Abstract
Sphingolipids and their metabolizing enzymes are beginning to be recognized as critical mediators in biological processes, specifically in inflammation and autoimmunity. Sphingosine kinases (SKs) and their lipid product sphingosine-1-phosphate (S1P) play essential roles in inflammatory signaling processes, as well as disease development and progression. SKs can be activated by numerous growth factors and cytokines, including TNF-α and IL-1β, leading to the generation of S1P. S1P exerts its biological effects on intracellular and extracellular targets, such as S1P receptors. In addition to roles in inflammatory signaling pathways SKs, S1P and S1P receptors have been implicated in immune cell function and trafficking, specifically in lymphocytes. This review will discuss the contribution of the bioactive sphingolipid S1P, its generating enzyme SK, and its cell surface receptors in the inflammatory and autoimmune diseases systemic lupus erythematosus, arthritis and inflammatory bowel disease.
Keywords: autoimmune, inflammation, sphingosine-1-phosphate, sphingosine kinase, TNF-α
Sphingolipids were initially thought to function as structural components of cell membranes, but have recently been appreciated as bioactive lipids with distinct biological actions [1]. Furthermore, sphingolipids have emerged as potential key players in inflammation and autoimmune diseases. Numerous sphingolipids and their metabolizing enzymes have been shown to be activated by proinflammatory cytokines and to play key roles in immune cell function and trafficking. Much of the research on sphingolipids and autoimmunity has focused on the potent bioactive lipid, sphingosine-1-phophosphate (S1P) and its synthetic enzyme sphingosine kinase (SK). Expression and activation of SK have been implicated both up- and down-stream of proinflammatory cytokines, such as IL-1β and TNF-α [2–4]. Moreover, S1P binding to its receptors (S1PRs) has been shown to be necessary for immune cell maturation, trafficking and function [5–10]. In this review, the authors will discuss the role of SKs and S1P in inflammation and autoimmune disease.
Sphingolipid metabolism
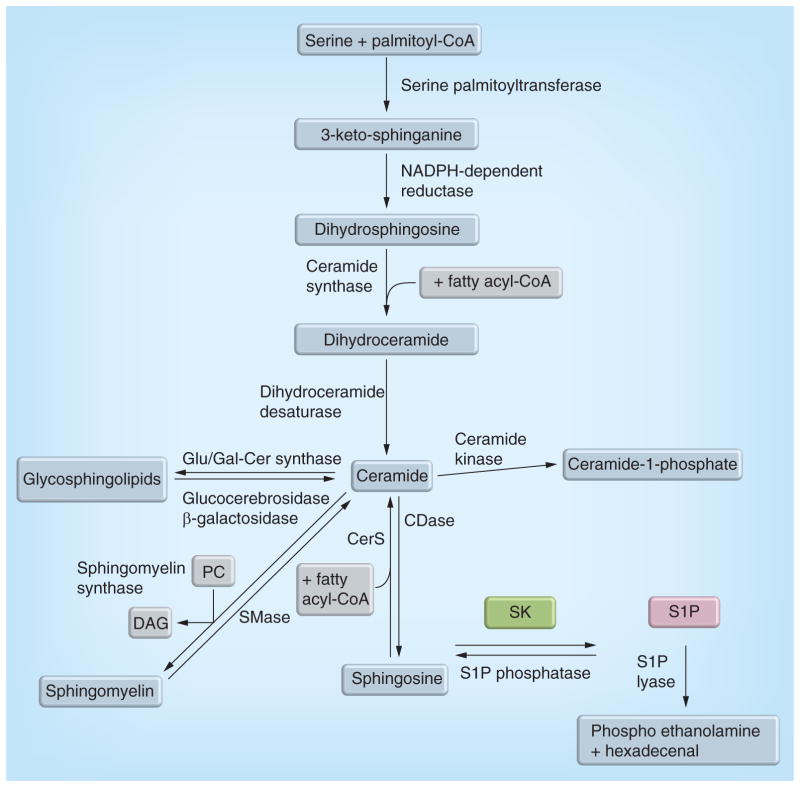
Sphingolipids and their metabolizing enzymes form a complex network of proteins and lipid signaling molecules (Figure 1). The initial step in de novo sphingolipid synthesis occurs with the condensation of serine and palmitoyl-coenzyme A to form 3-keto sphinganine, which is then rapidly reduced to dihydrosphingosine. Dihydrosphingosine is then acylated to form ceramide, the central sphingolipid, which has many potential fates in sphingolipid metabolism. More complex sphingolipids such as glycosphingolipids or sphingomyelin can be formed from ceramide via glucosylceramide or galactosylceramide synthase or sphingnomyelin synthase. Metabolic breakdown of sphingolipids occurs via breakdown of complex sphingolipids by cerebrosidases and sphingomyelin by sphingomyelinases to generate ceramide, which in turn can be phosphorlylated to ceramide-1-phosphate or de-acylated, by one of five ceramidases to sphingosine. Sphingosine can be phosphorylated to S1P by SKs. S1P then has two potential fates. S1P can either be dephosphorylated by a phosphatase, which allows for reformation of sphingosine, and potentially ceramide by ceramide synthases using sphingosine and fatty acyl-coenzyme A; or S1P can be irreversibly broken down by S1P lyase (S1PL) to ethanolamine phosphate and hexadecenal. Of particular interest to this review is SK, which is crucial in converting sphingosine to S1P, and as such, controls the relative levels of the bioactive sphingolipids ceramide, sphingosine and S1P.
Figure 1. Sphingolipid metabolism.
