Abstract
Objective
Retinol-binding protein 4 (RBP4) has recently been reported to be associated with insulin resistance (IR) and the metabolic syndrome by a number of researchers in various populations. However, controversies are present among different studies, which might be due to the differences between various ethnic, age, and sex groups. This study aimed to determine whether RBP4 can be assumed as a marker of IR and the metabolic syndrome in the Iranian obese children.
Methods
In the present longitudinal cross-sectional study, 100 5-17 years old obese children were recruited from January 1, 2011 to February 1, 2012. The patients’ information including the demographic variables, health status and behavior, and daily physical activity were collected. Moreover, serum RBP4 was measured and correlated with the homeostasis model assessment of IR index (HOMA-IR), components of the metabolic syndrome, and lipoprotein metabolism.
Findings
The results revealed a positively significant correlation between RBP4 and the HOMA-IR index (P=0.02). Partial Spearman test also revealed a significant correlation between RBP4 plasma concentrations and the components of the metabolic syndrome, including waist circumference, systolic (but not diastolic) blood-pressure, and fasting blood sugar (P<0.05). However, no significant correlation was observed between RBP4 and HDL (P=0.3) as well as triglycerides concentration (P=0.1). Moreover, plasma RBP4 level gradually increased with the increasing number of the metabolic syndrome components.
Conclusion
Regarding the results of the present study and previous investigations, RBP4 seems to be a suggestible predictive marker for both insulin resistance and metabolic syndrome in Iranian obese children; however, further studies are needed to be conducted among different ethnicities and age groups in order to determine the predictive value of this correlation.
Keywords: Retinol Binding Protein 4, Insulin Resistance, Metabolic Syndrome, Obesity, Children
Introduction
Obesity has turned out to be a global concern and has reached an epidemiologic proportion worldwide, which is mostly assumed to be because of the alterations in diet and sedentary lifestyle particularly among the children. Obesity is a major cause of Insulin Resistance (IR), especially due to the secretions of the active adipose tissue hormones, namely adipokines, leading to enhancing and/or impairing insulin action[1].
IR also plays a crucial role in pathogenesis and is known as a hallmark of Metabolic syndrome; a syndrome which may include a number of metabolic abnormalities, including dyslipidemia (decreased HDL level and increased serum triglycerides concentration), hypertension, and hyperglycemia[2, 3]. Moreover, the metabolic syndrome is related to the increased risk of cardiovascular diseases as well as diabetes mellitus type 2[4, 5]. Regarding the importance of global epidemics of obesity and its comorbidities, particularly IR and metabolic syndrome, identifying the key predictive factors, early diagnosis, and new intervention methods are of great importance in order to prevent the disease.
The relationship between IR and certain inflammatory factors, such as C-reactive protein (CRP), TNF-alpha, IL-6, and adipokines, specifically adiponectin, has been well documented[6, 7]. Retinol-binding protein 4 (RBP4) is a transport protein for retinol (vitamin A), which is mainly synthesized by hepatocytes and also adipose tissue and is secreted into circulation bound to retinol and transthyretin[8]. In addition to retinol transportation, RBP4 upregulation was reported to be correlated with obesity and IR[9]. RBP4 has received much attention as a potential predictor for IR and metabolic syndrome[9–13]. A noticeable increase in RBP4 plasma level was observed not only in animal models of obesity and insulin resistance, but also in diabetic human[8, 9]. Many studies have declared the relationship between the increased circulating RBP4 and different aspects of obesity[14–19], increased fasting plasma glucose levels (FPG)[15, 25], dyslipidemia, and IR[10–13, 20–23], whereas some others showed no relationship between plasma RBP4 and IR[14, 24]. The causes of such differences have not been clarified yet. Of course, varieties in the populations under study, age, sample size, and assaying methods may explain some of these differences. Hence, the correlation between plasma levels of RBP4 and IR and the predictive value of this marker for IR and metabolic syndrome are yet to be established.
Therefore, the present study aimed to investigate whether RBP4 plasma level is correlated with IR, metabolic syndrome, and its individual components in the 5-17 year old Iranian obese children.
