Abstract
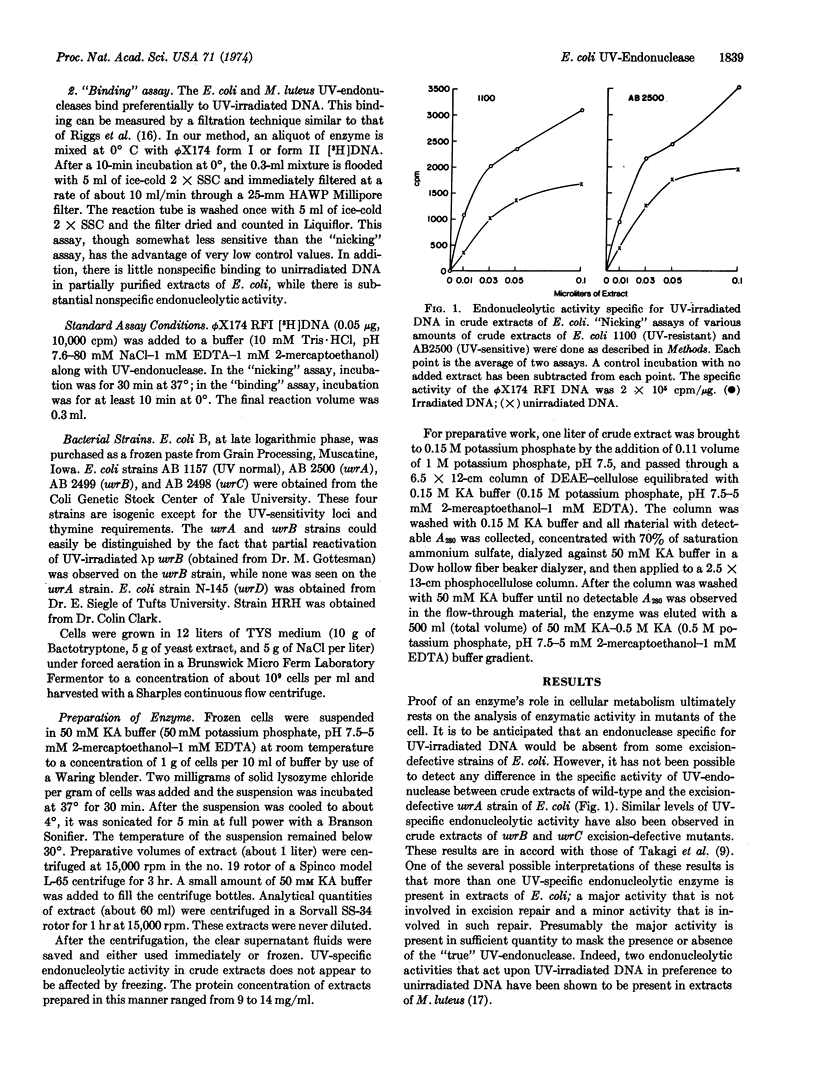
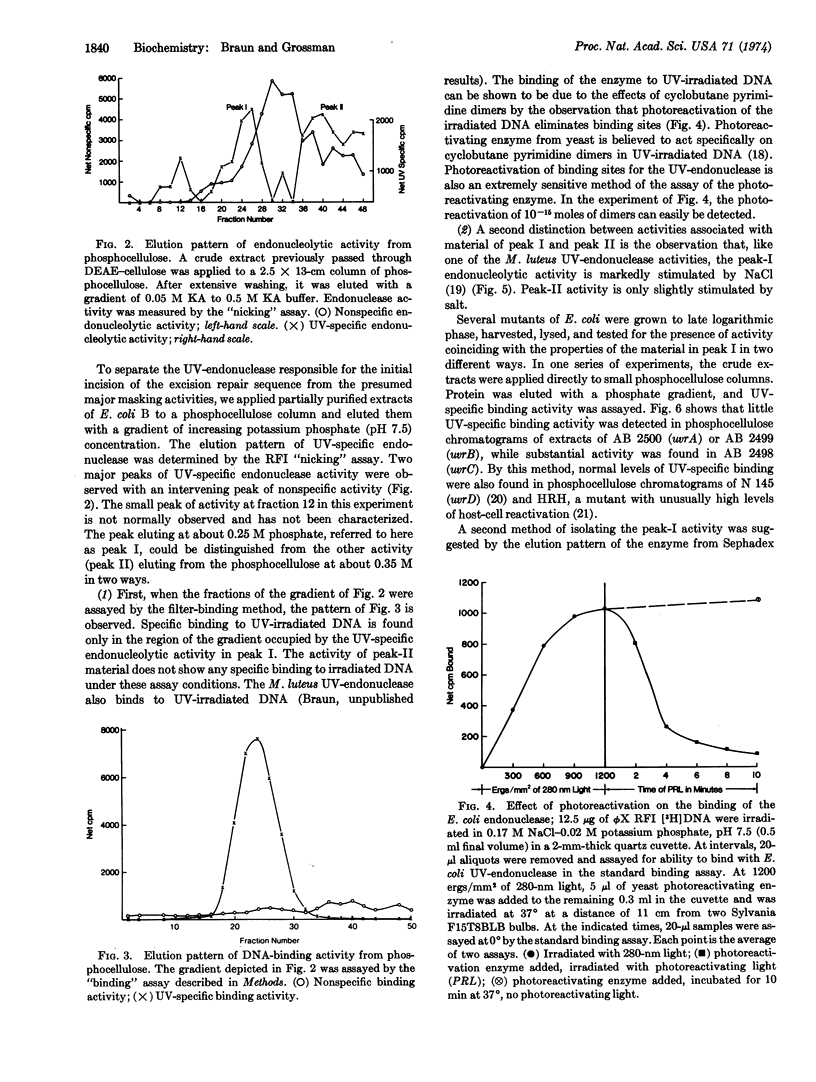
At least two endonucleolytic activities that preferentially incise ultraviolet (UV)-irradiated DNA exist in extracts of E. coli. These two activities can be separated by phosphocellulose chromatographic fractionation. The subject of this paper is one of these activities, which elutes from phosphocellulose with 0.25 M potassium phosphate, pH 7.5. This endonucleolytic activity specific for UV-irradiated DNA is absent from partially purified extracts of uvrA and uvrB mutants, which are defective in excision of pyrimidine dimers, but is present in normal amounts in the uvrC excision-defective mutant. The enzyme binds very tightly and specifically to UV-irradiated DNA. Binding can be prevented by prior treatment of the irradiated DNA with photoreactivating enzyme. This binding activity is absent in uvrA and uvrB mutants, but present in uvrC and uvrD mutants.
Keywords: DNA repair, DNA-protein binding, membrane filtration
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Richardson C. C. An endonuclease induced after infection of Escherichia coli with bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1970 Dec 10;245(23):6285–6291. [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA, 1. Purification of two enzymes involved in the excision of thymine dimers from ultraviolet-irradiated DNA. Proc Natl Acad Sci U S A. 1969 May;63(1):144–151. doi: 10.1073/pnas.63.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler I., Kushner S. R., Grossman L. In vivo role of the UV-endonuclease from Micrococcus luteus in the repair of DNA. Nat New Biol. 1971 Nov 10;234(45):47–50. doi: 10.1038/newbio234047a0. [DOI] [PubMed] [Google Scholar]