De novo sphingolipid production occurs via the condensation of serine and palmitoyl-CoA by serine palmitoyltransferase. Ceramide can be formed from the degradation of complex sphingolipids, such as sphingomyelin or glycosphingolipids. Phosphorlyation of ceramide by ceramide kinase can form ceramide-1-phosphate or deaclyation of ceramide can occur via one of five CDases. De-acylation results in the formation of sphingosine, which can be recycled back to ceramide by CerS or rapidly phosphorylated to form S1P by SKs. S1P can be dephosphorylated by S1P phosphatases or terminally degraded by S1P lyase to form hexadecanal and phosphoethanolamine. CDase: Ceramidase; CerS: Ceramide snythase; CoA: Coenzyme A; DAG: Diacylglycerol; Glu/Gal-Cer synthase: glucosylceramide/glalactosylceramide synthase; PC: Phosphatidylcholine; S1P: Sphingosine-1-phosphate; SK: Sphingosine kinase; SMase: Sphingomyelinase.
Ceramide, sphingosine and S1P, have critical and seemingly opposing signaling roles. Ceramide, a central lipid in sphingolipid metabolism and the backbone of all sphingolipids, has been implicated in cell death, cellular stress responses, senescence and cell cycle arrest [11,12]. Sphingosine, the metabolite of ceramide breakdown, has been associated with growth arrest and cell death [13,14]. Sphingosine is generally found in low abundance due to rapid phosphorylation by SK to form S1P, a potent bioactive lipid with roles in cellular proliferation, migration, invasion and inflammation. As a bioactive lipid, S1P can act intracellularly on recently determined intracellular targets or extracellularly by binding to a family of G-protein coupled receptors; namely, S1PRs [15]. Upon binding to one of these five known cell surface receptors, S1P initiates signal transduction leading to angiogenesis [16], inflammation [17], regulation of immune cell trafficking [10,18] and other prosurvival pathways.
Sphingosine kinase
Two isoforms of SK have been cloned, SK1 and SK2. Both isoforms of SK have the ability to phosphorylate sphingosine (and dihydrosphingosine) to form S1P (dihydro-S1P); however, with different substrate specificity. SK1 and SK2 are highly homologous, with SK2 possessing an extended N-terminus [19–21]. SK2 is thought to localize to the endoplasmic reticulum and the nucleus [22] has been implicated in apoptosis [23] and shown to regulate gene expression by associating with histone deacetylases [24]. SK1 is highly regulated and localized primarily in the cytosol. Translocation of SK1 to the plasma membrane has been demonstrated to occur upon phosphorylation [13,25], binding to calmodulin or CIB1 [26,27], as well as by association with TRAF2 [28,29]. This translocation, as well as other attributes of SK1, have been implicated downstream of promitogenic stimuli such as phorbol 12-myristate 13-acetate [26,30] and proinflammatory stimuli such as TNF-α [31–33]. In addition to TNF-α, other proinflammatory mediators such as IL-1β [34,35], complement 5a (C5a) [36] and lipopolysaccharide [37], have been shown to regulate SK1, suggesting that SKs, and SK1 in particular, may play a critical role in inflammatory processes.
The two isoforms of SK have been shown to demonstrate some functional redundancy, as SK2 has also been shown to bind calmodulin in cells and in vitro [27]. In addition, both SK1 and SK2 single knockout mice display no obvious phenotype; however, double knockout mice (loss of SK1 and SK2) are embryonic lethal due to defects in developmental angiogenesis [38,39]. The development of SK1 and SK2 knockout mice that have allowed us to decipher many of the physiological roles of these enzymes in disease models. Under normal conditions SK1−/− and SK2−/− mice appear physiologically and morphologically normal [40,41], with SK1−/− mice displaying a 50% decrease in serum S1P levels [40], while SK2−/− mice demonstrate approximately a fourfold increase in whole blood and plasma S1P levels [42]. Some studies have reported normal acute and chronic inflammatory responses in these mice; however, the more recent studies in animal models of disease have begun to elucidate distinct roles for these two lipid kinases.
Sphingosine-1-phosphate
S1P can be generated by either isoform of SK, and the downstream effects attributed to S1P are beginning to be attributed to specific isoforms of SK in specific cellular compartments. An appreciation for the intracellular generation of S1P has only recently arisen due to the identification of two novel intracellular targets. Generation of S1P by SK2 has been shown to occur in the nucleus and to play a role in epigenetic regulation of gene expression. This was the first study to establish a specific role for SK2-generated S1P. In a study by Hait et al., S1P was shown to directly interact with and inhibit histone deacetylases 1 and 2 [24]. An additional intracellular target of S1P is attributed to SK1-generated S1P, where by S1P and has been shown to bind to TRAF2 and be required for its E3 ubiquitin ligase activity upon TNF-α stimulation [29].
Many of the characteristic effects of S1P such as proliferation, migration, angiogenesis, inflammation and lymphocyte trafficking have been attributed to S1P binding extracellularly to the S1PRs (Figure 2) [43]. S1PRs have a distinct profile of binding to different G-protein subunits, permitting S1P to exert different biological effects due to cell-type distribution of S1PRs [16,44]. While it has been shown that S1P generated either intracellularly and/or extracellularly can bind to S1PRs, the contribution of each is not fully understood or appreciated. S1P generated in the cell has been shown to be transported extracellularly by several possible transporters, including the ABCC1 transporter [45,46]. This transport has been suggested to be dependent on protein kinase C in rat uterine leiomyoma cells [45]. Upon transport to the extracellular space, S1P can act in an autocrine or paracrine manner by binding to S1PRs [46]; thereby, demonstrating extracellular actions for intracellularly generated S1P. SK1 has also been shown to be secreted from monocytic cells and vascular endothelial cells where it can generate S1P extracellularly [47,48].
Figure 2. Sphingosine kinase, sphingosine-1-phosphate and sphingosine-1-phosphate receptor signaling.
