Abstract
Objective
To evaluate early aggressive vs. conservative nutrition and its effect on Retinopathy of Prematurity (ROP) in <32 weeks of gestation neonates.
Methods
A prospective, randomized, clinical study was conducted in NICU with a total of 75 preterm infants. In the intervention group, infants received early aggressive nutrition immediately after birth, in the control group infants were started on conventional parenteral nutrition (PN). Blood samples were obtained for Insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 3 (IGFBP3) levels before commencement of PN on the first postnatal day, and from week 1 to 6 every week. All the infants were examined for ROP.
Findings
Infants in the early aggressive group had a reduction in the risk of ROP of 5% (2 from 40); the number of infants needed treatment averaged 3.7 (2.7 to 5.2). A total of 11 neonates in the conventional group were detected having ROP (P<0.05). Overall, IGF-I levels were higher in the aggressive PN (APN) vs the conventional PN (CPN). ROP development was higher in the CPN compared to the APN. IGF-1levels were lower in ROP developers compared with non-ROP in the APN group. There was no difference in IGF-I levels in ROP developers versus non-ROP in the CPN group. IGF-1 levels were lower in the CPN group compared with the APN group in the third week in ROP developers. There was a correlation between ROP and IGF-1 levels. Through ROC analysis, IGF-1 was demonstrated as being a sensitive marker for ROP.
Conclusion
IGF-1 levels were higher in the APN group versus the CPN group. This may indicate that IGF-1 levels simply being higher is not enough; rather, that being higher above a cutoff value may prevent ROP.
Keywords: Parenteral nutrition, Prematurity, Retinopathy of prematurity, IGF-1, IGFBP3
Introduction
Insulin-like growth factor 1 (IGF-1) is required for Vascular endothelial growth factor (VEGF) activation of vascular endothelial cell proliferation and survival pathways. IGF-1 levels are deficient after premature birth, increasing the risk of retinal vascular loss and Retinopathy of Prematurity (ROP). ROP is related to oxygen-regulated vascular endothelial growth factor and to IGF-1. Preterm birth is associated with a rapid fall in serum IGF-1 level, as maternal sources of IGF-1 are lost and decreased serum concentrations of IGF-1 are associated with the development of ROP[1]. The bioavailability of IGF-1 is regulated by insulin growth factor binding protein (IGFBP). IGFBP3 is the principal carrier of IGF-1 during late intrauterine life[2, 3]. Preterm infants have lower serum levels of IGF-1 than term infants, and neonate's size at birth is correlated with the level of IGF-1 in the cord serum[4]. IGF-1 plays an important role in neovascularization, and it has been suggested that a lack of IGF-1 in the early weeks of life, followed by a slow increase, is likely to increase the risk of development of ROP[8, 9]. Serum IGF-1 levels in premature infants can predict which infants will develop retinopathy of prematurity, which further suggests that the early restoration of IGF-1 in premature infants to normal levels could prevent this disease[5].
In the present study, the primary outcome is defined as early aggressive parenteral nutrition increasing the levels of IGF-1 and IGFBP3, while the secondary outcome is defined as early aggressive PN reducing the incidence of ROP in <32 weeks of gestation preterm neonates.
