Abstract
Objective
We examined the preventive effect of probiotic and antibiotics versus antibiotics alone, in children with recurrent urinary tract infections (RUTI) in a preliminary randomized clinical trial.
Methods
Between March 2007 and April 2011, children with the history of RUTI and unilateral vesicoureteral reflux (VUR) were randomly assigned to receive concomitant probiotic and antibiotics (Lactobacillus acidophilus and bifidobacterium lactis, 107/ml, as 0.25 ml/kg three times a day regimen in addition to Nitrofurantoin, 1mg/kg daily (group I). In group II, all children received conventional prophylactic antibiotics alone (Nitrofurantoin, 1 mg/kg daily). Randomization was performed via using the random numerals table in a 1:1 manner with stratification by sex, age and grade of reflux. The urine examinations were done monthly and the incidence of UTI was evaluated in these two groups.
Findings
Forty-one children (age: 8.3±3.1 years) in group I and 44 children (age: 8.0±3.0 years) in group II were compared. During the course of three years, 39% in group I and 50% of participants in group II experienced RUTIs (P=0.4). Incidences of UTI - febrile and afebrile - reduced in both groups without any significant differences after two years of prophylaxis. Also, incidence of afebrile UTIs did not significantly differ (0.51±1.30 and 0.81±1.41 respectively, P =0.3); however, the incidence of febrile UTIs in particular were lower in group I (0.00±0.00 versus 0.13±0.40, P =0.03) in the last year.
Conclusion
The consumption of probiotic and antibiotics in children with RUTI is safe and more effective in reducing the incidence of febrile UTI in comparison to prophylactic antibiotics alone.
Keywords: Lactobacillus Acidophilus, Bifidobacterium Lactis, Antibiotic Prophylaxis, Vesicoureteral Reflux, Urinary Tract Infection
Introduction
Urinary tract infection (UTI) is a common entity in children. Up to the age of seven, 8% of girls and 2% of boys will experience at least one episode of UTI. Escherichia coli is responsible for about 80% of febrile and afebrile UTIs[1, 2]. Vesicoureteral reflux (VUR) is noticed in 70% of children with UTI and poor renal outcome is highly associated with the UTI in the setting of VUR[3, 4, 5]. In addition to surgical methods in prevention of symptomatic UTIs in children with VUR, antibiotics such as Cephalosporin, Trimethoprim-Sulfamethoxazole (TMP/SMX) and Amoxicillin–Clavulanic acid are prescribed at half the therapeutic doses[3, 4]. In a recent study, in 56 children with breakthrough UTI on prophylactic antibiotics 59% were resistant to the prophylactic antibiotic. Resistant uropathogens were more observed in children on Cefixime [6].
Antibiotics can prevent complications of infections such as pyelonephritis and renal scarring in susceptible children. Although long-term antibiotic prophylaxis reduces symptomatic UTIs, benefits should be considered against the risk of microbial resistance[7]. Many attempts have been made for a replacement method to prevent recurrent UTI (RUTI), but no approved medications have yet been suggested. Clinical searches suggest alternatives including the consumption of cranberry, mannose, and probiotics[8–10]. Bacteria in the stool are causative agents for almost all cases of ascending UTIs, so it is logical to assume that diet may affect the risk of UTI recurrences[8, 9].
Live microorganisms capable to confer a health effect on the host when consumed in adequate doses define probiotics[11]. Recently the benefits and safety of probiotics have been assessed in the field of gastroenterology from constipation to antibiotic associated diarrhea, irritable bowel syndrome to inflammatory bowel disease; however, the results were controversial[12–15]. Furthermore, the recent studies demonstrate the benefits of some probiotic strains like Lactobacillus rhamnosus and Lactobacillus fermentum against urogenital infections and in the urinary tract, however, many debates still exist on this issue[11, 14–16]. There have been some evidences that probiotics can prevent colonization of uropathogenic bacteria and have potential benefits on prevention of renal injury-inducing UTIs[17]. We designed a preliminary pilot study to investigate the efficacy of probiotic administration in addition to antibiotics for prevention of RUTI in children.