Subjects and Methods
Population
In this longitudinal cross-sectional study 100 obese children aged 5-17 years and BMI ≥ 95 percentile for age and sex[25] referred to the pediatric endocrinology clinics of Shiraz University of Medical Sciences, Shiraz, Iran from January 1, 2011 to February 1, 2012 were recruited. The inclusion criteria were: 1) BMI >95% percentile for age and sex, 2) Age 5 to 17 years, and 3) Confirmation by the parents to participate in the study after being orally presented with the objectives of the study and signing written informed consent. The exclusion criteria of the study were: 1) liver diseases except viral hepatitis, autoimmune hepatitis, Wilson, α1-antitripsin deficiency, and hemochromatosis, 2) acute and chronic renal failure, 3) Acute illnesses, 4)diabetes mellitus, 5) recent trauma, 6) consuming any medication, e.g. oral hypoglycemic agents, hypolipidemic agents, and alcohol, 7) cardiovascular and endocrine system diseases. The data on the demographic variables, health status, and behavior, and daily physical activity were collected by using a standardized questionnaire. All the participants were required to fast overnight (≥6 hrs) before the laboratory evaluations and physical examinations by a pediatric endocrinologist. The patients were also evaluated regarding height, weight, waist circumference (WC), hip circumference (HC), waist to hip circumference ratio (WC/HC), blood pressure, puberty Tanner staging, and clinical evidence of insulin resistance (eg Acanthosis nigricans). Then, blood samples were collected in tubes containing liquid EDTA, centrifuged at 4 °C, and kept at −80 °C until analysis.
Serological measurements
Serum triglyceride (TG), total cholesterol (Chol), high density lipoprotein (HDL), low density lipoprotein (LDL), and fasting plasma glucose (FPG) were assessed on a Kodak Ektachem 702 Analyzer with an enzymatic method (Eastman Kodak, Rochester, NY), and glycated hemoglobin (HbA1c) was measured using a commercial kit (Unimate HbA1c; Roche Diagnostics, Basel, Switzerland) by a single expert lab technician.
In addition, fasting insulin level (FI) was measured through a completely homologous radioimmunology assay (Linco Research inc., St. Charles, MO), which had less than 0.2% cross-reaction with proinsulin, with an assay sensitivity of 2.3 rU/ml, and intra- and inter-assay coefficient of variation of 7% and 9%, respectively. Finally, serum RBP4 (pg/ml) was estimated by an enzyme-linked immunosorbent assay (ELISA) (Sali-Savers®, ALPCO Diagnostics, Windham, NH) with a sensitivity of 8.94 ng/ml (IC20), 46.30 ng/mL (IC50), and 219.17 ng/mL (IC80), respectively. The intra-assay coefficient of variation was 1.9–4.6% and inter-assay was 6.7–8.8%.
Definition of metabolic syndrome and degree of Insulin resistance
According to the National Cholesterol Education Program (NCEP), children and adolescents meeting at least three of the following criteria are qualified as having the metabolic syndrome: elevated blood pressure ≥90th percentile for age and sex, HDL cholesterol ≤40 mg/dl, TG level ≥ 110 mg/dl, FPG level ≥100 mg/dl, and WC ≥90th percentile for age and sex.
The degree of IR was determined by the Homeostatic model assessment (HOMA-IR) using the following formula[26]: Fasting insulin (µU/ml) ×fasting blood sugar (mmol/L)/22.5; HOMA-IR higher than 3 was considered as insulin resistant[27].
Ethics
The protocol of this study was approved by Medical Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran.
Statistical analysis
The study data were analyzed using the SPSS statistical software (Version 16, Chicago, IL, USA). The normally distributed data were expressed as means±standard deviation (SD). Multivariable regression analysis was used to estimate the partial association between markers of the metabolic syndrome and the serum RBP4 concentration; moreover, correlation coefficients between RBP4 and metabolic features and HOMA-IR index were estimated by correlation analysis on ranks (Pearson correlation). P value ≤0.05 was considered as statistically significant.
Findings
The present study investigated 100 Iranian obese children (35 male and 65 female) 5-17 years old with characteristics demonstrated in Table 1 regarding RBP4 plasma concentration, components of insulin resistance, metabolic syndrome, and some other anthropometric parameters.
Table 1.
