Abstract
Chronic abuse of drugs can result in vast negative repercussions on behavioral and biological systems by altering underlying neurocircuitry. Long-term cannabinoid administration in rats leads to detrimental cellular and dendritic morphology changes. Previous studies have found that chronic treatment with delta-9-THC selectively decreases dendritic morphology and spine density in the dentate gyrus of young rats (Rubino et al., 2009), however, whether these changes are specific to a particular developmental age is not known. The present study evaluated the effects of chronic exposure (7 or 21 days) to WIN 55, 212-2 (i.p., 3.7 mg/kg), a potent cannabinoid agonist, on dendritic morphology of dentate gyrus neurons in adult rats. Upon completion of treatment brains were processed for Golgi-Cox staining. No significant effects of WIN 55, 212-2 exposure were observed for dendritic branching or length. Spine density was quantified in the inner (proximal), middle, and outer (distal) thirds of the dendritic fields selected to approximate the spatial loci of afferents comprising the associational-commissural pathway, medial perforant path, and lateral perforant path, respectively. Compared to vehicle controls there was a significant reduction in spine density (~1 spine/10μm) in the inner and middle dendritic segments. The spine density reduction was significant in inner segments following 7 days of treatment. These results suggest that chronic cannabinoid treatment specifically alters spine density in the dendritic targets of the associational-commissural afferents and medial perforant path projections, but not lateral perforant path. The resulting loss of dendritic spine density may be an important factor underlying cannabinoid induced memory impairments.
Keywords: Cannabinoid, Dendritic spines, Hippocampus, Dentate Gyrus, Synaptic Plasticity, Golgi-Cox
1. Introduction
Long-term exposure to exogenous cannabinoids can result in persistent changes in dendritic morphology and spine density (Kolb et al., 2006; Rubino et al., 2009). Changes to dendritic morphology represent potential mechanisms by which cannabinoid exposure may influence behavioral and cognitive processes. Previous studies have demonstrated that chronic treatment with delta-9-tetrahydrocannabinol (THC) after 10–12 days selectively alters dendritic morphology of neurons depending on developmental age and region of interest. In adolescent rats THC administration altered dendritic morphology of dentate gyrus granule cells (Rubino et al., 2009), while in adult rats THC administration increased dendritic morphology of medium spiny neurons of the nucleus accumbens shell and pyramidal neurons of the medial prefrontal cortex with no changes to the CA1 field of the hippocampus, striatum, orbital frontal cortex, parietal cortex, or occipital cortex (Kolb et al., 2006). Accompanying the changes in granule cell dendritic morphology (i.e., lowered dendritic length, branching, spine density) produced by chronic exposure to cannabinoids in adolescence, Rubino et al. 2009 found deficits in spatial working memory in a radial arm maze, decreased protein expression (GFAP, VAMP2, PSD95) and NMDA receptor levels across the hippocampus. When taken together, previous studies imply chronic cannabinoid abuse in adolescence results in decreased synaptic plasticity and long-term cognitive deficits in adulthood. The impact of cannabinoids with use beginning in adulthood remains an open area of research, as little has been reported in this area. Whether the pattern of hippocampal alterations seen by Rubino et al., 2009 is observed in adult animals that begin cannabinoid use in adulthood has yet to be determined. Also, given that previous differences in dendritic morphology were found with THC, it is pertinent to see if the effects generalize to other cannabinoid agonists with different receptor binding affinities.
Cannabinoids cause memory deficits in a wide assortment of behavioral paradigms (Riedel and Davies, 2005). The most commonly reported outcomes of cannabinoid use or exposure are robust short-term memory deficits (Robinson and Riedel, 2004) mediated by CB1 receptors in the CA1 region of the hippocampus (Wise et al., 2009). Cannabinoids also disrupt long-term spatial memory storage by interfering with memory consolidation processes mediated by CB1 receptors in the dorsal hippocampus (Yim et al., 2008). It is likely CB1 receptors in other regions of the hippocampal formation modulate other forms of memory and consolidation processes.