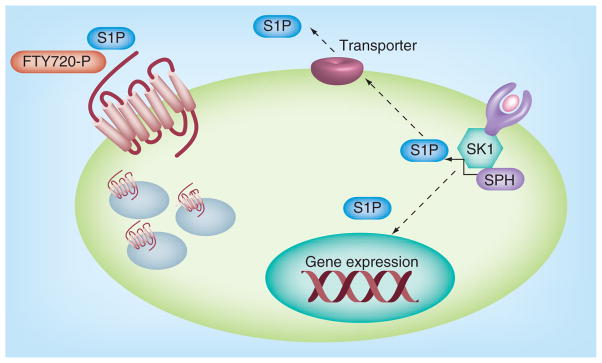
Agonists such as TNF-α or lipopolysaccharide bind to and activate their receptors leading to the activation of SKs. Activation of SK increases generation of S1P, which can act on intracellular targets or be exported out of the cell by transporters. Intracellular targets of S1P, as well as the extracellular S1P receptors have both been shown to exert numerous downstream functions, including gene expression. Similarly FTY720, phosphorylated primarily by SK2, can bind S1P receptors and induce receptor internalization and downregulation.
S1P: Sphingosine-1-phosphate; SK: Sphingosine kinase; SPH: Sphingosine.
S1P has been demonstrated to play a particularly important role in the transduction of inflammatory signaling. The first major suggestions that SKs, and SK1 in particular, could play an important role in inflammation came from the indication that SK1 could be activated downstream of proinflammatory cytokines [34,35,49,50]. Activation of SK1 by TNF-α has been shown to induce expression of VCAMs and ICAMs in endothelial cells [50]. TNF-α and IL-1β have also been shown to induce COX-2 and increase prostaglandin production in A549 lung cancer cells and L929 mouse fibroblasts in an SK1-dependent manner [34,35]. Since these first key studies into the potential role of SKs in inflammation, SK1 and S1P have also been shown to be essential for some aspects of NF-κB signaling in cells [29].
S1P and its receptors have also been implicated in immune cell trafficking as S1P levels in blood have been shown to influence lymphocyte trafficking [10]. This line of research has been augmented with the recent use of FTY720 and other S1PR agonists to modulate S1PR function and expression. FTY720 is phosphorylated primarily by SK2 [51]; although recent studies suggest that it can be phosphorylated by SK1 [52]. Upon phosphorylation, FTY720 binds to any of the five known S1PRs, with the exception of S1PR2. The actions of FTY720 have primarily been attributed to its binding to S1PR1 on lymphocytes, leading to activation and ubiquitin-mediated degradation of the receptor [53]. This degradation of S1PR1 leads to sequestration of lymphocytes in lymph nodes [40]. FTY720 has been extensively used in animal models for inflammation and autoimmune diseases and is currently used to maintain and slow the progression of multiple sclerosis under the trade name Gilenya™ (Novartis, Surrey, UK). Interestingly, FTY720 and some of its derivatives have also been shown to induce proteosomal degradation of SK1 [54,55]. Since the introduction of FTY720, several other more selective S1PR1 modulators have been introduced and utilized to influence lymphocyte trafficking, including KRP-203 [56,57], W-061 [58] and ponesimod [59].
SKs and S1P play critical roles in numerous cell signaling pathways including proliferation, migration and inflammation. The roles and regulation of SK/S1P in inflammatory processes and immune cell functions are beginning to be elucidated and better understood not only in cells, but also in vivo (Table 1).
Table 1.
Modulation of the sphingosine kinase 1/sphingosine-1-phosphate pathway in in vivo models of disease.
| Disease | Model | Modulation | Response | Ref. |
|---|---|---|---|---|
| Lupus | MRL/lpr | FTY720 S1PR agonist |
Improved survival; reduced autoantibodies to ds-DNA and IgG deposition | [62] |
| NZB/W F1 | FTY720 S1PR agonist |
Decreased histological lesions; no change in mesangial expansion or interstitial infiltrates | [63] | |
| MRL/lpr | KRP-203 S1PR agonist |
Increased survival; decreased proteinuria; decreased infiltrating T cells | [57] | |
| BXSB | FTY720 S1PR agonist |
Improved survival; reduced mesangial proliferation and inflammatory infiltrate; no change in IgG or C3 deposition | [64] | |
| MRL/lpr | ABC294640 SK2 inhibitor | No protection from disease | [61] | |
|
| ||||
| Arthritis | Collagen-induced arthritis | LX2931 and LX2932 S1PL inhibitor |
Decreased circulating lymphocyte counts and joint swelling | [73] |
| SKG mouse | FTY720 S1PR agonist |
Suppressed bone destruction; enhanced IL-4 production by CD4+ T cells | [76] | |
| hTNFtg | SK1−/− mice | Decreased paw swelling, deformity and inflammation, and COX-2 protein; fewer mature osteoclasts | [3] | |
| hTNFtg | SK2−/− mice and ABC294640 | No significant impact on severity or progression Significantly more severe arthritis | [70] | |
| Collagen-induced arthritis | DMS SK Inhibitor SK1 siRNA |
Decreased disease severity, articular inflammation and joint destruction Decreased incidence and disease activity |
[66] | |
| Collagen-induced arthritis | SK1 siRNA SK2 siRNA |
Decreased incidence, disease severity and articular inflammation Increased severity of disease | [69] | |
| Collagen-induced arthritis | TASP0277308 S1PR1 antagonist |
Suppressed the incidence of disease; decreased paw swelling | [77] | |
| MIA-induced arthritis | ABC294640 SK2 inhibitor | Reduced histology in knee joint and reduced pain | [71] | |
| Adjuvant-induced arthritis | Ponesimod S1PR1 agonist |
Prevented paw swelling and cytokine production | [59] | |
|
| ||||
| IBD | Oxazolone-induced colitis | FTY720 S1PR agonist |
Reduced Th2 cytokines, histologic damage, colon shortening and myeleoperoxidase activity | [82] |
| T cell transfer | FTY720 S1PR agonist |
Prevented development of colitis | [87] | |
| DSS and TNBS | ABC294640 SK2 inhibitor |
Decreased cytokine production and disease index | [4,81] | |
| IL-10−/− mice | KRP-203 S1PR agonist |
Reduced T and B cells in lamina propria and production of proinflammatory cytokines | [56] | |
| DSS | SK2−/− | Enhanced SK1 expression, S1P production and severity of colitis | [52] | |
| DSS | SK1−/− | Decreased histology, circulating white blood cells, COX-2 and granulocytic infiltration | [80] | |
| TNBS | FTY720 S1PR agonist |
No protection from weight loss, diarrhea or pathology | [83] | |
| DSS | S1PR4−/− | Decreased weight loss and IL-6 production | [85] | |
| DSS | W-061 S1PR1 agonist |
Decreased Th17-cell infiltrate into lamina propria; no change in Tregs | [58] | |
| DSS | S1PR1−/− | Increased colonic vascular permeability and bleeding | [88] | |
In vivo results from studies modulating the SK1/S1P pathway in lupus, arthritis and IBD are shown.