Characteristics of study participants a
| Variable | Mean (SD) |
|---|---|
| Age (years) | 11.37 (3.06) |
| Height (cm) | 141.88 (16.4) |
| Weight (cm) | 62.17 (26.27) |
| BMI (kg/m2) | 30.29 (8.21) |
| Acanthosis nigricans (yes) | 53.8% |
| Systolic blood pressure (mm Hg) | 116.9 (13.2) |
| Diastolic blood pressure (mm Hg) | 74.06 (7.6) |
| waist circumference (cm) | 87.43 (15.83) |
| Hip circumference (cm) | 92.1 (15.56) |
| Waist to hip ratio | 0.94 (0.02) |
| Fasting plasma glucose (mmol/l) | 5.24 (0.49) |
| Fasting insulin (µU/ml) | 21.07 (13.97) |
| Triglycerides (mg/dl) | 119.3 (91.6) |
| Total Cholesterol (mg/dl) | 159.45 (28.9) |
| Low density lipoprotein (mg/dl) | 86.6 (21.2) |
| High density lipoprotein (mg/dl) | 45.3 (10.21) |
| Retinol-binding protein 4 (pg/ml) | 19397 (3121) |
| Homeostasis model assessment of IR index | 5.52 (3.79) |
These variables were log transformed before analyses; SD: Standard deviation
The relationship between RBP4 and Insulin resistance
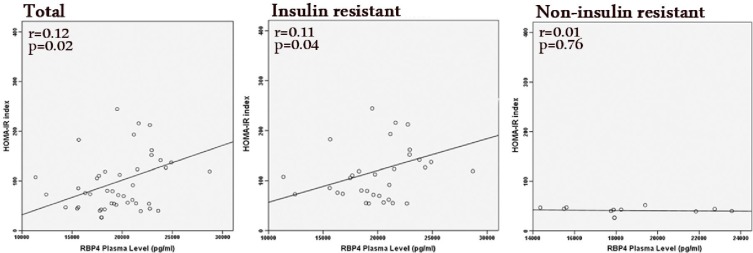
As it is exhibited in Table 2 there are positive correlations between RBP4 and HbA1c (P=0.03), fasting insulin (P=0.03), FPG (P=0.04), and expectedly the HOMA-IR index (P=0.02), which indicated the relationship between RBP4 plasma level and IR. 16 patients fulfilled 3 out of 5 characteristics of metabolic syndrome. The results of regression analysis between RBP4 and HOMA-IR index were consequential among the entire study population and the group of insulin resistant cases in particular (HOMA-IR > 3) while among non-insulin resistant group it was not noticeable (Fig. 1). However, no significant relationship was observed between the presence of the clinical symptoms of IR, such as Acanthosis nigricans and the RBP4 level (P=0.08). Furthermore, no significant difference was found between male and female patients regarding RBP4 concentrations.
Table 2.
Partial Spearman correlation coefficient among Retinol-binding protein 4 values, metabolic syndrome components, and other metabolic parameters
| RBP4 | BMI | WC | SBP | DBP | TG | FPG | HDL | LDL | Chol | HbA1c | Fasting Insulin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | 0.28 | |||||||||||
| WC | 0.42 a | 0.81a | ||||||||||
| SBP | 0.35 b | 0.52 a | 0.46 a | |||||||||
| DBP | 0.02 | -0.01 | 0.09 | 0.25 | ||||||||
| TG | 0.22 | -0.07 | 0.20 | 0.08 | 0.12 | |||||||
| FPG | 0.31 b | 0.21 | 0.36 b | 0.01 | -0.07 | 0.05 | ||||||
| HDL | -0.15 | -.02 | -0.10 | -0.19 | -0.17 | -0.62 a | -0.01 | |||||
| LDL | 0.11 | 0.02 | -0.19 | 0.16 | 0.19 | -0.01 | -0.17 | 0.32 b | ||||
| Chol | 0.18 | -.01 | 0.13 | 0.15 | 0.19 | 0.04 | -0.07 | 0.38 a | 0.96 a | |||
| HbA1c | 0.31 b | -0.11 | 0.02 | -0.18 | 0.17 | 0.21 | 0.39 a | -0.01 | 0.15 | 0.19 | ||
| Fasting insulin | 0.34 b | 0.33 b | 0.35 b | 0.16 | -0.07 | 0.24 | 0.47 a | -0.03 | 0.03 | 0.06 | 0.12 | |
| HOMA-IR index | 0.35 b | 0.25 | 0.32 b | 0.16 | 0.08 | 0.36 b | 0.45 a | -0.18 | 0.01 | 0.06 | 0.17 | 0.99 a |
RBP4: Retinol binding protein 4; BMI: Body mass index; WC: Waist circumference; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; TG:, triglycerides; FPG, fasting plasma glucose; HDL: High density lipoproteins; LDL: Low density lipoproteins; Chol: Total cholesterol; HOMA-IR: Homeostasis model assessment of IR; HbA1c; Glycated hemoglobin (HbA1c)
P ≤0.005
P ≤0.05
Fig. 1.