The dentate gyrus acts as a gateway into the hippocampal formation, specifically with regards to medial and lateral perforant path projections from the entorhinal cortex representing the major neocortical afferents to the hippocampus; and therefore, may be a key location cannabinoids exert influence to disrupt memory. Mossy cells of the dentate hilus contain the highest levels of CB1 receptors amongst excitatory hippocampal neurons (Kawamura et al., 2006; Monory et al., 2006). There is also a dense representation in the inner third of the molecular layer of the dentate gyrus (Katona et al., 2006), which corresponds spatially to the associational-commissural projection, where mossy cells form synapses onto dendrites of the granule cells (Johnston and Amaral, 2004). Afferents to dentate gyrus granule cells also include the medial perforant path and lateral perforant path projections from the entorhinal cortex, which form synapses in the middle third and outer third (distal) of the granule cell dendritic fields. The medial and lateral entorhinal inputs have been shown by Hargreaves et. al. (2005) to relay different types of information, with spatial information conveyed by the medial perforant path and non-spatial information conveyed by the lateral perforant path. It is important to further understand the role cannabinoids have on the dentate gyrus because this region contains the highest amount of CB1 receptors, has well-defined synaptic circuitry and synaptic plasticity, and is critically involved in spatial memory consolidation.
The present study was designed to examine changes in dendritic morphology produced by chronic treatment with a potent cannabinoid agonist WIN 55, 212-2. The drug treatment began once the animals were mature adults (6 months of age), which distinguishes this study from previous reports. Adult rats were given daily i.p. injections of WIN 55, 212-2 or vehicle for 7 or 21 days after which the brains were processed for Golgi-Cox staining. Dendritic branching and length were quantified in granule cells sampled from the medial portion of the upper blade of the dentate gyrus, Zilles’ area DG (Zilles, 1985) (Fig. 1). By focusing on the spatial distribution of dentate gyrus afferents, it is also possible to infer how cannabinoids impact various hippocampal projections and their targets. Toward this goal, spine density was quantified in segments sampled from the inner, middle and outer thirds of the dendritic fields, relative to the soma, to estimate changes in the targets of the associational-commissural projection, medial perforant path projection, and lateral perforant path projection, respectively (Fig. 2).
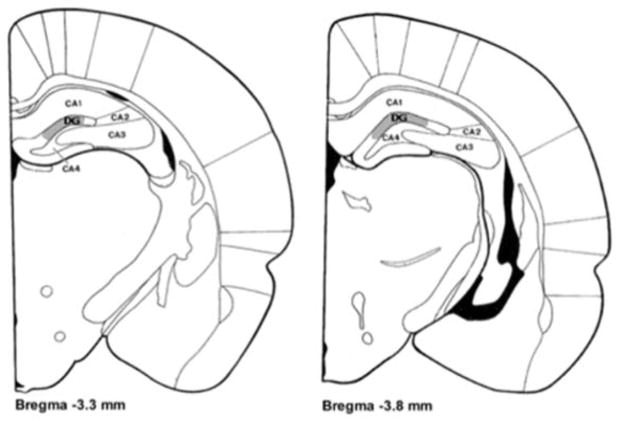
FIGURE 1.
Coronal sections [adapted from Zilles (1985)] showing the region from which dentate granule cells were drawn. Sampling occurred in the upper blade of the Dentate Gyrus (DG), highlighted in gray, in sections ranging from 3.3 mm to 3.8 mm posterior to Bregma.
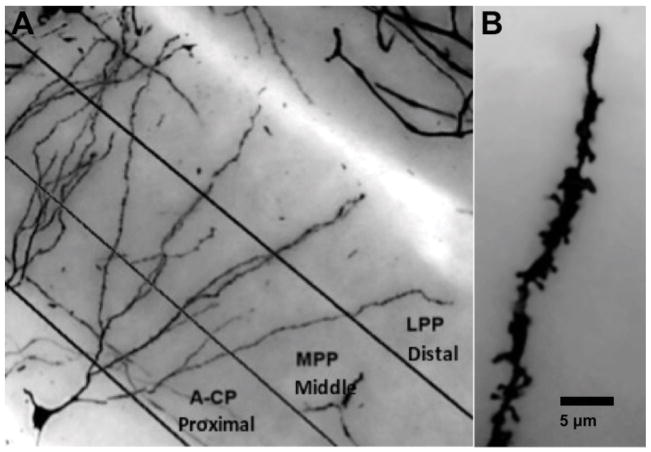
FIGURE 2.