Subjects and Methods
Study design
A prospective, randomized, double blind, clinical trial was conducted in the neonatal intensive care units of Sisli Children's Hospital, Istanbul from April 2009 to December 2010. Preterm infants appropriately sized for a gestational age of <32 weeks were eligible for participation in the study. Exclusion criteria included transfer to another hospital within 48 hours of birth, intrauterine growth retardation, small-for-gestational age (SGA) and large-for-gestational age (LGA) birth weights, congenital (cardiac, pulmonary, or gastrointestinal) anomalies or metabolic diseases known to affect energy or nutrient metabolism, severe asphyxia characterized by seizures or severe metabolic acidosis on the first day of life, and evidence of infection. The study protocol was approved by the ethical committee of Sisli Children's Hospital, and written informed consent from a parent was obtained for each child prior to the study. There was no funding source relevant to the study. Gestational age was calculated from maternal history or estimated from the Ballard score[6]. Appropriate for gestational age was defined according to Usher and McLean criteria[7]. Infants were randomly allocated within six hours after birth to receive early and aggressive nutrition (APN) or conventional parenteral nutrition (CPN). An independent researcher used a computer-generated randomization table based on blocks of four to assign the infants to Group 1 or 2, which corresponded to batch numbers on the parenteral nutrition products. Eligible neonates entered the study based on a 1:1 parenteral nutrition allocation to APN or CPN group. Parenteral nutrition was prepared by the hospital pharmacy. In this way, investigators, parents, and nursing staff were blinded to treatment allocation. The code of the batch numbers was broken after data analysis had been performed.
Sample size calculation
We hypothesized that preterm infants with serum IGF-1 concentrations below 33 ng/ml for a prolonged period have an increased risk of later development of ROP[8]. On the basis of previous findings, we calculated that a sample size of 75 (40 infants in the study group and 35 in the control group) would allow us to detect a difference in IGF-1 levels between the two groups (α=.05, power =80%)[9]. The α level was set at 0.05 on the basis of a two-sided, two-sample t test.
Nutritional protocols
1. Parenteral nutrition:
As soon as consent was obtained and the infant was clinically stabilized, parenteral nutrition was given through an indwelling central venous catheter. Infants who received parenteral nutrition starting with 3.0 g/kg/d amino acids (Primene 10%, Baxter/Clintec, Maurepance, France) and 2.0 g/kg/d lipids (Intralipid 20%, Fresenius KABI, Uppsala, Sweden) increasing by 1.0 g/kg/day with an aimed intake of 4.0 g/kg/d amino acids and 3.0 g/kg/d lipids on the second day of life were considered to belong to the early APN. Infants who received parenteral nutrition starting with 1.5 g/kg/d amino acids and 1.0 g/kg/d lipids on the first day of life increasing by 1.0 g/kg/day with an aimed intake of 4.0 g/kg/d amino acids and 3.0 g/kg/d lipids on the third day of life were considered to belong to the CPN. Intake of fluid, glucose, and electrolytes was ordered by the attending neonatologist and not dictated by the experimental protocol. Glucose infusion (Dextrose 20%, 10%, 5%, Baxter/Clintec, Maurepance, France) was started at 6-8 mg/kg/min during the first day of life and increased gradually to 12 mg/kg/min in order to maintain blood glucose concentration between 80-100 mg/dl while avoiding any hyperglycemia (Chemstrip >150 mg/dl plus glycosuria). Parenteral amino acids and lipids dosage was reduced when the enteral feedings supplied 0.5 g/kg/day protein and 1g/kg/day lipids. They were terminated when enteral feedings supplied 100-140 cc/kg/day of total nutrition volume.
2. Enteral nutrition:
Infants were initially fed unfortified expressed breast milk in addition to parenteral nutrition when clinically stable within the first day of life. Trophic enteral feeding was initiated at 10 ml/kg/day for infants weighing less than 1250 g at birth and 15-20 ml/kg/day for infants weighing ≥1250 g at birth, and slowly advanced (10-20 ml/kg/day) after feeding volumes were tolerated. If breast milk reached 100 cc/kg/day, human milk fortifier (Eoprotin® Milupa, GmbH, Friedrichsdorf, Germany) was added at 1 spoon for 30 cc breast milk. Achievement dates of full enteral feeds (140-150 cc/kg/day) for groups were recorded. Weight measurements were calculated weekly for the first 6 weeks.