RBP4 and HOMA-IR index in the entire population, the group of insulin resistant cases (HOMA-IR > 3), and in the group of non-insulin dependent subjects. The regression analysis reveals significant association between RBP4 and HOMA-IR in the total and insulin resistant groups while this relationship is inconsiderable in the non-insulin dependent patients
Regarding the Tanner staging system of puberty, 52.9%, 19.6%, 15.7%, and 11.8% of the patients were in stages 1, 2, 3, and 4, respectively, with inconsequentially different plasma RBP4 levels (P=0.4).
The relationship between RBP4 and Metabolic syndrome components as well as other metabolic parameters
Pearson correlation analysis revealed a significant positive correlation between RBP4 plasma concentrations and the components of the metabolic syndrome, including WC (P=0.006), SBP (P=0.009), and FPG (P=0.04), while no significant relationship was observed between RBP4 and DBP (P=0.6), HDL (P=0.4), and TG (P=0.1).
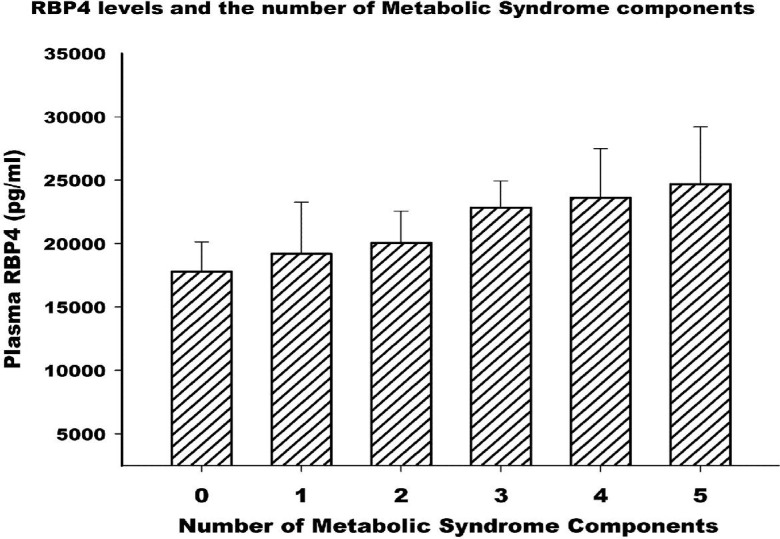
Moreover, plasma RBP4 concentrations gradually increased with the increasing number of present metabolic syndrome components (Fig. 2). The mean (±SD) values of RBP4 levels (pg/dl) for those with zero to five components were 17791.43 (±2340.16), 19197.5 (±4063.37), 20059.01 (±2507.46), 22841.67 (±2095.11), 23621.43 (±3857.15), and 24695.01 (±4512.45), respectively. The correlations between RBP4 and other metabolic parameters which are involved in the development of the metabolic syndrome (e.g. LDL and Cholesterol) are demonstrated in Table 2.
Fig. 2.
Plasma RBP4 levels according to the number of metabolic syndrome components. Data are exhibited as mean±SD; P≤0.05 for trend.