(A) For spine analysis the dentate granule cell dendritic field was divided into 3 layers (proximal, middle, and distal) to correspond to major afferent pathways: A-CP (Associational-Commissural Pathway), MPP (Medial Perforant Path), and LPP (Lateral Perforant Path). (B) Example illustrating the staining quality of granule cell spines (1200X final magnification).
2. Results
Analyses of Variance (ANOVAs) were performed using SPSS (version 18 for Macintosh). All test statistics reported here were significant at p < .05 unless otherwise noted. For all analyses Drug condition (Vehicle, WIN 55, 212-2) and Exposure length (7-day, 21-day) were between-subjects factors. Within-subjects factors included in separate ANOVAs for branch order, length, and spine density were distance from soma (Sholl analysis), branch order, or segment (spine density only). Effect sizes (partial eta squared; η) are reported for all effects. One Vehicle animal was identified as an outlier with respect to spine density quantification and excluded from analysis because its spine density values were greater than 3 standard deviations below the group mean, consistent with suboptimal staining. There were a total of 23 brains included in analyses of dendritic length, branching, and spine density (11 Vehicle and 12 WIN 55, 212-2).
2.1. Dendritic length and branching
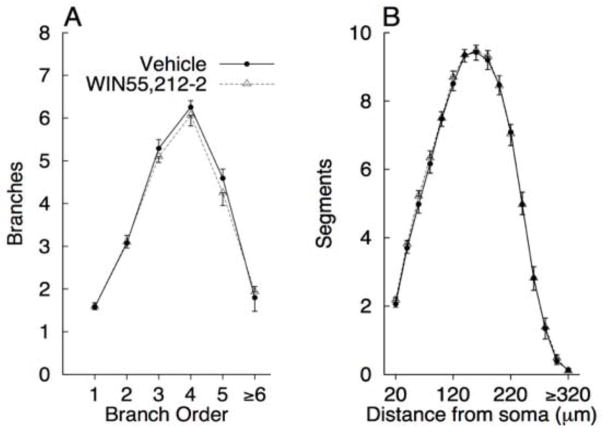
Mean dendritic branching and length are presented in Figure 3A and B. Because there were no significant effects involving duration of exposure, the data were collapsed over this factor to simplify presentation of the results. There were no significant main effects or interactions for branch order [all ps > 0.355] or total branches [MVEH = 22.317, MWIN = 22.042, all ps > 0.439]. There were also no significant main effects or interactions with distance from soma for dendritic length [all ps > 0.226], or for total dendritic length [MVEH = 32.891, MWIN = 33.725, all ps > 0.275].
FIGURE 3.
Mean (+SEM) of total branches for first through sixth (and greater) branch orders (A) and dendritic length as a function of distance from the soma (B) for Vehicle and WIN 55, 212-2 exposed rats.
2.2. Spine density
Mean spine density for the 3 segments of interest: inner, middle, and outer thirds (association-commissural path, medial perforant path, and lateral perforant path, respectively), in the Dentate Gyrus are presented in Figure 4. Because there were no interactions involving duration of exposure and drug treatment the data were collapsed over levels of exposure duration to simplify presentation of the results. The Segment X Drug interaction approached significance [F(2, 38)=3.126, p= 0.055, η = 0.141]. Inspection of the means suggests that WIN 55,212-2 exposure reduced spine density on the order of 1 spine per 10 microns in the inner and middle segments. Comparisons of the drug groups for each segment revealed significant differences for the inner segment [F(1, 21) = 5.511, p = 0.029, η = 0.208] and middle segment [F(1, 21) = 4.661, p = 0.043, η = 0.182], but not the outer segment [p = 0.557].
FIGURE 4.