Subjects and Methods
Setting and participants
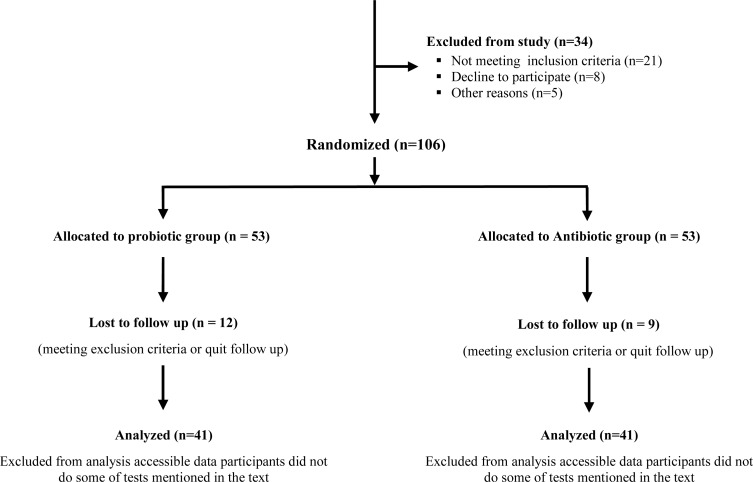
We performed this prospective pilot study at Children's Medical Center, Pediatric Center of Excellence (Tehran University of Medical Sciences, Tehran, Iran) from November 2007 to November 2011. Ethics committee of Tehran University of Medical Sciences approved this study. Participant's parents signed the informed consent indicating the aim of study and interventions. One hundred and forty children were assessed for eligibility and 106 of them who met the inclusion criteria were randomized. In this study, according to literature, UTI was defined as an inflammatory response of the urothelium to bacterial invasion that is usually associated with bacteriuria and pyuria. Bacteriuria was considered when a single bacterial species was isolated in concentrations equal or greater than 100000 colony forming units/ml of mid-stream clean-catch urine samples. Pyuria was also diagnosed when greater than or equal to 10 leucocytes per µl were reported. In addition, RUTI was defined as a recurrent infection that occurs after documented successful resolution of an antecedent infection. Children aged 3 to 15 years with the history of RUTIs in the setting of unilateral VUR and poor response to antibiotics prophylaxis (with four or more times incidence of symptomatic UTIs one year prior to enrollment into this study) were included in the study. Exclusion criteria included history of secondary VUR, bilateral VUR and urogenital anomalies except unilateral VUR (e.g. urethral anomalies, ano-rectal malformations, neuropathic bladder, ureterocele, concomitant upper tract anomalies, urethra-pelvic junction obstruction, megaureter etc.). Voiding dysfunction and secondary VUR were also ruled out according to full urologic and neurologic examination, evaluation for dysfunctional elimination syndrome and performing urodynamic study, including voiding diary and annual uroflowmetry. In addition, children who required interventions that interfered with our study protocol as well as those with concurrent co-morbidities and bilateral VUR were excluded (Fig. 1). Patients with constipation were also enrolled in the study only after they were treated appropriately.
Fig. 1.
Flow diagram of the study
Allocation and intervention
The number of required patients for this pilot study was calculated according to previous guidelines[18]. Randomization was performed via using the random numerals table in a 1:1 manner (computer generated) with stratification by sex, age and grade of reflux. In this regard, patients were randomly assigned to each one of the probiotic plus antibiotic and antibiotic groups. Group I (probiotic + antibiotic group) received daily probiotic supplementation as probiotic yoghurt (0.25 ml/kg from 100 ml yoghurt containing 107 colony forming-unit (CFU)/ml of Lactobacillus acidophilus (LA5) and Bifidobacterium lactis (BB12) (R&D department of Iran Dairy Industries Pegah Co., Tehran, Iran) in addition to Nitrofurantoin 1 mg per kg at night. However, group II (antibiotic group) received only conventional daily antibiotics for prophylaxis (Nitrofurantoin1 mg per kg at night). All the patients in both arms underwent a run in period of receiving plain yogurt 100 ml daily which was produced by the same company and did not contain any probiotics for two weeks. Whenever patients had febrile UTI suggestive of pyelonephritis, therapeutic doses of antibiotics were used according to approved guidelines in both groups.