- Minato S., Werbin H. Spectral properties of the chromophoric material associated with the deoxyribonucleic acid photoreactivating enzyme isolated from baker's yeast. Biochemistry. 1971 Nov 23;10(24):4503–4508. doi: 10.1021/bi00800a025. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Murayama I. Deoxyribonucleic acid damage by monofunctional mitomycins and its repair in Escherichia coli. J Bacteriol. 1972 Feb;109(2):475–483. doi: 10.1128/jb.109.2.475-483.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Setlow R. B. Endonucleolytic activity from Micrococcus luteus that acts on -ray-induced damage in plasmid DNA of Escherichia coli minicells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2927–2931. doi: 10.1073/pnas.69.10.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive, radiation-sensitive mutant of Escherichia coli. II. DNA replication. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1195–1202. doi: 10.1073/pnas.64.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Schekman R. W., Iwaya M., Bromstrup K., Denhardt D. T. The mechanism of replication of phi X174 single-stranded DNA. 3. An enzymic study of the structure of the replicative form II DNA. J Mol Biol. 1971 Apr 28;57(2):177–199. doi: 10.1016/0022-2836(71)90340-8. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Johansen I. Incisions in ultraviolet irradiated circular bacteriophage lambda DNA molecules in excision proficient and deficient lysogens of E. coli. Mol Gen Genet. 1973;123(2):173–184. doi: 10.1007/BF00267333. [DOI] [PubMed] [Google Scholar]
- Takagi Y., Sekiguchi M., Okubo S., Nakayama H., Shimada K., Yasuda S., Nishimoto T., Yoshihara H. Nucleases specific for ultraviolet light-irradiated DNA and their possible role in dark repair. Cold Spring Harb Symp Quant Biol. 1968;33:219–227. doi: 10.1101/sqb.1968.033.01.025. [DOI] [PubMed] [Google Scholar]
- Taketo A., Yasuda S., Sekiguchi M. Initial step of excision repair in Escherichia coli: replacement of defective function of uvr mutants by T4 endonuclease V. J Mol Biol. 1972 Sep 14;70(1):1–14. doi: 10.1016/0022-2836(72)90160-x. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]