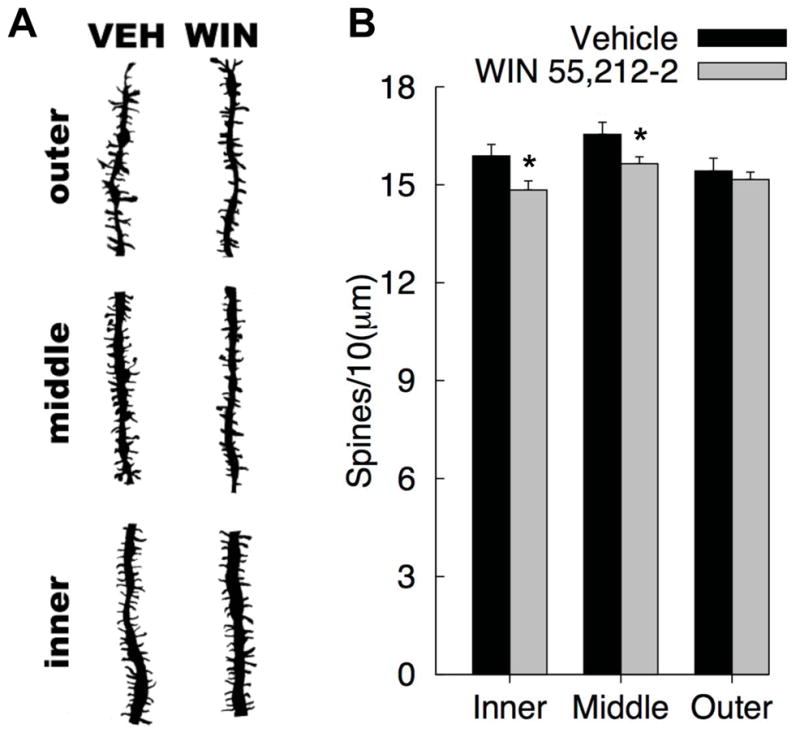
Dentate granule cell dendritic field spine density in Vehicle and WIN 55, 212-2 exposed rats: (A) Representative camera lucida drawings of the cell layers from each group (1200X final magnification), (B) Mean (+SEM) spine density from the inner, middle, and outer segments, corresponding to the Associational-Commissural Pathway, Medial Perforant Path, and Lateral Perforant Path (respectively) [*p<0.05].
In addition, there was a main effect of Segment [F(2, 38) = 17.106, η = 0.474], which was due to increased spine density in the middle segment compared to each of the other two segments [all ps < 0.0004]. There was also a significant interaction between Segment X Exposure duration [F(2, 38)=9.775, η = 0.340]. Rats that received injections (vehicle or drug administration) for 7 days had greater spine density in the inner segment than rats that received injections for 21 days [M7d = 15.876, M21d = 14.750, F(1, 21) = 6.704, η = 0.242]. No other main effects or interactions were significant.
3. Discussion
Long-term cannabinoid administration was associated with a robust reduction in dendritic spine density within granule cells of the dentate gyrus. More specifically, the spine density loss (1 spine per 10 μm) was limited to the inner and middle dendritic segments of the granule cell, which correspond spatially to targets of the associational-commissural afferent and medial perforant path afferent, respectively. These observations do not reflect a generalized reduction in overall dendritic morphology, as no differences were found between drug and vehicle groups in measures of dendritic length and branching, but instead indicate select spine sensitivity in spatially relevant afferents from the entorhinal cortex following long-term cannabinoid exposure. When taken together, these results suggest long-term WIN 55, 212-2 treatment specifically alters targets of the associational-commissural path and medial perforant path afferent from entorhinal cortex but not the lateral perforant path afferent from entorhinal cortex.