Discussion
Previous studies revealed a positive correlation between circulating RBP4, a transport protein for vitamin A, and the biochemical markers of carbohydrate metabolism as well as the factors contributing to the metabolic syndrome and insulin resistance state. Specifically, circulating RBP4 has been found to be positively correlated with FPG, FI, HbA1c[10], and HOMA-IR index[15] and negatively correlated with glucose disposal rate[10, 15, 17]. Plasma RBP4 level was shown to be negatively associated with peripheral insulin sensitivity[28]. Some investigations declared that RBP4 plasma concentration is higher in the patients with metabolic syndrome in comparison to those without the metabolic syndrome[11, 29]. Moreover, RBP4 has shown correlations with the number of the metabolic syndrome parameters as well as the value of each parameter, among which TG has the strongest and most steady correlation with RBP4 level[11, 29], while the weakest and the least frequent association has been reported for FPG[11, 30]. von-Eynatten et al (2007) reported no significant relationship between RBP4 and HOMA-IR index or HbA1c for insulin resistance; however, they found significant positive correlations between RBP4 and Chol, LDL, TG, and hepatic lipase activity in the patients with type 2 diabetes[31]. Qibin et al (2007) also conducted a study on Chinese population and reported RBP4 levels to be positively associated with BMI, WC, TG, LDL, blood pressure, fasting insulin, and HOMA-IR index while negatively related to HDL. They also showed that the level of RBP4 increased with the increase in the number of the metabolic syndrome components[11]. In a study conducted by Aeberli et al (2007), RBP4 was seen to be significantly correlated with TG and fasting insulin among normal and obese children of Northern Switzerland aged between 6 and 14 years[32]. Moreover, Yang et al showed that RBP4 caused IR in a laboratory model by reducing the GLUT4 expression in the adipose tissue but not in the muscle tissue[9]. Furthermore, Graham et al (2006) reported that serum RBP4 levels were associated with the magnitude of IR in obesity, impaired glucose tolerance, and type 2 diabetic cases, and also non-obese, non-diabetic individuals with a strong family history of type 2 diabetes. They also found that RBP4 concentration was positively correlated with the components involved in the metabolic syndrome, including BMI, WC/HC, TG, and SBP while negatively correlated with HDL. A reduction in serum RBP4 was also observed in the subjects who had improvement from IR state[10].
The present study on the Iranian obese children aged 5 to 17 years showed that RBP4 plasma concentration was positively associated with some metabolic syndrome components, including WC, FPG, and blood pressure. Moreover, we demonstrated that with the increase in the number of the components of metabolic syndrome, RBP4 level was increased. In this investigation, RBP4 was revealed to be significantly associated with HOMA-IR index, as an indicator of IR state.
One of the limitations of the present study was that serum retinol level, RBP4 to serum ratio, serum ferritin concentration, C-reactive protein, plasma uric acid levels[33], and renal function, and some other confounding factors, were not considered. Among these, serum ferritin was introduced as an independent determinant of poor metabolic control in the diabetic patients, and also a marker of IR[34]. According to a study conducted by Yudkin et al, it is declared that inflammatory state and plasma level of C-reactive protein, that we did not estimate in this study, may induce insulin resistance and affect the level of RBP4[35]. Serum uric acid level was also reported to have a positive correlation with IR by Rathmann et al[33]. Due to the large amount of blood sampling for other important serum tests in our study, we omitted these examinations and tried to rule out the inflammation through medical history, physical examination, and WBC counts of the patients.
Conclusion
Overall, considering previous researches and the present study, RBP4 plasma concentration is correlated with metabolic syndrome, constitutional factors as well as insulin resistance among 5-17 years old Iranian children. Although further longitudinal studies are still needed to be conducted on different ethnic populations and various age groups to realize whether these associations are dependent on any environmental variable and/or genetical background, our findings provide a novel insight into the potential role of RBP4 in pathogenesis of the metabolic syndrome and insulin resistance.
Acknowledgment
This paper was extracted from the Endocrinology fellowship thesis of Dr Foroogh Saki with the ID number 4086. This study was supported by Student Research Committee and Pediatric Endocrinology Research Committee of Shiraz University of Medical Sciences, Shiraz, Iran. The authors would like to thank the Research Improvement Center of Shiraz University of Medical Sciences and Ms. A. Keivanshekouh for improving the use of English in the manuscript.