Probiotic yogurt preparation and production
For this special probiotic yogurt production, milk was heated at 85°C for 30 minutes and cooled to the fermentation temperature. After inoculation with the starter culture (Lactobacillus acidophilus (LA-5), Bifidobacterium (BB-12) along with yogurt bacteria (Chr. Hansen, Denmark), it was distributed into the 100 ml plastic retail container, sealed and incubated (at 37°C) and finally cooled and stored at 4-6°C. The shelf time was 2-3 weeks without significant reduction in total bacterial count. The probiotic yogurt prepared for group I was produced with different flavors and thus was attractive enough to ensure adequate adherence of children to the 100 ml daily regimen. Additionally, to ensure that children sufficiently consumed the distributed yogurts/pills, their parents were convinced and instructed accordingly, and the yogurts/pills were delivered to the parents at the office every two weeks.
The meantime of therapy with probiotic for each patient in group I was two years. In this regard, every patient received 100 ml package of probiotic yogurts as 0.25 ml/kg three times a day regimen for two and half months. Afterwards, the patient did not receive probiotic for 15 days. This schedule was repeated for 2 times in the first 6 months. For the second 6 months, the patients underwent three episodes comprising 45 days of probiotic-received with 15 days probiotic-free intervals. In the third 6 months, they received probiotics for 1 month, and in the next one month, they did not receive any probiotic at all. Finally, for the last 6 months, they received probiotics for 15 days and in the remaining 45 days, they did not receive any yogurt. It is noteworthy that according to the current literature, Nitrofurantoin - at the prescribed doses - does not affect the bacterial flora of the gastrointestinal system.
The bacterial count was checked and if the initial bacterial count was more than 10 million /ml, project carrier transferred the yogurt in cool containers to our clinic for immediate distribution. From each of the yogurts that was delivered to the patients, a sample was obtained, kept at standard conditions for 10 days and sent for assessing bacterial count to ensure that the initial bacterial count was constant 10 days after the distribution. During the study period, only two samples were contaminated with molds that were due to inappropriate closing of the containers and the bacterial count of other samples were constant for the obtained samples. Moreover, the bacterial species applied in the production of the yoghurt in our study were unique and specifically different from that of other dairy products at shops (i.e. ice cream, drinking yoghurt, etc.). The technology for production of this type of yogurt with special probiotic count was unique and so we had patent formula and production process in our country under our names.
Outcomes and follow up
Patients with positive urine culture (10 5CFU/ml or more counts) of a pathogenic organism in midstream urine sample were considered to have UTI. Pediatric nephrologist/urologist assessed presence of urinary tract infection and systemic symptoms (such as fever, flank tenderness, shivering etc.), in order to detect febrile UTI as soon as possible. Considering that timed voiding is an important factor in the prevention of UTI, all of the parents of participants were explained to appropriately train their children for voiding on a regular basis every two hours. Moreover, the patients have been instructed not to be in rush at voiding time. All of the patients were instructed by their parents to stay seated in the bathroom in order to empty their bladder completely. Such instructions were also given to the school counselors. Urine analysis (U/A), urine culture (U/C) and patient examination was done monthly. In addition to our planned investigations, participants and their parents were educated to refer to our hospital when they showed symptoms that were suggestive of UTI. Asymptomatic and fine patients occasionally faxed their U/A and U/C reports, but ill patients referred to the hospital for performing more laboratories and imaging assessments.
In addition to dimercaptosuccinic Acid (DMSA) scan, VCUG was performed for grading of VUR in boys and RNC was performed in girls at the first and end of the study as well as at the follow up visits on an annual basis. The definition for DMSA was based on nuclear medicine service report, kidney size, renal function and scar as well as new scar formation. In addition, the International Reflux Study in Children classification was used to grade reflux from I to V on VCUG[19]. For RNC, a mild, moderate and severe grading was applied.