Previous studies that examined dendritic morphology after long-term THC exposure in adolescence found reductions in dendritic branching, length and spines in the dentate gyrus that persisted into adulthood (Rubino et al., 2009). The current study extends these findings by showing that spine loss in the dentate gyrus also occurs in adult rats that began chronic treatment in adulthood after the brain was fully matured; therefore, some changes in dendritic morphology following long-term cannabinoid administration are not specific to a particular developmental age. Conversely, the fact that we did not find differences in overall dendritic branching and length in adult rats implies that adolescent granule cells may be more sensitive to cannabinoids. There is clear evidence for adolescent cannabinoid sensitivity that results in persistent, long-term decreases in hippocampal dendritic morphology, protein expression, NMDA receptor levels, and deficits spatial working memory (Rubino et al. 2009). Given that the negative consequences observed after adolescent exposure far outnumber the effects observed after mature adulthood exposure, there is an indication that hippocampal cannabinoid sensitivity may partially resolve by adulthood. In fact, cannabinoid-induced structural plasticity was only found in very specific circuits (associational-commissural path and medial perforant path) within the hippocampus and only in relation to spine density within those circuits. Reductions in overall dendritic branching and length in adult rats might have been expected given that there were reductions in spine density; however, no significant reductions in branching or length were observed. One possibility is that the lack of spine density reductions in the most distal (outer) portions of the granule cell dendritic fields helped prevent overall reductions in length and branching. Taken together with the current observations, the robust reductions in branching and length that have been reported in adolescent rats suggests that granule cells are more sensitive to cannabinoids prior to adulthood. Another study that examined dendritic morphology following long-term THC exposure in adult rats found increased dendritic morphology on medium spiny neurons of the nucleus accumbens and pyramidal neurons of the medial prefrontal cortex and a lack of drug differences in pyramidal neurons in the CA1 field of the hippocampus (Kolb et al., 2006). These authors did not investigate morphology differences in the dentate gyrus, but taken together the available data indicate that regionally specific changes within the hippocampus and hippocampal formation occur following long-term cannabinoid exposure. Potentially, increases in dendritic morphology in other brain regions may compensate for reductions in dendritic spine density in the dentate gyrus. The current study also provides evidence that a different cannabinoid agonist WIN 55, 212-2, with higher binding affinities for the CB1 receptor than THC, produces similar changes to dendritic morphology following long-term administration; therefore, the changes in dendritic morphology may be a consequence of all cannabinoid agonists. Previously it was shown that WIN 55, 212-2 administration elevated spine densities in the zebra finch area X and XVC song regions following long-term exposure (Gilbert and Soderstrom, 2011), thus, future studies should further examine the pattern of increases and decreases in spine density following exposure to WIN 55, 212-2.
The current study examined differences in dendritic morphology 24 hours after the last drug injection. With this design it is possible to examine the immediate changes produced by WIN 55, 212-2, but it remains unknown if the effects are long lasting, and more research is needed to verify the persistence of spine density changes observed following exposure and withdrawal. Previous cannabinoid research using THC by Kolb et al. 2006 and Rubino et al. 2006 found the morphology changes produced by cannabinoids to be long-lasting and persistent even after several weeks of withdrawal (from 1 to 2 months). Therefore, it is the likely the WIN 55, 212-2 spine density alterations are long-lasting. Although, following cocaine treatment Kolb et al. (2003) found that persistence of spine alterations (in this case enhancements) varies depending on drug treatment regiment, which may also play an important role in the persistence of changes observed with cannabinoids.