DMS: Dimethyl-sphingosine; DSS: Dextran sulfate sodium; IBD: Iinflammatory bowel disease; MIA: Monosodium iodoacetate; SK: Sphingosine kinase; S1P: Sphingosine-1-phosphate; S1PL: Sphingosine-1-phosphate lyase; S1PR: Sphingosine-1-phosphate receptor; TNBS: 2,4,6-trinitrobenzene sulfonic acid.
Lupus
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease. This disease is characterized by humoral autoimmunity followed by both cellular and innate immune responses. These immune responses lead to inflammation and fibrosis in numerous organs, including the joints, skin and kidneys. SK/S1P and the S1PRs have been implicated in the immune cell trafficking and function in SLE, as well as in the production of proinflammatory mediators in SLE. Patients with juvenile onset SLE demonstrate elevations in serum S1P [60]. Our recent study demonstrated that serum S1P and dihydro-S1P are increased in the MRL/lpr murine model for SLE [61]. This study also demonstrated that dihydro-S1P was elevated in kidney tissues from these mice. Interestingly, inhibition of SK2, which has a higher affinity for dihydro-S1P than SK1, failed to prevent disease in these mice. These studies suggest that SK1 may prove to be a beneficial therapeutic target.
Many of the recent studies have focused on the immune modulator FTY720. In the MRL/lpr mouse model for SLE the use of FTY720 has been shown to increase survival, decrease the occurrence of auto-antibodies to dsDNA, and IgG deposition in lupus nephritis [62]. In addition, in the NZB/W mouse model FTY720 decreased both proteinuria and histologic lesions in lupus nephritis, but failed to prevent mesangial expansion [63]. This study compared FTY720 to cyclophosphamide and suggested that FTY720 may be an acceptable alternative therapeutic in lupus nephritis. Interestingly, in the BXSB model FTY720 did not decrease the production of auto-antibodies, and IgG and C3 were detected in the glomeruli in FTY720-treated mice [64]. In this study, mice treated with FTY720 exhibited decreased proteinuria and increased survival with similar results as were demonstrated in the study using MRL/lpr mice. However, in BXSB mice treated with FTY720, mesangial cell proliferation and inflammatory cell infiltration was suppressed, contrary to what was observed in the NZB/W mouse model. The differences in responses to FTY720 are unlikely to be owing to dose or administration of the compound, as the studies all used similar dosing regimen suggesting that the differences are more likely owing to the murine model utilized for their studies. These studies strongly suggest that FTY720 may prove to be a potential therapeutic for SLE. This is partially confirmed by a study using a more selective agonist, KRP-203. Data with this agonist suggest that KRP-203 is a selective agonist of S1PR1. In the MRL/lpr mouse model, administration of KRP-203 increased survival by 50% and decreased both proteinuria and infiltration of CD4+ and CD8+ T cells in glomeruli and interstitium [57]. Taken together, these studies implicate S1PRs as a potential novel therapeutic target for reduction of lupus nephritis associated with SLE.
Arthritis
Arthritis is a disease involving inflammation and degradation of cartilage, subchondral bone disruption and pain in joint tissues. The current modalities for treatment include anti-inflammatories, such as NSAIDs and steroids, with more recent therapeutics being developed to target proinflammatory cytokines (anti-TNF-α), especially for rheumatoid arthritis (RA). Sphingolipids and sphingolipid metabolizing ezymes, specifically SK and S1P, have been implicated in the processes involved in both osteoarthritis (OA) and RA. Synovial fluid levels of S1P have been demonstrated to be elevated in patients with RA when compared with those from patients OA [65,66], and elevated levels of SK2 protein have been demonstrated in synovial fibroblasts from RA patients [67]. Similarly, S1PR1 expression has been shown to be increased in the vascular endothelial cells, synovial lining cells and inflammatory mono-nuclear cells of RA synovium when compared with OA synovium [68]. These studies suggest that S1P and the S1PR1 may play a clinically relevant role in RA and perhaps to a lesser extent OA.
A potential role for SKs and S1P in the development and progression of arthritis have been substantiated by the use of knockout mice and chemical inhibitors. Initially, the use of mice lacking either SK1 or SK2 demonstrated no differences in disease incidence or severity when compared with wild-type mice in a collagen-induced arthritis (CIA) model [38]. However, the use of siRNA in vivo, which is a controversial technique, for either SK1 or SK2 in the CIA model demonstrated differences between mice lacking either enzyme and control mice. In this study, arthritis incidence and severity were decreased in mice treated with SK1 siRNA [69]; however, mice treated with SK2 siRNA demonstrated increased disease severity and incidence. Additional studies investigating the role of SKs in arthritis have been conducted using SK1−/− and SK2−/− mice in the human transgenic TNF (hTNF) model for RA. In the study by Baker et al., hTNF/SK1−/− mice exhibited significantly less symptoms of RA than their hTNF/SK1+/+ mice as evidenced by less paw swelling, deformity and joint inflammation. This was attributed in part to decreased COX-2 and fewer mature osteoclasts in the ankle joints of hTNF/SK1−/− mice [3]. However, deletion of SK2 did not significantly impact severity or progression of disease in the hTNF murine model of arthritis [70]. These studies further support the suggestion that SK1 and SK2 have distinct biological roles.