ROP Classification
Retinopathy of prematurity was classified according to the International Classification of Retinopathy of Prematurity[10] and subdivided into stage 1 (demarcation line), stage 2 (ridge), stage 3 (ridge with extraretinal fibrovascular proliferations), stage 4 (subtotal retinal detachment), and stage 5 (total retinal detachment). In all of the gestational weeks, each child was classified according to the most advanced ROP stage observed. Infants were examined according to a routine protocol, and were examined for ROP after 4 weeks. Screening was initiated when the baby reached a postmenstrual age (PMA) of 32 weeks, and was performed once or twice per week depending on the severity of the disease until the retina was fully vascularized. The pupils were dilated with cyclopentolate hydrochloride (0.25%), tropicamide (0.25%), and neosynephrine hydrochloride (1.25%), one hour before examination. The examination was performed by indirect ophthalmoscopy with a 25-diopter lens using indentation and a lid speculum by a trained pediatric ophthalmologist who had no knowledge of the IGF-1 and IGFBP3 levels or nutritional protocols. Care was taken to minimize pain and stress during the examinations. Retinopathy of prematurity in stages 0 to 4 (International Classification of Retinopathy of Prematurity) and treatment (by laser ablation) were noted.
Laboratory tests and methods
Serum IGF–I levels were determined by ELISA kits (Biosource Human IGF–1 Ctyelisa, Biosource, Rue de l'Industrie 8, B–1400 Nivelles; sensitivity 4.9 ng/ml), and IGFBP3 levels were determined by ELISA kits (Biosource Human IGFBP3 Ctyelisa, Biosource, Rue de l'Industrie 8, B–1400 Nivelles; sensitivity 10.5 ng/ml). We measured IGF-1 and IGFBP3 plasma levels in the postnatal first two hours of life and then weekly from week 1 to 6. Blood samples were taken through an indwelling venous catheter, by venipuncture. All samples were drawn at least two hours after the last peroral feeding, except when the infant received continuous feeding through a nasogastric tube. The EDTA tubes were spun immediately at 1000 g for ten minutes, and the plasma was separated and frozen at 70°C until analysis[11].
Statistical analysis
Statistical analysis was performed with SPSS 11.5.0 (Statistical Package for the Social Sciences SPSS Inc. 2002, Chicago, IL), MedCalc 8.1.0.0 (MedCalc Statistical Software for Biomedical Research, 2005 Frank Schoonjans, Mariakerke, Belgium) and StatsDirect (release 2.4.3, Cheshire, UK). Student's t-test was used for normally distributed continuous variables. Categorical data were analyzed using chi square and the Fisher exact test. An analysis of variance test for repeated measures was used to evaluate the changes in all laboratory values. The number needed to treat (NNT) was calculated with StatsDirect. Specificity and sensitivity tests for IGF-1 and IGFBP3 in infants who were diagnosed as ROP were calculated by ROC analysis. P<0.05 was considered to be statistically significant.
Findings
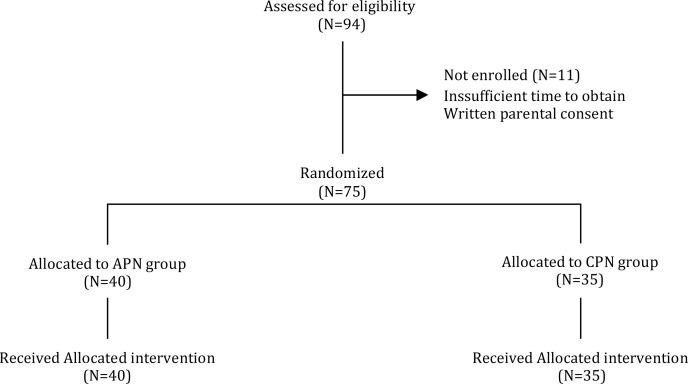
The total number of neonates of 25-31 weeks gestation admitted to the participating units during the study period was 94. Of these, eight were not eligible: four had intrauterine growth retardation, three had congenital heart anomalies (two suffered from tetralogy of Fallot and one had an atresia of tricuspid valve), one had severe asphyxia characterized by seizures. Of the remaining 86 infants, 11 were not randomized because of insufficient time to obtain written parental consent. 75 neonates were included in the study (40 neonates in the APN group and 35 neonates in the CPN group (Fig. 1). Forty-two (56%) neonates were male and 33 (44%) female. Demographic and anthropometric characteristics of the groups are shown in Table 1. Nutritional characteristics of the groups are presented in Table 2.
