behaviour; this attenuation was associated with upregulation of GLT1 levels in the NAc and PFC17.
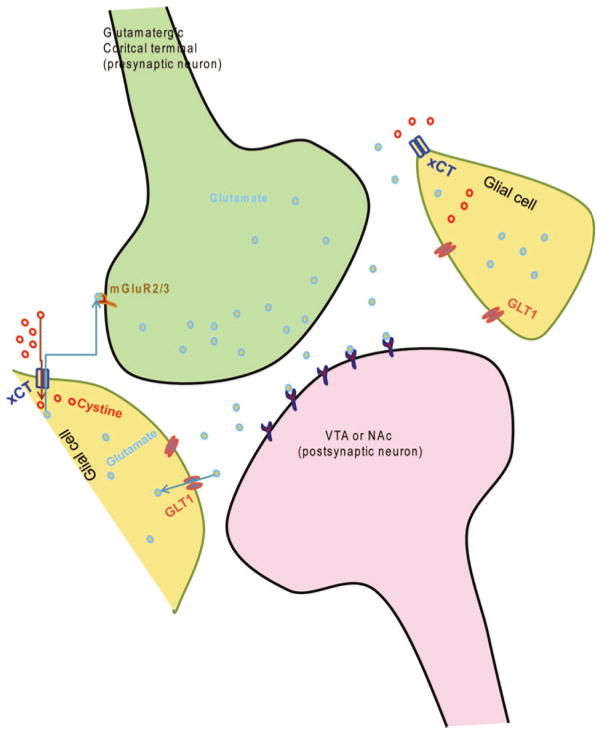
Evidence demonstrated that extracellular glutamate levels might be different between drugs of abuse, including alcohol and cocaine23. For example, basal extracellular levels are lower in the NAc of rats self-administered with cocaine as compared to rats chronically exposed to alcohol36,37. It is important to note that ceftriaxone treatment has similar effects between these two models of cocaine self-administration and alcohol intake, including the relapse paradigm14–18,32. Kalivas et al. and Knackstedt et al. reviewed recently that a decrease in the levels of basal extracellular glutamate in the cocaine-self-administration chronic paradigm might be associated with a decrease in the down-regulation of GLT1 and cystine-glutamate exchanger (xCT) levles in the NAc17,24. However, increased glutamate release at the synapse was found in the NAc during cue-induced relapse to cocaine-seeking behaviour. This increase in glutamate release found in the NAc was associated with activation of the PFC38. It is important to note that basal extracellular glutamate levels are different between cocaine-seeking behaviour and alcohol intake models23, and yet, ceftriaxone was found to attenuate alcohol intake, relapse-like ethanol-drinking behaviour and cue-induced relapse to cocaine-seeking behaviour15,16,17,32. This is probably associated with the effects of ceftriaxone in restoring GLT1 or xCT levels, which are found downregulated after cocaine or alcohol intake in the NAc and PFC17,18. xCT has been shown to play a key role in glutamate homeostasis (Figure 1). For example, findings reported that administration of N-acetylcysteine restored the levels of extracellular glutamate and attenuated cocaine-induced seeking behaviour through mGluR2/3 receptors39. Thus, GLT1, xCT and mGluR2/3 receptors are critical in regulating drug-seeking behaviour, including alcohol, through modulation of glutamate homeostasis (Figure 1).
Figure 1.
Schematic diagram showing the glutamatergic cortical terminal in contact with the postsynaptic neuron from the VTA or NAc. Both glial GLT1 and xCT regulate glutamate homeostasis to modulate dependence and seeking behaviour to drugs, including alcohol. In addition, mGluR5 located at the glutamatergic cortical terminal is also involved in glutamate homeostasis. GLT1, glutamate transporter1; mGluR5 metabotropic glutamate receptor subtype 5; NAc, nucleus accumbens; VTA, ventral tegmental area; xCT, cystine-glutamate exchanger.