Chemical inhibitors for SK, modulation of S1P levels and the S1PRs have also been demonstrated in murine models of arthritis. Inhibition of SKs has traditionally been achieved by the use of non-specific inhibitors, such as dimethyl-sphingosine. Dimethyl-sphingosine has been shown to reduce arthritis severity and cytokine production in CIA-induced arthritis [66]. A novel inhibitor of SK2 has recently been tested in two models of arthritis, namely the hTNF transgenic and injection of monosodium iodoacetate into the knee joint of rats. Contrary to the genetic deletion of SK2, where no differences were detected in the hTNF model, inhibition of SK2 with ABC294640 led to increased severity of disease when compared with vehicle-treated hTNF mice [70]. Interestingly, use of this specific SK2 inhibitor in the monosodium iodoacetate-induced arthritis model resulted in less severe knee joint histology scores [71]. It is of note that this inhibitor has also recently been shown to bind and inhibit the estrogen receptor, indicating that some effects of this inhibitor may be owing to off-target effects [72].
Modulation of S1P levels and S1PRs has also been examined in murine models of arthritis. A novel indirect inhibitor of S1PL, which would increase S1P levels, yielded a dose-dependent decrease in circulating lymphocyte counts and showed a therapeutic effect in rodent models of RA [73]. Use of this inhibitor, the first clinically studied inhibitor of S1PL, in Phase I clinical trials reduced circulating lymphocytes in patients in a dose-dependent and reversible fashion and was well tolerated at dose levels of up to 180 mg daily. The reduction of circulating lymphocytes demonstrated with this S1PL inhibitor is similar to that seen with FTY720. Indeed, there have been several studies using FTY720 or other modulators of S1PRs in animal models of arthritis. FTY720 has been shown to decrease edema, joint destruction and lymphocyte infiltration CIA-induced arthritis and adjuvant-induced arthritis in rats [74,75]. FTY720 also decreased cytokine production and inflammatory infiltrate in the SKG genetic model for arthritis [76]. Recently, more specific S1PR agonists/antagonists have been developed and tested in some of these animal models of arthritis. Specifically, S1PR1-selective antagonists TASP0277308, suppressed arthritis development in CIA-induced arthritis [77]. In addition, an orally bioavailable S1PR1 agonist ponesimod prevented edema and increases in paw volume and joint inflammation in the arthritis and adjuvant model of arthritis in rats [59]. Recent clinical trials in Europe have also indicated that ponesimod can produce transient bradycardia, but is well tolerated [78].
These studies suggest that modulation of S1P production via SK1, and potentially via SK2, may prove to be a valuable potential therapeutic. The development and use of novel SK1 specific inhibitors will be useful in determining the specific roles for each SK isoform in arthritis development and progression. Similarly, these studies suggest that modulation of specific S1PRs may also prove to be fruitful potential therapeutic targets in arthritis.
Inflammatory bowel disease
Ulcerative colitis and Crohn’s disease are two distinct forms of inflammatory bowel disease (IBD). Both forms of IBD involve production of proinflammatory cytokines, specifically TNF-α, and infiltration of immune cells into the intestine. The entire GI tract can be affected by Crohn’s disease, whereas inflammation owing to ulcerative colitis is restricted to the colon. Traditional modalities of treatment for IBD include immunosuppressants, such as corticosteroids and modified chemotherapeutics. Recently, as seen with RA, therapeutics targeting the overproduction of proinflammatory cytokines, such as anti-TNF-α therapies, have begun gaining ground in the treatment of IBD. As previously mentioned, TNF-α has been shown to stimulate the activity of SK1 in several types of cells, including intestinal epithelial cells [79].
Genetic deletion or inhibition of SK has also been implicated in IBD. The author’s laboratory demonstrated that genetic deletion of SK1 partially protected against acute dextran sulfate sodium (DSS)-induced colitis, specifically reducing granulocytic infiltration into colon tissue and preventing the induction of COX-2 in colon tissues [80]. Genetic deletion of SK2 seems to highlight opposing functions for the SK isoforms in IBD. In a model of DSS-induced colitis, SK2−/− demonstrated increased disease and mice lacking SK2 failed to recover following the removal of DSS [52]. This increased severity of disease was attributed to increases in SK1 and S1P; however, in the studies with SK1−/− mice, no compensation of SK2 was detected. Interestingly, as seen with RA chemical inhibition of SK2 resulted in opposing outcomes. ABC294640, an inhibitor of SK2, has been shown to improve disease and cytokine production in DSS-induced colitis, in both acute and chronic models [4]; with similar results in 2,4,6-trinitrobenzene sulfonic acid-induced disease in rats [81]. These studies suggest there may be some interplay between SK1 and SK2 in the modulation of IBD. In addition, there may be obvious differences observed between genetic deletion and chemical inhibition of SK2 in IBD, similar to what has been shown with modulation and deletion of S1PR1. A better understanding of the function and compensation between SKs would also significantly benefit from the development of a potent and specific SK1 inhibitor for use in these models.
Use of FTY720 and other S1PR modulators has been suggested for IBD similar to what has been discussed above with lupus and arthritis. FYT720 has been reported to protect against disease in several different mouse models of IBD. In a mouse model using oxazolone-induced colitis, mice administered FTY720 demonstrated decreased macroscopic and microscopic disease and, most notably, a significant decrease in Th2 cytokine production [82]. Similarly in a T-cell transfer model of colitis, FTY720 protected against colitis in mice lacking lymphoid tissues [4]. However, in the 2,4,6-trinitrobenzene sulfonic acid model for IBD, which is commonly used to mimic Crohn’s disease, FTY720 did not protect against weight loss associated with 2,4,6-trinitrobenzene sulfonic acid administration [83]. Furthermore, administration of FTY720 could produce deleterious effects owing to its immunosuppressant functions, as demonstrated in the study of gastrointestinal infection with Citrobacter rodentium [84]. This study demonstrated that mice administered FTY720 were unable to clear C. rodentium, which could have implications for human Escherichia coli infection.