Due to the fact that there is a dense band of CB1 receptors present in the lower layer of the dentate gyrus stratum moleculare (Katona et al., 2006) this area is likely to be affected by long-term cannabinoid exposure. This region also corresponds to the ipsilateral associational-commissural projection originating from axons of the mossy cells of the hilus that form excitatory synapses back onto granule cells creating a feedback loop to regulate dentate gyrus responsiveness (Johnston and Amaral, 2004; Laurberg and Sorensen, 1981). Interestingly, endocannabinoids have been shown to signal at glutamatergic synapses on dendritic spines throughout the hippocampus. Katona et. al. (2006) found that diacylglycerol lipase α, the precursor to 2-arachidonoyl-glycerol, is highly concentrated on the heads of dendritic spines indicating involvement with retrograde signaling at glutamatergic synapses to presynaptic CB1 receptors. Expanding on these results, Uchigashima et. al (2011) showed that within the dentate gyrus specifically, mossy cells contained abundant presynaptic CB1 receptors and granule cell spines released 2-arachidonoyl-glycerol forming a bridge for retrograde signaling to adjust network activity in the dentate gyrus following excitation. If the CB1 receptors are located at the end of a mossy cells synapses releasing glutamate onto granule cell spines, following exogenous cannabinoid agonist stimulation the Gi/o coupled CB1 receptor would prevent glutamate release and the postsynaptic spine may retract due to loss of excitation. Within the associational-commissural afferents may also be where exogenous cannabinoids are most disruptive by creating feedback inhibition disruption. If cannabinoids inhibit mossy cell excitation of proximal granule cell dendrites following feedback inhibition, the end result may be reduced excitation of the granule cell, reduced synaptic plasticity, and reduced hippocampal-dependent learning and memory. Given that the medial perforant path is involved in long-term potentiation following high frequency stimulation of entorhinal inputs, the medial perforant path is a critical site of memory processing into the hippocampus. It has been shown that during high frequency stimulation of the medial perforant path in vivo, CB1 activation increases glutamate release from perforant path synapses while inhibiting release of GABA from local interneurons (Sokal et al., 2008). Additionally it has been shown that medial perforant path stimulation preferentially recruits inhibitory basket cells in a feedforward fashion, bypassing excitatory granule cell inputs and feedback inhibition (Ewell and Jones, 2010). The cumulative effects of cannabinoids on these processes may contribute to the pattern of spine density loss observed here.
Alterations in spine density resulting from long-term cannabinoid exposure in adult rats may be an important contributing factor underlying behavioral findings of cannabinoid induced memory impairment and memory consolidation deficits. Segment specific changes in spine density may have important consequences for spatial learning and memory processes in the dentate gyrus, especially considering the pattern of reductions observed here. Hargreaves et. al. (2005) showed that the medial perforant path transferred spatial information from the medial entorhinal cortex, while the lateral perforant path transferred nonspatial information. Alterations in the synaptic targets of medial perforant path projection in the dentate gyrus may contribute to spatial learning and memory impairments, possibly through reductions in processes involved in consolidation of spatial information. WIN 55, 212-2 given post-training in the Morris water task impaired long-term spatial memory consolidation by activating CB1 receptors in the dorsal hippocampus (Yim et al., 2008). Prior work from our laboratory found similar Morris water task post-training administration consolidation deficits following a 24-hour consolidation time-frame (Candelaria-Cook, 2009; Candelaria-Cook and Hamilton, 2008). We also found that the WIN 55, 212-2 spatial memory consolidation deficit could be recovered by administration of the cannabinoid antagonist AM 251 (Candelaria-Cook and Hamilton, 2010), further supporting the role of CB1 receptors in spatial memory consolidation. Future research should examine the relationship betweem cannabinoid induced reductions in spine sensity and memory consolidation. One prediction derived from the present data would be that cannabinoids may have a more profound effect on spatial memory consolidation than consolidation of non-spatial information processed via the lateral perforant path.
A few limitations of the current study should be taken into consideration. The present study only evaluated dendritic morphology within the dentate gyrus and its afferents. It is likely that different, yet complementary changes in morphology exist in other regions of the hippocampus and in other regions of the brain, as indicated by Kolb et. al. (2006). The present study was also limited to analysis of males. Future work should address if sex differences exist in granule cell dendritic morphology. The present study was also limited to a single WIN 55, 212-2 drug dose. A high dose was selected based on previous literature on cannabinoid-induced deficits on spatial memory. Although the results obtained here with a high dose of cannabinoids were subtle and limited to spine density, in the future different doses ranging from low to high should be investigated.
In summary, the present findings demonstrate a significant reduction in dendritic spine density of the dentate gyrus following long-term administration of WIN 55, 212-2 in adult rats. This loss in spine density on the granule cells was specific to the portions of the dendritic fields that receive associational-commissural afferents from within the hippocampal formation and medial perforant path afferents from the entorhinal cortex, and did not generalize to overall changes in dendritic length and branching. Given the involvement of granule cell plasticity in memory, these observations suggest exposure to exogenous cannabinoids can have profound and long-lasting effects on long-term spatial memory storage and consolidation.