Our primary outcome measure was investigating the incidence of UTI in children who received probiotic plus antibiotics in comparison with the antibiotic group. Our secondary outcome measures were to investigate new scarring and kidney function after prophylaxis, comparison of different causative organisms of recurrent UTI in both groups and their susceptibility to Nitrofurantoin.
Statistical analysis
Data were analyzed using SPSS (Statistical Package for the Social Sciences, version 16.0, SPSS Inc, Chicago, Illinois, USA). The numerical outcome measures were tested using Student's t test. The chi square test was used for comparing categorical variables. P. values less than 0.05 were considered statistically significant.
Findings
At the end of the study, in group I (probiotic+ antibiotic) 41 children (26 female and 15 male) and in the group II (antibiotic) 44 children (30 female and 14 male) remained for analysis. Mean (standard deviation) age of patients in intervention and control groups were 8.3 (3.1) and 8.0 (3.0), respectively. Other demographic data and grading of VUR in our subjects have been shown in Table 1.
Table 1.
Grading of vesicoureteral reflux in probiotic + antibiotic and antibiotic group (P>0.05)
| VUR Grade | Probiotic + Antibiotic group | Antibiotic group | ||
|---|---|---|---|---|
| Variable | Before treatment | After treatment | Before treatment | After treatment |
| Normal | 0.0 | 20 (48.9) | 0.0 | 21 (47.7) |
| Grade I | 11 (26.8) | 6 (14.6) | 13 (29.5) | 8 (18.2) |
| Grade II | 13 (31.7) | 6 (14.6) | 15 (34.1) | 7 (15.9) |
| Grade III | 11 (26.8) | 6 (14.6) | 10 (22.8) | 5 (11.4) |
| Grade IV | 4 (9.8) | 2 (4.9) | 4 (9.1) | 2 (4.5) |
| Grade V | 2 (4.9) | 1 (2.4) | 2 (4.5) | 1 (2.3) |
VUR: Vesicoureteral reflux
Incidence of UTI was assessed annually and decreased in both groups throughout three years of receiving prophylaxis. Comparison of incidence of UTI per person per year during the years of study showed no significant difference between the two groups as shown in Table 2. Interestingly, in the last year of treatment, no febrile UTI was observed in the children who received probiotic and antibiotic prophylaxis in comparison with antibiotic treated group (P = 0.03) (Table 2).
Table 2.
Comparison of incidences of UTIs (per person per year) between two groups of study
| Groups | Year 1 | Year 2 | Year 3 | P. Value | |||
|---|---|---|---|---|---|---|---|
| 1-2a | 1-3b | 2-3c | |||||
| Total UTIs* | Probiotic + antibiotic | 1.29 (2.58) | 0.70 (1.69) | 0.51 (1.30) | 0.2 | 0.9 | 0.4 |
| Antibiotic | 1.54 (2.45) | 1.13 (1.95) | 0.95 (1.61) | 0.3 | 0.1 | 0.6 | |
| P . Value | 0.6 | 0.3 | 0.2 | - | - | - | |
| Febrile UTIs* | Probiotic + antibiotic | 0.07 (0.26) | 0.04 (0.21) | 0 | 0.3 | 0.08 | 0.2 |
| Antibiotic | 0.25 (0.65) | 0.15 (0.42) | 0.13 (0.40) | 0.4 | 0.3 | 0.7 | |
| P . Value | 0.1 | 0.1 | 0.03 | - | - | - | |
| Afebrile UTIs* | Probiotic + antibiotic | 1.21 (2.44) | 0.63 (1.71) | 0.51 (1.30) | 0.4 | 0.1 | 0.5 |
| Antibiotic | 1.29 (2.13) | 0.97 (1.69) | 0.81 (1.41) | 0.4 | 0.2 | 0.6 | |
| P . Value | 0.9 | 0.4 | 0.3 | - | - | - | |
Data are expressed as mean ± standard deviation; UTI: Urinary tract infection
Data of each group in the first and second year of follow up compared (using paired sample t test)
Data of each group in the first and third year of follow up compared (using paired sample t test)
Data of each group in the second and third year of follow up compared (using paired sample t test)
The number of patients with the experience of RUTI was higher in group II (22/44 versus 16/41, P = 0.4). In group I, 5/16 (31%) of patients experienced recurrent febrile UTI and 11/16 (69%) were afebrile. In group II, 7/22 (32%) of recurrences of UTI were febrile and 15/22 (68%) afebrile. Recurrences of UTI were seen more in females than in male participants in a way that female/male ratio in group I was 9:7 and 16:6 in group II without significant difference in the two arms (P > 0.05). After stratification by VUR grade, no statistical difference was observed between the two arms in each grade (P > 0.05).