The development and use of FTY720 in numerous models of autoimmunity and inflammation has also spawned the generation of several other S1PR modulators and examination of the role of S1PRs in IBD. Use of KRP-203 in the IL-10-deficient mouse model for IBD demonstrated decreases in infiltrating lymphocytes in the lamina propria in colon tissues [56]. This S1PR1 agonist also prevented the production of TNF-α, IFN-γ and IL-12 in the IL-10−/− mice. Another S1PR1 agonist, W-061, decreased CD4+ T cells, as well as both Th1 and Th17 cells, in the colon lamina propria of mice with DSS-induced colitis, but had no effect on Tregs [58]. Genetic deletion of S1PR1 or S1PR4 have been demonstrated in DSS-induced colitis with opposing results. Mice lacking S1PR4 exhibited protection from DSS-induced colitis, as evidenced by decreased weight loss and serum IL-6 levels [85]. Interestingly, loss of S1PR1 decreased vascular integrity and enhanced bleeding in DSS-induced colitis. These studies suggest that modulation of S1PRs may prove beneficial in IBD, but that particular attention will need to focus on the specific receptors involved.
Conclusion
The interplay of sphingolipid metabolizing enzymes and their bioactive sphingolipid products, such as SKs and S1P, as well as the S1PRs, have been demonstrated to play essential roles in initiating/transmitting inflammatory signaling pathways, recruiting specific immune cells to tissue sites of injury, and in the progression of numerous inflammatory and autoimmune diseases. We are beginning to better understand how to manipulate and modulate the SK/S1P pathway to reduce disease progression in these inflammatory diseases. In the future, improvement upon our current understanding may lead to more specific modulators of the SK/S1P pathway for potential therapeutics.
Future perspective
Much progress has been made in the last decade on the role of bioactive sphingolipids and their roles in numerous disease states, including inflammatory and autoimmune diseases. Much of the recent progress for the roles of bioactive sphingolipids has grown out of the studies using FTY720 to modulate S1PR function. The next step is to improve upon this generation of S1PR modulators and develop modulators for specific receptors, which is currently underway. Development of specific S1PR modulators will potentially prevent some of the off target effects seen with FTY720. Another initiating factor for much of this progress stems from the generation of knockout mice. The field is currently improving upon the traditional knockout mouse models with the generation of conditional knockouts. Elegant studies in these condition knockouts are determining the roles of sphingolipid enzymes in specific cell populations and how these enzymes and their lipid products modulate disease states. However, sphingolipid research in inflammation and disease would greatly benefit from specific inhibitors for each of the SK enzymes (as well as other sphingolipid enzymes). This may be more feasible with the recent publication revealing the structure of SK1 [86]. As a whole, the next decade should see development of more specific modulators of sphingolipid enzymes and their lipid products with great potential for clinical development.
Executive summary.
Sphingolipid metabolism
Sphingolipids and their metabolizing enzymes form a complex network of proteins and lipid signaling molecules.
Ceramide, sphingosine and sphingosine-1-phosphate (S1P) are three potent bioactive lipids with distinct bioactive functions.
Sphingosine kinase
There are two isoforms of sphingosine kinase (SK), SK1 and SK2, of which SK1 seems to be more tightly regulated.
SK1 can be activated by TNF-α and IL-1β and is required for the downstream induction COX-2 both in cells and in vivo.
Sphingosine-1-phosphate
S1P can be generated by either SK1 or SK2 to act on intracellular targets or to be transported from the cell to act on cell surface S1P receptors (S1PRs).
S1P and S1PRs have been extensively studied for their roles in immune cell function and trafficking.
Lupus
Patients with juvenile onset systemic lupus erythematosus demonstrate elevations in serum S1P.
S1PR modulators including FTY720 and KRP-203 and increase survival in animal models of systemic lupus erythematosus.
Inhibition of SK2 in a murine model of systemic lupus erythematosus does not protect from disease.
Arthritis
S1P levels are higher in synovial fluid from patients with rheumatoid arthritis than those from patients osteoarthritis.
Genetic deletion of SK1, but not SK2, partially protects against disease in a murine model of rheumatoid arthritis.
Modulation of S1PRs with FTY720, TASP0277308 and ponesimod are effective at reducing arthritis in animal models.
Inflammatory bowel disease
Genetic deletion of SK1, but not SK2, partially protects against dextran sulfate sodium-induced colitis.
Inhibition of SK2 partially protects against colitis in two animal models.
FTY720, W-061 and KRP-203 have been shown to protect in animal models of disease through modulation of S1PRs and lymphocytes.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Disclaimer
The content of this material does not represent the views of the US Department of Veterans Affairs or the US Government.