Fig. 1.
Flow diagram of eligible newborns.
The number of neonates not randomized and the reasons for non-enrolment are provided.
APN: Aggressive Parenteral Nutrition; CPN: Conventional Parenteral Nutrition
Table 1.
Demographic and anthropometric characteristics of the groups
| Parameter | APN (n = 40) | CPN (n = 35) | P. value |
|---|---|---|---|
| Weeks of gestation, (range) | 28.7 (1.5) [25-31] | 29.0 ± 1.1 [25-31] | 0.3 |
| Birth weight (g), | 1210 (176.2) | 1278 (145.5) | 0.07 |
| Birth height (cm), | 41.1 (3.4) | 40.7 (3.2) | 0.6 |
| Head circumference (cm), | 29.4 (2.2) | 29.1 (2.0) | 0.5 |
| Maternal age | 24.4(3.6) | 23.1(4.2) | 0.2 |
| Cesarean section, n (%) | 25.0 (68) | 23.0 (76) | 0.07 |
| Maternal steroids, n (%) | 27.0(68) | 25.0 (76) | 0.9 |
| Need for laser ablation, n (%) | 0 | 2.0(5.7) | 0.2 |
APN: Aggressive parenteral nutrition; CPN: Conventional parenteral nutrition, mean (SD)
Table 2.
Nutritional characteristics of the groups
| Parameter | APN (n = 40) | CPN (n = 35) | P. Value |
|---|---|---|---|
| Duration of parenteral nutrition (days) | 13.1 (3.5) | 13.5 (3.0) | 0.6 |
| Full enteral feeding time (days) | 15.1 (5.8) | 14.8 (5.5) | 0.8 |
| Energy intake (kcal/kg/day) | 121,5(35.5) | 115,5(28.5) | 0.4 |
| Protein intake (g/kg) | 3.52 (0.7) | 3.2 (0.5) | 0.02 |
| Lipid intake (g/kg) | 6.6 (1.5) | 6.1 (0.6) | 0.03 |
| Weight gain (g) | 115(75) | 100(80) | 0.4 |
* Student's t-test, mean (SD); APN: Aggressive parenteral nutrition; CPN: Conventional parenteral nutrition
The ROP ratio was detected for 2 (5.0%) neonates in the APN group and 11 (31.4%) neonates in the CPN group (P=0.004). There were two neonates with ROP stage 2 in APN group. There were five neonates with ROP stage 1 and six neonates with ROP stage 2. The APN group showed a ROP risk reduction of 5% (2 from 40); the number of infants that needed treatment averaged 3.7 (2.7 to 5.2).
Serum IGF-1 and IGFBP3 levels were significantly different in the APN group between the ROP diagnosed and non-ROP neonates from third week of life. The IGF-1 and IGFBP3 levels were markedly lower among the CPN group with ROP developed after the postnatal third week of life (29.1±1.9 vs 24.1±1.6 ng/ml, P<0.05) and (1220±54 vs 980±95 ng/ml, P<0.05) (Table 3, 4). ROP was found to correlate negatively with IGF-1 and IGFBP3 levels at postnatal third week [IGF-1 (r2:-0.65), IGFBP3 (r2:-0.51)].
Table 3.