4. Experimental procedures
4.1. Animals
Subjects were 24 male Long-Evans rats acquired from Harlan Laboratories (Indianapolis, IN). All rats were 6 months of age at the beginning of experiment. Rats were pair-housed in standard clear plastic cages and maintained in a temperature and humidity controlled vivarium with food and water available ad libitum. The University of New Mexico Institutional Animal Care and Use Committee (IACUC) approved all experimental procedures.
4.2. Drugs treatments
The cannabinoid agonist WIN 55, 212-2 (Sigma-Aldrich), was dissolved in 5% DMSO (Sigma-Aldrich) plus 0.1% Tween 80 (Sigma-Aldrich) and brought to volume with sterile Dulbecco’s phosphate buffered saline (Sigma-Aldrich). WIN 55, 212-2 was prepared at a concentration of 3.7 mg/ml. The control solution consisted of the drug vehicle. Both drug and vehicle solutions were prepared fresh daily and injected in a volume of 1 ml/kg of body weight. All drug doses and vehicle preparations were selected according to previous literature (Pamplona et al., 2006; Pamplona and Takahashi, 2006; Schneider et al., 2005; Yim et al., 2008). The rats were divided into 4 experimental groups (n=6): 7 day Control, 7 day WIN, 55-212-2, 21 day Control, and 21 day WIN 55, 212-2. Shortly after the beginning of the light cycle (1000 h), rats were weighed in the colony room and given an intraperitoneal (i.p.) injection of WIN, 55, 212-2 or vehicle control, once daily, for 7 or 21 days. Rats were immediately returned to their cage following injection.
4.3. Golgi-Cox staining and analysis
At the conclusion of the experiment, one day following their last injection, rats were given an overdose of sodium pentobarbital and perfused transcardially with 0.9% (wt/vol) saline. The brains were extracted and immediately immersed in 30 ml of Golgi-Cox solution (Glaser and Van der Loos, 1981) for 14 days, followed by immersion in 30% (wt/vol) sucrose for 3 days. Coronal sections, 200 μm thick, were cut on a vibrating blade microtome, mounted on 2% gelatinized slides, stained, dehydrated, cleared, and cover slipped, as described by Gibb and Kolb (1998).
Granule cells of the dentate gyrus, Zilles’ area DG (Zilles, 1985), were selected for analysis from the medial portion of the upper blade (Figure 1). An Olympus light microscope (Model BX51) equipped with a drawing attachment was used for analysis. Five neurons from each hemisphere (10 neurons per rat) were traced using the camera lucida technique (250X final magnification). Selection was limited to unobscured, complete, and well-impregnated neurons with representative dendritic morphology for the region of interest. Sampling included sections ranging from 3.3 mm to 3.8 mm anterior to Bregma. An experimenter blind to drug conditions performed all morphology, spine tracing, and analysis.
Dendritic branching was measured by counting bifurcations on each dendrite (Coleman and Riesen, 1968). First-order branches were dendritic segments prior to the first bifurcation from the soma and branch order was incremented by 1 for each subsequent bifurcation on a given dendritic branch. The number of first through sixth-order (and higher) branches was quantified and an estimate of total branches was determined from these values. Dendritic length was measured using a Sholl analysis (Sholl, 1981). A printed transparency of a series of 20 μm concentric ring intersections (calibrated to 250X final magnification) was centered over the cell body and the total number of intersections between each ring and dendritic branch were counted. The Sholl values were converted to estimates of dendritic length as a function of distance from the soma.
For spine density analysis, each dendritic branch was divided into 3 different segments at various distances from the cell body: an inner (proximal) segment, middle segment and outer (distal) segment (see Figure 2). Each segment was approximately 50 μm in length. These divisions were selected to correspond to the 3 major afferent pathways projecting into the dentate gyrus: associational-commissural path, medial perforant path, and lateral perforant path, respectively. Selection was limited to branches for which all 3 segments were continuous (i.e., not uninterrupted by a branch point). Spine density was measured by tracing each dendritic segment at high power (2000X final magnification) followed by tracing all spines present in each segment. Total spine density per 10 μm was calculated from these values. Spine density was quantified on 10 granule cells, 5 cells per hemisphere. Mean spine density was calculated on 10 measurements per segment, 3 segments per cell. The unit of analysis for each rat was mean spine density for the entire dendritic field or for each layer.