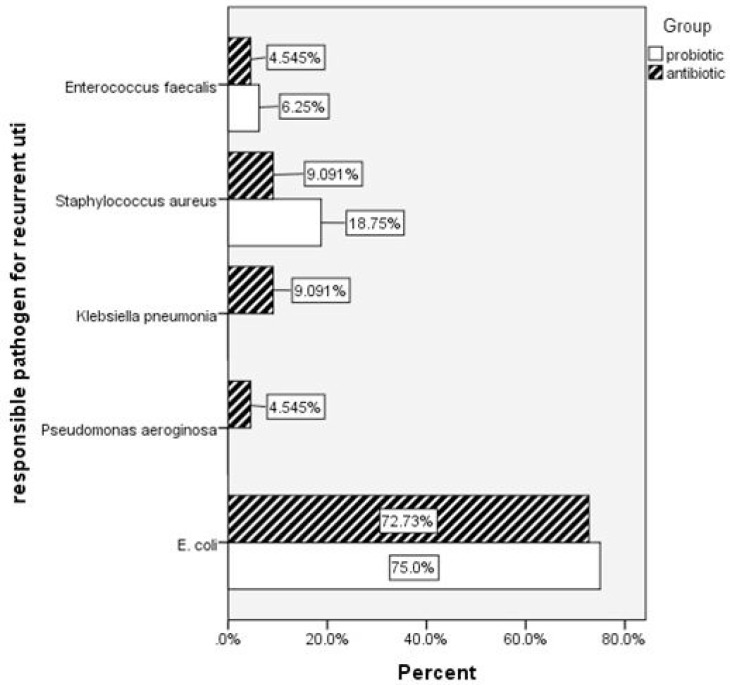
E. coli microorganism, caused most of the infections in both of study arms, but in group I no infection was seen by Pseudomonas aeroginosa and Klebsiella pneumoniae (Fig. 2). RUTIs were caused by more sensitive E. coli strains to Nitrofurantoin in probiotic and antibiotic group in the last year (P = 0.02). Development of new renal scar was 6/44 (13%) in children who received antibiotic prophylaxis and 2/41 (4%) in children who received probiotic + antibiotic prophylaxis with no statistical difference (P = 0.2) (Table 3). Of note to say that no side effect was observed due to either probiotics or antibiotics.
Fig. 2.
Recurrent Urinary tract infections and responsible pathogens in two groups of study (P=0.3)
Table 3.
E. coli and its sensitivity to Nitrofurantoin in cases of RUTI through three year follow up and development of new renal scar after RUTIs
| Follow up period or scar formation | Probiotic+ antibiotic | Antibiotic | P. Value | |
|---|---|---|---|---|
| Year1 | Sensitive | 5 | 9 | 0.9 |
| Resistant | 2 | 3 | ||
| Year2 | Sensitive | 5 | 5 | 0.2 |
| Resistant | 0 | 4 | ||
| Year3 | Sensitive | 5 | 2 | 0.02 |
| Resistant | 0 | 6 | ||
| New scar development | Negative | 39 | 38 | 0.2 |
| Positive | 2 | 6 |
RUTI: Recurrent Urinary tract infection
Discussion
In this study, significant difference was observed between antibiotic alone and probiotic + antibiotic prophylaxis in the prevention of febrile UTIs after three years consumption of probiotic yoghurt. Episodes of UTIs reduced throughout the three years of follow up in both groups.