Comparison of Insulin-like growth factor-1 (ng/mL) levels in the study groups
| Time | APN and ROP (n = 2) | APN and no ROP (n = 38) | P. value* | CPN and ROP (n = 11) | CPN and no ROP (n = 24) | P. value* |
|---|---|---|---|---|---|---|
| First day | 18.2 (1.1) | 19.1 (1.4) | 0.4 | 17.6 (1.6) | 18.0 (1.8) | 0.5 |
| 1st week | 20.3 (1.2) | 21.5 (1.1) | 0.1 | 14.5 (2.1) | 15.6 (1.5) | 0.08 |
| 2ndweek | 24.1 (1.7) | 25.4 (1.3) | 0.2 | 20.2 (1.5) | 21.1 (1.8) | 0.2 |
| 3rd week** | 29.1 (1.9) | 31.8 (1.5) | 0.01 | 24.1 (1.6) | 25.2 (1.8) | 0.08 |
| 4th week | 34.7 (3.2) | 37.5 (1.8) | 0.04 | 26.3 (1.8) | 27.2 (1.6) | 0.1 |
| 6th week | 37.9 (3.7) | 39.8 (1.1) | 0.04 | 31.2 (2.9) | 32.8 (2.1) | 0.07 |
APN: Aggressive parenteral nutrition; CPN: Conventional parenteral nutrition
ANOVA t-test
3rd week correlation coefficient was revelaled [IGF-1 (r2:-0.65), IGFBP3 (r2:-0.51)]
Table 4.
Comparison of insulin growth factor binding protein-3 levels in the study groups
| Time | APN and ROP (n = 2) | APN and no ROP (n = 38) | P. value* | CPN and ROP (n = 11) | CPN and no ROP (n = 24) | P. value* |
|---|---|---|---|---|---|---|
| First day | 535 (74) | 552 (65) | 0.7 | 488 (98) | 496 (75) | 0.8 |
| 1st week | 825 (55) | 867 (42) | 0.2 | 674 (65) | 702 (52) | 0.2 |
| 2ndweek | 1065 (45) | 1102 (48) | 0.3 | 896 (75) | 927 (61) | 0.2 |
| 3rd week** | 1220 (54) | 1268 (31) | 0.04 | 980 (95) | 1024 (52) | 0.08 |
| 4th week | 1578 (79) | 1632 (28) | 0.01 | 1134 (132) | 1198 (94) | 0.1 |
| 6th week | 1674 (97) | 1736 (35) | 0.03 | 1328 (90) | 1377 (81) | 0.1 |
APN: Aggressive parenteral nutrition; CPN: Conventional parenteral nutrition
ANOVA t-test
3rd week correlation coefficient was revelaled [IGF-1 (r2:-0.65), IGFBP3 (r2:-0.51)]
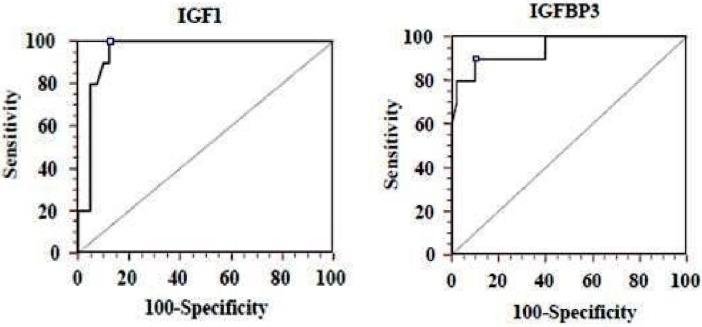
According to the ROC curve, IGF-1 levels were found to be 100% sensitive (CI: 69.0 to 100.0) and 87.5% specific for the cut-off point 30 ng/ml CI: 73.2 to 95.8), and IGFBP3 levels were found 90%sensitive (CI: 55.5 to 98.3) and 90% specific (CI: 76.3 to 97.1) for the cut-off point 1300 ng/ml at the third week (Fig. 2). Need for laser ablation therapy ratios of the groups are shown in Table 1. Laser ablation was applied to two of the CPN group neonates. One of the neonates developed plus disease in zone 1, while the other neonate developed stage 2 ROP with plus disease in zone 2. There was no difference between the need for laser ablation and ROP structural outcome.