Financial & competing interests disclosure
This work was supported by a VA Career Development Award. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1■■.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. Seminal review of sphingolipids, their generating enzymes and their functions. [DOI] [PubMed] [Google Scholar]
- 2.Sun WY, Pitson SM, Bonder CS. Tumor necrosis factor-induced neutrophil adhesion occurs via sphingosine kinase-1-dependent activation of endothelial {alpha}5{beta}1 integrin. Am J Pathol. 2010;177(1):436–446. doi: 10.2353/ajpath.2010.091016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J Immunol. 2010;185(4):2570–2579. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maines LW, Fitzpatrick LR, French KJ, et al. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53(4):997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishimaru N, Yamada A, Nitta T, et al. CCR7 with S1P1 signaling through AP-1 for migration of Foxp3+ regulatory T-cells controls autoimmune exocrinopathy. Am J Pathol. 2012;180(1):199–208. doi: 10.1016/j.ajpath.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Bode C, Graler MH. Immune regulation by sphingosine 1-phosphate and its receptors. Arch Immunol Ther Exp (Warsz) 2012;60(1):3–12. doi: 10.1007/s00005-011-0159-5. [DOI] [PubMed] [Google Scholar]
- 7.Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci. 2011;32(1):16–24. doi: 10.1016/j.tips.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigert A, Weis N, Brune B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology. 2009;214(9–10):748–760. doi: 10.1016/j.imbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158(5):1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 11.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270(51):30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 12.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 13.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J Biol Chem. 2002;277(38):35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 14.Seufferlein T, Rozengurt E. Sphingosine induces p125FAK and paxillin tyrosine phosphorylation, actin stress fiber formation, and focal contact assembly in Swiss 3T3 cells. J Biol Chem. 1994;269(44):27610–27617. [PubMed] [Google Scholar]
- 15.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582(1–3):72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 16.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682(1–3):48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Jolly PS, Bektas M, Olivera A, et al. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199(7):959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham TH, Baluk P, Xu Y, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207(1):17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19■.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374(5–6):413–428. doi: 10.1007/s00210-007-0132-3. One of the first reports of an intracellular target of sphingosine-1-phosphate in the nucleus. [DOI] [PubMed] [Google Scholar]
- 20.Okada T, Ding G, Sonoda H, et al. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280(43):36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Sugiura M, Nava VE, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 22.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278(47):46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Toman RE, Goparaju SK, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278(41):40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 24■.Hait NC, Allegood J, Maceyka M, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–1257. doi: 10.1126/science.1176709. One of the first reports of an intracellular target of sphingosine-1-phosphate in the nucleus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitson SM, Xia P, Leclercq TM, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201(1):49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarman KE, Moretti PA, Zebol JR, Pitson SM. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium and integrin binding protein 1. J Biol Chem. 2009;285(1):483–492. doi: 10.1074/jbc.M109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland CM, Moretti PA, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM. Thecalmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J Biol Chem. 2006;281(17):11693–11701. doi: 10.1074/jbc.M601042200. [DOI] [PubMed] [Google Scholar]
- 28.Xia P, Wang L, Moretti PA, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277(10):7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez SE, Harikumar KB, Hait NC, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitson SM, Moretti PA, Zebol JR, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22(20):5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahelin RV, Hwang JH, Kim JH, et al. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem. 2005;280(52):43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- 32.Sethu S, Mendez-Corao G, Melendez AJ. Phospholipase D1 plays a key role in TNF-alpha signaling. J Immunol. 2008;180(9):6027–6034. doi: 10.4049/jimmunol.180.9.6027. [DOI] [PubMed] [Google Scholar]
- 33.Snider AJ, Orr Gandy KA, Obeid LM. Sphingosine kinase: role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie. 2010;92(6):707–715. doi: 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17(10):1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35■.Pettus BJ, Bielawski J, Porcelli AM, et al. Thesphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17(11):1411–1421. doi: 10.1096/fj.02-1038com. Sphingosine kinase (SK)1 regulation by proinflammatory cytokines and downstream consequences. [DOI] [PubMed] [Google Scholar]
- 36.Melendez AJ, Ibrahim FB. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis. J Immunol. 2004;173(3):1596–1603. doi: 10.4049/jimmunol.173.3.1596. [DOI] [PubMed] [Google Scholar]
- 37.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2007;85(3–4):107–114. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580(19):4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 39■.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25(24):11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. Critical understanding of SK and sphingosine-1-phosphate in embryogenesis and development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40■■.Allende ML, Sasaki T, Kawai H, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279(50):52487–52492. doi: 10.1074/jbc.M406512200. Generation of SK1-knockout mice, a role for SK2 in FTY720-mediated lymphopenia. [DOI] [PubMed] [Google Scholar]
- 41.Zemann B, Urtz N, Reuschel R, et al. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol Lett. 2007;109(1):56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Kharel Y, Raje M, Gao M, et al. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem J. 2012;447(1):149–157. doi: 10.1042/BJ20120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4(5):397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 44.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15(5):513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Tanfin Z, Serrano-Sanchez M, Leiber D. ATP-binding cassette ABCC1 is involved in the release of sphingosine 1-phosphate from rat uterine leiomyoma ELT3 cells and late pregnant rat myometrium. Cell Signal. 2011;23(12):1997–2004. doi: 10.1016/j.cellsig.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103(44):16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammad SM, Taha TA, Nareika A, Johnson KR, Lopes-Virella MF, Obeid LM. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostaglandins Other Lipid Mediat. 2006;79(1–2):126–140. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Venkataraman K, Thangada S, Michaud J, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397(3):461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274(48):34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]