Although antibiotics such as Trimethoprim–sulfamethoxazole, Amoxicillin-clavulanic acid and Cephalosporin are widely used in the treatment of acute UTI, the effective approach for prevention of UTI is still obscure[7, 20]. To date, antibiotic prophylaxis and surgical correction of VUR are accepted approaches for prevention of UTI[3, 21]. Costs and side effects of these approaches and debates about their efficacy in prevention of RUTI have encouraged the researchers to find alternative methods[20]. Recently natural approaches have gained popularity among scientists for management of human diseases. Very few researches assessed the efficacy of cranberry juice in prevention of UTI in women and children. They showed that consumption of cranberry juice prevents recurrences of UTIs in women but not in the children[22, 23]. It has been suggested that cranberry juice has an effective role in UTI prophylaxis through the modulation of the microbial flora of the intestinal and urogenital environment[22, 24]. On the other hand, there are studies suggesting that probiotics (Lactobacillus rhamnosus GR-1 and L. reuteri RC-14) can prevent the colonization of uropathogenic bacteria[25, 26]. Furthermore, there are debates on the usefulness of probiotics in inflammatory bowel disease and constipation[12–15]. These controversial effects may be due to strains that were studied and dosage and duration of probiotic supplementation, therefore further studies were suggested.
Reid and his colleagues in 1985, assessed the efficacy of lactobacilli in prevention of UTI in rats for the first time [27]. They injected five strains of periurethral uropathogens into the urinary bladder and then instilled an isolate of Lactobacillus casei GR1 within rat's bladders. They noted the prevention of colonization in 84% of rats[27]. Beneficial probiotic strains for the UTI prophylaxis can produce bacteriocin, biosurfactant barrier and hydrogen peroxide and adhere to the sites of uropathogenic bacteria. By such mechanisms, they can interfere with the colonization of uropathogens[28]. Osset et al observed that Lactobacilli from hemagglutination group III had greater capacity to block uropathogen adherence than other strains. The most susceptible uropathogens were Pseudomonas aeroginosa PA5, Klebsiella pneumonia KP7 and Staphylococcus aureus SA11, while Proteus mirabilis PM1 was the most resistant uropathogen to blockage[29]. Similarly, based on the results of this study no infection by Pseudomonas aeroginosa and Klebsiella pneumoniae was recorded in children who received probiotic + antibiotic prophylaxis. A human clinical trial showed that instillation of L. rhamnosus GR-1 or L. fermentum RC-14 through the vagina reduced the recurrence rate of UTI in women[30]. Another study by Kontiokari et al showed that consuming L. acidophilus yogurt for at least three times per week was associated with significant reduction in episodes of UTI breakthrough. Administration of vaginal suppositories containing the strain Lactobacillus crispatus GAI 98322 can significantly reduce the recurrence rate of UTI in women, without any adverse complication[31].
Contrary to many searches on the usefulness, safety and efficacy of probiotic strains for prevention of UTI in women, the data in the field of pediatrics are very poor[32]. To our knowledge there has only been one clinical trial, which assessed the efficacy of probiotics versus antibiotic prophylaxis for RUTIs in children[33]. They found that consumption of probiotics (Lactobacillus acidophilus 108 CFU/g 1 g bid) for prophylaxis was as effective as TMP/SMX (2/10 mg/kg) antibiotic prophylaxis in children with persistent primary VUR. Similarly, we found a better protection of antibiotic and probiotic in compare to antibiotic alone against febrile and resistant UTIs. Additionally, the incidence of UTIs in our study was higher than those they recorded with the predominance of afebrile UTIs. One suggested reason is that urine samples of our participants were evaluated monthly to detect any asymptomatic infection. However, their participants were children with persistent primary VUR and their history of UTI was not stated. While VUR is highly frequent among children with recurrent UTI, all the children with VUR will not experience UTI. In this study, we recruited children with unilateral VUR who developed recurrent UTIs under antibiotic prophylaxis.