Fig. 2.
Left: Cut-off point level 30 ng/ml for IGF-1 (sensitivity 100%, specificity 87.5%)
Right: Cut-off point level 1300 ng/ml for IGFBP3 (sensitivity 90%, specificity 90%)
IGF-1: Insulin-like growth factor 1
Discussion
Progress in neonatal intensive care in recent years has led to an increased survival of very low birth weight infants with an increasing incidence of ROP[12]. ROP incidence had been linked according to countries’ levels of development, neonatal intensive care, and survival of the preterm infants. In a recently reported study in Turkey, ROP incidence was shown to be 32.1%, while another study reported ROP incidence 32% in <2000g infants in Iran. In our study, ROP incidence results were similar to these studies, except for the results of APN group[13, 14].
Guidelines for protein and energy requirements have recently been revised to consider the fetal reference related to lean body mass and protein gain, rather than weight gain, with revised protein intakes up to 4.4 g/kg/day at 26 to 30 weeks gestation. Early initiation of PN increased the positive nitrogen balance, and a higher energy intake was achieved without increasing the risk of metabolic acidosis, hypercholesterolemia or hypertriglyceridemia, and provided a decreased loss of amino acid in premature infants in the first days of life[15, 16].
The IGF-1 level is a nutrition-dependent parameter, and increased IGF-1 levels correlate positively with gestational age and birth weight[17]. Previous studies have demonstrated that a prolonged period of a low level of serum IGF-1 in preterm infants is strongly associated with ROP. Engström et al[18] reported that the relationship between protein intake and IGF-1 was positive, as was the relationship between weight gain and IGF-1. According to Yeung et al[19], the IGF-1 level correlated positively with protein intake, and nitrogen retention. Smith et al[20] provided normative data for IGF-1 and IGFBP3 in premature infants, and confirmed the relationship of protein intake to the maintenance of serum levels of these peptides. In our study overall IGF-1 and IGFBP3 levels were higher in the APN versus the CPN group, and ROP development was higher in the CPN compared to the APN group. This study also showed that early APN positively affects IGF-1 and IGFBP3 levels, and the incidence of ROP is reduced by increasing the levels of IGF-1 and IGFBP3 in neonates <32 weeks of gestation. Although both groups received equal amounts of protein and lipid from the third day of life, mean weight gain and full enteral feeding time of both groups were similar but the levels of IGF-1 and IGFBP3 were different. For these diversities, we might speculate that the two-day difference in receiving the amounts of proteins and lipids had effects on the levels of IGF-1 and IGFBP3. van den Akker et al[21] demonstrated that protein anabolism produced by administration of early amino acids is accomplished through an increase in protein synthesis and not from a decrease in protein breakdown. This increase in protein synthesis might be caused by increased synthesis of IGF-1 and IGFBP3. However the exact mechanism was not clear.
In our recently published study, we tested the hypothesis that giving higher amounts of amino acids and lipids to infants born at <34 gestational weeks might improve growth in the 40th week of gestation and had a positive preventive effect on development of ROP in 50 preterm infants. A positive relationship between high protein intake and IGF-1 and IGFB3 levels were seen. However, we measured IGF-1 and IGFBP3 levels only at postnatal 40th week. We thought not obtaining serial measurements of IGF-1 and IGFBP3 levels might restrict the results. In this present study, we wanted to confirm the relationship between ROP and serial measurements of IGF-1 and IGFBP3 in gestational age of < 32 weeks preterm infants.
IGFBP3 concentrations in serum were reduced by protein-calorie malnutrition and raised with refeeding, particularly when high protein nutrition was used[22].