Conclusion
It is concluded here that dysfunction of glutamate transmission has been suggested to impair neurocircuits involved in alcohol dependence, which affect the neuroplasticity associated with ethanol intake. Alcohol-seeking behaviour is promoted by increased glutamate transmission in key regions of the mesocorticolimbic reward circuit, including the PFC and NAc. GLT1 is a key player in the regulation of the majority of glutamate uptake and consequently, modulating ethanol consumption. We have identified two compounds, ceftriaxone and GPI-1046 known to upregulate GLT1 levels in the central reward brain regions for the attenuation of ethanol intake. Ceftriaxone was also effective in the attenuation of cue-induced relapse to cocaine. Ceftriaxone and GPI-1046 treatments inversed the effects of ethanol consumption via the upregulation of GLT1 levels in the NAc. These findings indicate that GLT1 is a potential therapeutic target for the treatment of alcohol dependence.
Acknowledgments
This work was supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
Abbreviations list
- ADE
alcohol deprivation effect
- ALS
amyotrophic lateral sclerosis
- GLT1
glutamate transporter 1
- HD
Huntington’s disease
- mGluR5
metabotropic glutamate receptor subtype 5
- NAc
nucleus accumbens
- NMDA
N-methyl-D-aspartate
- PFC
prefrontal cortex
- VTA
ventral tegmental area
- xCT
cystine-glutamate exchanger
Footnotes
Competing interests: none declared. Conflict of Interests: none declared.
All authors contributed to the conception, design, and preparation of the manuscript, as well as read and approved the final manuscript.
All authors abide by the Association for Medical Ethics (AME) ethical rules of disclosure.
References
- 1.Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003 Jul;99(1):79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Michaelis ML, Michaelis EK. Effects of chronic ethanol treatment on the expression of calcium transport carriers and NMDA/glutamate receptor proteins in brain synaptic membranes. J Neurochem. 1997 Oct;69(4):1559–69. doi: 10.1046/j.1471-4159.1997.69041559.x. [DOI] [PubMed] [Google Scholar]
- 3.Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996 Aug;40(1):71–8. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- 4.Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995 Sep;283(1–3):177–83. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- 5.Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003 Nov;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- 6.Grahn RE, Kalman BA, Brennan FX, Watkins LR, Maier SF. The elevated plusmaze is not sensitive to the effect of stressor controllability in rats. Pharmacol Biochem Behav. 1995 Nov;52(3):565–70. doi: 10.1016/0091-3057(95)00141-i. [DOI] [PubMed] [Google Scholar]
- 7.Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, et al. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005 Jul;25(30):7054–61. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000 Oct;32(1):1–14. [PubMed] [Google Scholar]
- 9.Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–56. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- 10.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001 Sep;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 11.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995 Jul;38(1):73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005 Jan;156(2):233–9. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005 Jan;433(7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 14.Sari Y, Franklin KM, Alazizi A, Rao PS, Bell RL. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience. 2013 Jun;241:229–38. doi: 10.1016/j.neuroscience.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011 May-Jun;46(3):239–46. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009 Jul;29(29):9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010 Jan;67(1):81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012 Dec;227:327–35. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002 Oct;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999 Jan;156(1):11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998 Dec;28(3):309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 22.Kalivas PW. Glutamate systems in cocaine addiction. C Curr Opin Pharmacol. 2004 Feb;4(1):23–9. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem. 2012;19(30):5148–56. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56( Suppl 1):169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003 Jul;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 26.Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007 Jan;150(1):5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spreux-Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Le Forestier N, et al. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J Neurol Sci. 2002 Jan;193(2):73–8. doi: 10.1016/s0022-510x(01)00661-x. [DOI] [PubMed] [Google Scholar]
- 28.Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, et al. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008 Apr;153(1):329–37. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, et al. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001 Mar;21(6):1876–83. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, et al. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002 Aug;22(15):6372–9. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999 Dec;19(24):10767–77. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol. 2013 Jun;27(6):541–9. doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J Biomed Sci. 2010 Jul;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001 Aug;25(8):1140–50. [PubMed] [Google Scholar]
- 35.Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, et al. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000 Jan;24(1):8–16. [PubMed] [Google Scholar]
- 36.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004 Feb;24(7):1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miguéns M, Del Olmo N, Higuera-Matas A, Torres I, García-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008 Feb;196(2):303–13. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 38.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003 Apr;23(8):3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005 Jul;25(27):6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]