No significant effects were observed for dendritic branching or length.
WIN 55,212-2 treatment reduced spine density in inner and middle dendritic segments.
Cannabinoids alter associational-commissural pathway and medial perforant pathway.
Cannabinoids do not alter lateral perforant pathway.
Spine density loss may be important contributing factor behind memory deficits.
Acknowledgments
This research was supported in part by NIH/NIGMS grant 5R25-GM060201 to FCC, Quad-L Foundation support to FCC, and UNM-RWJF Center for Health Policy Dissertation Fellowship to FCC and NIH grant AA019462 to DH. We thank Clark Bird, Ph.D., for his comments on the final manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Candelaria-Cook FT. Cannabinoids impair acquisition, consolidation and reconsolidation of rodent spatial memory. University of New Mexico; Albuquerque: 2009. p. 71. [Google Scholar]
- Candelaria-Cook FT, Hamilton DA. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Cannabinoid administration impairs acquisition, consolidation, and reconsolidation of rodent spatial memory. Program No. 295.3/SS53. 2008. Online. [Google Scholar]
- Candelaria-Cook FT, Hamilton DA. 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2010. Post-training cannabinoid administration impairs 24-hour consolidation of rodent spatial memory. Program No. 510.9/NNN4. 2008. Online. [Google Scholar]
- Coleman PD, Riesen AH. Environmental effects on cortical dendritic fields. I Rearing in the dark. J Anat. 1968;102:363–374. [PMC free article] [PubMed] [Google Scholar]
- Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30:12597–12607. doi: 10.1523/JNEUROSCI.1854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Gilbert MT, Soderstrom K. Late-postnatal cannabinoid exposure persistently elevates dendritic spine densities in area X and HVC song regions of zebra finch telencephalon. Brain Res. 2011;1405:23–30. doi: 10.1016/j.brainres.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain section by obverse-reverse computer microscpoy - application of a new, high clarity golgi-nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Johnston D, Amaral DG. Hippocampus. In: Shepherd G, editor. The Synaptic Organization of the Brain. 5. Oxford Univeristy Press; New York: 2004. pp. 455–498. [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung K, Piomelli D, Mackie K, Freud TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li YL, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. PNAS. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Laurberg S, Sorensen KE. Associational and commissural collaterals of neurons in the hippocampal-formation (hilus fasciae dentatae and subfield CA3) Brain Res. 1981;212:287–300. doi: 10.1016/0006-8993(81)90463-7. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Woelfel B, Dodt HU, Zieglgaensberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RDS, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology. 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Takahashi RN. WIN 55,212-2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci Lett. 2006;397:88–92. doi: 10.1016/j.neulet.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handbook of Experimental Pharmacology. 2005;168:445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- Robinson L, Riedel G. Cannabinoid function in spatial learning: an update. Curr Neuropharmacology. 2004;2:125–143. [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol. 2005;16:447–454. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- Sholl DA. The organization of the cerebral cortex. Methuen; London: 1981. [Google Scholar]
- Sokal DM, Bennetti C, Girlanda E, Large CH. The CB1 receptor antagonist, SR141716A, prevents high-frequency stimulation-induced reduction of feedback inhibition in the rat dentate gyrus following perforant path stimulation in vivo. Brain Res. 2008;1223:50–58. doi: 10.1016/j.brainres.2008.05.065. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Yamazaki M, Yamasaki M, Tanimura A, Sakimura K, Kano M, Watanabe M. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci. 2011;31:7700–7714. doi: 10.1523/JNEUROSCI.5665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB1 receptors mediate the memory impairing effects of delta(9)-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim TT, Hong NS, Ejaredar M, McKenna JE, McDonald RJ. Post-training CB1 cannabinoid receptor agonist disrupts long-term consolidation of spatial memories in the hippocampus. Neurosci. 2008;151:929–936. doi: 10.1016/j.neuroscience.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Zilles K. The cortex of the rat: a stereotaxic atlas. Springer; Berlin: 1985. [Google Scholar]