Löfqvist et al[23]. suggested that IGFBP3, acting independently of IGF-1, helped to prevent oxygen-induced vessel loss and promote vascular regrowth after vascular destruction in vivo in a dose-dependent manner, resulting in less retinal neovascularization. In the present study, we found that IGFBP3 levels similar to IGF-1 levels were higher in the APN group and were negatively correlated with ROP.
Löfqvist et al[24] reported that low serum IGF-1 levels in the third postpartum week correlated with increased risk of ROP. Hellstrom et al[25] found IGF-1 levels lower than 30 ng/ml in preterms between 30-35 weeks of gestation as a risk factor for the development of ROP stages 2-5. Low IGF-1 levels for a long period of time affected the development of ROP. In another study, it was reported that IGF-1 levels lower than 24 ng/ml at postnatal 4-6 weeks were related to ROP. Additionally it was shown that early nutrition could increase IGF-1 levels in early periods and prevent ROP stage 1[23]. In our study, a negative correlation between ROP and IGF-1 levels was confirmed, and through ROC analysis at the postnatal third week IGF-1 was demonstrated to be a sensitive marker for ROP. These results might point to a cut-off value for IGF-1 protective effect for ROP, ie, when overall IGF-1 levels were higher in the APN versus the CPN group, but in the APN group some neonates developed ROP, this might mean that IGF-1 levels simply being higher is not enough; rather, being higher above a cutoff value might prevent ROP.
Timely treatment for ROP is effective in preventing severe vision impairment. Recent evidence has shown benefit from earlier treatment[10]. Our cases were treated according to a consensus statement of an international group of retinopathy of prematurity experts. According to the concensus[10], laser ablation was applied to two neonates with ROP in the CPN group. No complications after treatment were noted.
Limitations of the research: There are some limitations in our study that should be noted: Oxygen therapy and monitoring, oxygen levels, hypercapnia, diagnosis of bronchopulmonary dysplasia, and a need for blood transfusion might be possible confounding factors in this study.
Conclusion
IGF-1 levels were higher in the aggressive PN group versus the conventional PN group. IGF-1 was demonstrated as being a sensitive marker for ROP for neonates < 32 weeks of gestation. However, we suggest that further and larger randomized controlled studies are needed to confirm the relationship between aggressive parenteral nutrition, IGF-1, IGFBP3 and ROP.
Acknowledgment
The authors would like to thank nurses of NICU at Sisli Children's Hospital.
Conflict of Interest
None
References
- 1.Hagadorn JI, Richardson DK, Schmid CH, et al. Cumulative illness severity and progression from moderate to severe retinopathy of prematurity. J Perinatol. 2007;27(8):502–9. doi: 10.1038/sj.jp.7211780. [DOI] [PubMed] [Google Scholar]
- 2.Bang P, Westgren M, Schwander J, et al. Ontogeny of insulin-like growth factor-binding protein-1, -2, and -3: quantitative measurements by radioimmuno-assay in human fetal serum. Pediatr Res. 1994;36(4):528–36. doi: 10.1203/00006450-199410000-00020. [DOI] [PubMed] [Google Scholar]
- 3.D'Ercole AJ, Willson DF, Underwood LE. Changes in the circulating form of serum somatomedin-C during fetal life. J Clin Endocrinol Metab. 1980;51(3):674–6. doi: 10.1210/jcem-51-3-674. [DOI] [PubMed] [Google Scholar]
- 4.Langford K, Nicolaides K, Miell JP. Maternal and fetal insulin-like growth factors and their binding proteins in the second and third trimesters of human pregnancy. Hum Reprod. 1998;13(5):89–93. doi: 10.1093/humrep/13.5.1389. [DOI] [PubMed] [Google Scholar]