Conflict of Interest
None
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92(5):593–6. doi: 10.1016/s0092-8674(00)81125-3. [DOI] [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzo C, Okoloise M, Williams K, et al. The Metabolic Syndrome as Predictor of Type 2 Diabetes. Diabetes Care. 2003;26(11):3153–9. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without c-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108(4):414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 7.Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christou GA, Tselepis AD, Kiortsis DN. The metabolic role of retinol binding protein 4: an update. Horm Metab Res. 2012;44(1):6–14. doi: 10.1055/s-0031-1295491. [DOI] [PubMed] [Google Scholar]
- 9.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 10.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354(24):2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 11.Qi Q, Yu Z, Ye X, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92(12):4827–34. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 12.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(31):12587–94. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DC, Lee JW, Im JA. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007;56(3):327–31. doi: 10.1016/j.metabol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Janke J, Engeli S, Boschmann M, et al. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55(10):2805–10. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 15.Stefan N, Hennige AM, Staiger H, et al. High circulating retinol-binding protein 4 is associated with elevated liver fat, but not with total-, subcutaneous-, visceral-, or intramyocellular fat in humans. Diabetes Care. 2007;30(5):1173–8. doi: 10.2337/dc06-2342. [DOI] [PubMed] [Google Scholar]
- 16.Haider DG, Schindler K, Prager G, et al. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2007;92(3):1168–71. doi: 10.1210/jc.2006-1839. [DOI] [PubMed] [Google Scholar]
- 17.Gavi S, Stuart LM, Kelly P, et al. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92(5):1886–90. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 18.Balagopal P, Graham TE, Kahn BB, et al. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab. 2007;92(5):1971–4. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 19.Saki F, Karamizadeh Z, Honar N, et al. Association of plasma retinol binding protein-4 (RBP4) and sonographic grading of fatty liver in obese Iranian children. Hepat Mon. 2012;12(12):e7103. doi: 10.5812/hepatmon.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho YM, Youn BS, Lee H, et al. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29(11):2457–61. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 21.Broch M, Vendrell J, Ricart W, et al. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. 2007;30(7):1802–6. doi: 10.2337/dc06-2034. [DOI] [PubMed] [Google Scholar]
- 22.Erikstrup C, Mortensen OH, Pedersen BK. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355(13):1393–4. [PubMed] [Google Scholar]
- 23.Silha JV, Nyomba BL, Leslie WD, et al. Ethnicity, insulin resistance, and inflammatory adipokines in women at high and low risk for vascular disease. Diabetes Care. 2007;30(2):286–91. doi: 10.2337/dc06-1073. [DOI] [PubMed] [Google Scholar]
- 24.Yagmur E, Weiskirchen R, Gressner AM, et al. Insulin resistance in liver cirrhosis is not associated with circulating retinol-binding protein 4. Diabetes Care. 2007;30(5):1168–72. doi: 10.2337/dc06-2323. [DOI] [PubMed] [Google Scholar]
- 25.Williams CL, Campanaro LA, Squillace M, et al. Management of childhood obesity in pediatric practice. Ann N Y Acad Sci. 1997;817:225–40. doi: 10.1111/j.1749-6632.1997.tb48209.x. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and (-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Tresaco B, Bueno G, Pineda I, et al. Homeostatic model assessment (HOMA) index cut-off values to identify the metabolic syndrome in children. J Physiol Biochem. 2005;61(2):381–8. doi: 10.1007/BF03167055. [DOI] [PubMed] [Google Scholar]
- 28.Ribel-Madsen R, Friedrichsen M, Vaag A, et al. Retinol-binding protein 4 in twins: regulatory mechanisms and impact of circulating and tissue expression levels on insulin secretion and action. Diabetes. 2009;58(1):54–60. doi: 10.2337/db08-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingelsson E, Sundström J, Melhus H, et al. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009;206(1):38–9. doi: 10.1016/j.atherosclerosis.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Lai MM, Li TC, et al. Relationship between serum retinol-binding protein 4 and visfatin and the metabolic syndrome. Diabetes Res Clin Pract. 2009;85(1):24–9. doi: 10.1016/j.diabres.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 31.von-Eynatten M, Lepper PM, Liu D, et al. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50(9):1930–7. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- 32.Aeberli I, Biebinger R, Lehmann R, et al. Serum Retinol-Binding Protein 4 Concentration and Its Ratio to Serum Retinol Are Associated with Obesity and Metabolic Syndrome Components in Children. J Clin Endocrinol Metab. 2007;92(11):4359–65. doi: 10.1210/jc.2007-0468. [DOI] [PubMed] [Google Scholar]
- 33.Rathmann W, Funkhouser E, Dyer AR, et al. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: The CARDIA Study. Ann Epidemiol. 1998;8(4):250–61. doi: 10.1016/s1047-2797(97)00204-4. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Real JM, Ricart-Engl W, Arroyo E, et al. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care. 1998;21(1):62–8. doi: 10.2337/diacare.21.1.62. [DOI] [PubMed] [Google Scholar]
- 35.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects:associations with obesity, insulin resistance, and endothelial dysfunction a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]