- 50.Xia P, Gamble JR, Rye KA, et al. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95(24):14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kharel Y, Lee S, Snyder AH, et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280(44):36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 52.Liang J, Nagahashi M, Kim EY, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23(1):107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oo ML, Thangada S, Wu MT, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282(12):9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 54.Lim KG, Tonelli F, Li Z, et al. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J Biol Chem. 2011;286(21):18633–18640. doi: 10.1074/jbc.M111.220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tonelli F, Lim KG, Loveridge C, et al. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22(10):1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song J, Matsuda C, Kai Y, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324(1):276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 57.Wenderfer SE, Stepkowski SM, Braun MC. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 2008;74(10):1319–1326. doi: 10.1038/ki.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanada Y, Mizushima T, Kai Y, et al. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS ONE. 2011;6(9):e23933. doi: 10.1371/journal.pone.0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piali L, Froidevaux S, Hess P, et al. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther. 2011;337(2):547–556. doi: 10.1124/jpet.110.176487. [DOI] [PubMed] [Google Scholar]
- 60.Watson L, Tullus K, Marks SD, Holt RC, Pilkington C, Beresford MW. Increased serum concentration of sphingosine-1-phosphate in juvenile-onset systemic lupus erythematosus. J Clin Immunol. 2012;32(5):1019–1025. doi: 10.1007/s10875-012-9710-3. [DOI] [PubMed] [Google Scholar]
- 61.Snider AJ, Ruiz P, Obeid LM, Oates JC. Inhibition of sphingosine kinase-2 in a murine model of lupus nephritis. PLoS ONE. 2013;8(1):e53521. doi: 10.1371/journal.pone.0053521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okazaki H, Hirata D, Kamimura T, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J Rheumatol. 2002;29(4):707–716. [PubMed] [Google Scholar]
- 63.Alperovich G, Rama I, Lloberas N, et al. New immunosuppresor strategies in the treatment of murine lupus nephritis. Lupus. 2007;16(1):18–24. doi: 10.1177/0961203306073136. [DOI] [PubMed] [Google Scholar]
- 64.Ando S, Amano H, Amano E, et al. FTY720 exerts a survival advantage through the prevention of end-stage glomerular inflammation in lupus-prone BXSB mice. Biochem Biophys Res Commun. 2010;394(3):804–810. doi: 10.1016/j.bbrc.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 65.Limaye V, Xia P, Hahn C, et al. Chronic increases in sphingosine kinase-1 activity induce a pro-inflammatory, pro-angiogenic phenotype in endothelial cells. Cell Mol Biol Lett. 2009;14(3):424–441. doi: 10.2478/s11658-009-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai WQ, Irwan AW, Goh HH, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181(11):8010–8017. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 67.Kamada K, Arita N, Tsubaki T, et al. Expression of sphingosine kinase 2 in synovial fibroblasts of rheumatoid arthritis contributing to apoptosis by a sphingosine analogue, FTY720. Pathol Int. 2009;59(6):382–389. doi: 10.1111/j.1440-1827.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 68.Kitano M, Hla T, Sekiguchi M, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54(3):742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 69.Lai WQ, Irwan AW, Goh HH, Melendez AJ, Mcinnes IB, Leung BP. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J Immunol. 2009;183(3):2097–2103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]
- 70.Baker DA, Eudaly J, Smith CD, Obeid LM, Gilkeson GS. Impact of sphingosine kinase 2 deficiency on the development of TNF-alpha-induced inflammatory arthritis. Rheumatol Int. 2012 doi: 10.1007/s00296-012-2493-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fitzpatrick LR, Green C, Maines LW, Smith CD. Experimental osteoarthritis in rats is attenuated by ABC294640, a selective inhibitor of sphingosine kinase-2. Pharmacology. 2011;87(3–4):135–143. doi: 10.1159/000323911. [DOI] [PubMed] [Google Scholar]
- 72.Antoon JW, White MD, Meacham WD, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151(11):5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bagdanoff JT, Donoviel MS, Nouraldeen A, et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl) ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53(24):8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 74.Matsuura M, Imayoshi T, Okumoto T. Effect of FTY720, a novel immunosuppressant, on adjuvant- and collagen-induced arthritis in rats. Int J Immunopharmacol. 2000;22(4):323–331. doi: 10.1016/s0192-0561(99)00088-0. [DOI] [PubMed] [Google Scholar]
- 75.Wang F, Tan W, Guo D, He S. Reduction of CD4 positive T cells and improvement of pathological changes of collagen-induced arthritis by FTY720. Eur J Pharmacol. 2007;573(1–3):230–240. doi: 10.1016/j.ejphar.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 76.Tsunemi S, Iwasaki T, Kitano S, Imado T, Miyazawa K, Sano H. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin Immunol. 2010;136(2):197–204. doi: 10.1016/j.clim.2010.03.428. [DOI] [PubMed] [Google Scholar]
- 77.Fujii Y, Hirayama T, Ohtake H, et al. Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J Immunol. 2012;188(1):206–215. doi: 10.4049/jimmunol.1101537. [DOI] [PubMed] [Google Scholar]
- 78.Brossard P, Derendorf H, Xu J, Maatouk H, Halabi A, Dingemanse J. Pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P receptor modulator, in the First-in-Human Study. Br J Clin Pharmacol. 2013 doi: 10.1111/bcp.12129. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawamori T, Osta W, Johnson KR, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20(2):386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 80.Snider AJ, Kawamori T, Bradshaw SG, et al. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 2009;23(1):143–152. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maines LW, Fitzpatrick LR, Green CL, Zhuang Y, Smith CD. Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn’s disease. Inflammopharmacology. 2013;18(2):73–85. doi: 10.1007/s10787-010-0032-x. [DOI] [PubMed] [Google Scholar]
- 82.Daniel C, Sartory NA, Zahn N, et al. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol Immunol. 2007;44(13):3305–3316. doi: 10.1016/j.molimm.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 83.Radi ZA, Heuvelman DM, Masferrer JL, Benson EL. Pharmacologic evaluation of sulfasalazine, FTY720, and anti-IL-12/23p40 in a TNBS-induced Crohn’s disease model. Dig Dis Sci. 2011;56(8):2283–2291. doi: 10.1007/s10620-011-1628-8. [DOI] [PubMed] [Google Scholar]
- 84.Murphy CT, Hall LJ, Hurley G, et al. The sphingosine-1-phosphate analogue FTY720 impairs mucosal immunity and clearance of the enteric pathogen Citrobacter rodentium. Infect Immun. 2012;80(8):2712–2723. doi: 10.1128/IAI.06319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schulze T, Golfıer S, Tabeling C, et al. Sphingosine-1-phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25(11):4024–4036. doi: 10.1096/fj.10-179028. [DOI] [PubMed] [Google Scholar]
- 86■■.Wang Z, Min X, Xiao SH, et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure. 2013;21(5):798–809. doi: 10.1016/j.str.2013.02.025. First to describe the crystal structure of SK1. [DOI] [PubMed] [Google Scholar]
- 87.Fujii T, Tomita T, Kanai T, et al. FTY720 suppresses the development of colitis in lymphoid-null mice by modulating the trafficking of colitogenic CD4+ T cells in bone marrow. Eur J Immunol. 2008;38(12):3290–3303. doi: 10.1002/eji.200838359. [DOI] [PubMed] [Google Scholar]
- 88.Montrose DC, Scherl EJ, Bosworth BP, et al. S1P(1) localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J Lipid Res. 2013;54(3):843–851. doi: 10.1194/jlr.M034108. [DOI] [PMC free article] [PubMed] [Google Scholar]