- 5.Hellstrom A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87(7):3413–6. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 6.Ballard JL, Khoury JC, Wedig K, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 7.Usher R, McLean F. Intrauterine growth of live-born Caucasian infants at sea level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks of gestation. J Pediatr. 1969;74(6):901–10. doi: 10.1016/s0022-3476(69)80224-6. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: Direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98(10):5804–8. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Can E, Bülbül A, Uslu S, et al. Effects of aggressive parenteral nutrition on growth and clinical outcome in preterm infants. Pediatr Int. 2012;54(6):869–74. doi: 10.1111/j.1442-200X.2012.03713.x. [DOI] [PubMed] [Google Scholar]
- 10.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 11.Kajantie E, Dunkel L, Rutanen EM, et al. IGF-I, IGF binding protein (IGFBP)-3, phosphoisoforms of IGFBP-1, and postnatal growth in very low birth weight infants. J Clin Endocrinol Metab. 2002;87(5):2171–9. doi: 10.1210/jcem.87.5.8457. [DOI] [PubMed] [Google Scholar]
- 12.Kim TI, Sohn J, Pi SY, et al. Postnatal risk factors of retinopathy of prematurity. Paediatr Perinat Epidemiol. 2004;18(2):130–4. doi: 10.1111/j.1365-3016.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 13.Feghhi M, Altayeb SM, Haghi F, et al. Incidence of retinopathy of prematurity and risk factors in the south-western region of Iran. Middle East Afr J Ophthalmol. 2012;19(1):101–6. doi: 10.4103/0974-9233.92124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpay A, Uğurbaş SH. Incidence and risk factors for retinopathy of prematurity in the west Black Sea region, Turkey. Turk J Pediatr. 2012;54(2):113–8. [PubMed] [Google Scholar]
- 15.Parish A, Bhatia J. Early aggressive nutrition for the premature infant. Neonatology. 2008;94(3):211–4. doi: 10.1159/000143724. [DOI] [PubMed] [Google Scholar]
- 16.Simmer K. Aggressive nutrition for preterm infants-benefits and risks. Early Hum Dev. 2007;83(10):631–4. doi: 10.1016/j.earlhumdev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Lo HC, Tsao LY, Hsu WY, et al. Relation of cord serum levels of growth hormone, insulin-like growth factors, insulin-like growth factor binding proteins, leptin, and interleukin-6 with birth weight, birth length, and head circumference in term and preterm neonates. Nutrition. 2002;18(7-8):604–8. doi: 10.1016/s0899-9007(01)00811-5. [DOI] [PubMed] [Google Scholar]
- 18.Engström E, Niklasson A, Wikland KA, et al. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005;57(4):605–10. doi: 10.1203/01.PDR.0000155950.67503.BC. [DOI] [PubMed] [Google Scholar]
- 19.Yeung MY, Smyth JP. Nutritionally regulated hormonal factors in prolonged postnatal growth retardation and its associated adverse neurodevelopmental outcome in extreme prematurity. Biol Neonate. 2003;84(1):1–23. doi: 10.1159/000071438. [DOI] [PubMed] [Google Scholar]
- 20.Smith WJ, Underwood LE, Keyes L, et al. Use of insulin-like growth factor I (IGF-I) and IGF-binding protein measurements to monitor feeding of premature infants. J Clin Endocrinol Metab. 1997;82(12):3982–8. doi: 10.1210/jcem.82.12.4452. [DOI] [PubMed] [Google Scholar]
- 21.van den Akker CH, te Braake FW, Wattimena DJ, et al. Effects of early amino acid administration on leucine and glucose kinetics in premature infants. Pediatr Res. 2006;59(5):732–5. doi: 10.1203/01.pdr.0000214990.86879.26. [DOI] [PubMed] [Google Scholar]
- 22.Löfqvist C, Chen J, Connor KM, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci USA. 2007;104(25):10589–94. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löfqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124(12):1711–8. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 24.Löfqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulin like growth factor I. Arch Ophthalmol. 2009;127(5):622–7. doi: 10.1001/archophthalmol.2009.69. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87(7):3413–6. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]