This study has several limitations. One hundred and forty children were primarily assessed for eligibility to reach the calculated sample size. Only 106 children met the inclusion criteria and were enrolled. Considering that we excluded patients who presented with any disease at any time during the study period (e.g. upper respiratory tract infections, food or seasonal allergy, etc.), in addition to the patients who did not follow receiving treatments, data from another 21 patients were not included in the final analysis, further reducing the power of our study. Thereby, the power of study was less than 80% (α = 0.05 and β = 0.2). Another limitation of this study is the absence of any control (placebo) group. In fact, our ethical board committee did not permit us to stop antibiotic prophylaxis and put the patients on plain yogurts as the third arm.
Conclusion
The results of this pilot study suggest that probiotic + antibiotic administration in patients with unilateral VUR could be more effective in the prevention of febrile UTI in comparison with antibiotic therapy alone. Further randomized clinical trials with larger study population are needed to elucidate the safety and obscure aspects of probiotic administration in children. Evaluation of other probiotic strains in other geographical areas for prophylaxis of UTI is also recommended.
Acknowledgment
The authors thank the authorities in charge of R&D department of Iran Dairy Industries Co. (Pegah). The authors would like to thank Mrs. Zahra Rahimi and Maryam Alijani, the liable and the expert of Urodynamic laboratory and Biofeedback at pediatric urology ward, Pediatric Center of Excellence, respectively. Furthermore, we thank Miss Sara Harsini and Dr. Ali Tourchi for their assistance in preparing the preliminary draft of this manuscript. We also, thank Dr. Forough-zaman Nouri because of her great efforts in examination of females’ genitalia. Finally, we highly appreciate Kiana Kajbafzadeh (Faculty of Land and Food Systems, University of British Columbia, Canada) for her valuable revision of the manuscript.
Conflict of Interest
Yoghurt containing Lactobacillus acidophilus and Bifidobacterium lactis was provided by Research and Development Department of Iran Dairy Industries Co. (Pegah).
References
- 1.Hellström A, Hanson E, Hansson S, et al. Association between urinary symptoms at 7 years old and previous urinary tract infection. Arch Dis Child. 1991;66(2):232–4. doi: 10.1136/adc.66.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998;87(5):549–52. doi: 10.1080/08035259850158272. [DOI] [PubMed] [Google Scholar]
- 3.Peters CA, Skoog SJ, Arant BS, Jr, et al. Summary of the AUA guideline on management of primary vesicoureteral reflux in children. J Urol. 2010;184(3):1134–44. doi: 10.1016/j.juro.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 4.Leroy S, Romanello C, Smolkin V, et al. Prediction of moderate and high grade vesicoureteral reflux after a first febrile urinary tract infection in children: construction and internal validation of a clinical decision rule. J Urol. 2011;187(1):265–71. doi: 10.1016/j.juro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Sorkhi H, Nooreddini HG, Amiri M, et al. Prediction of vesicoureteral reflux in children with first urinary tract infection by dimercaptosuccinic Acid and ultrasonography. Iran J Pediatr. 2012;22(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Nateghian AR, Robinson JL, Mohandessi S, et al. Resistance patterns of breakrhrough urinary tract infections in children on antibiotic prophylaxis. J Infec Pub Health. 2009;2(3):147–52. doi: 10.1016/j.jiph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011;365(3):239–50. doi: 10.1056/NEJMra1007755. [DOI] [PubMed] [Google Scholar]
- 8.Gurley BJ. Cranberries as antibiotics? Comment on “Cranberries vs antibiotics to prevent urinary tract infections: a randomized double-blind nonin-feriority trial in premenopausal women”. Arch Intern Med. 2011;171(14):1279–80. doi: 10.1001/archinternmed.2011.332. [DOI] [PubMed] [Google Scholar]
- 9.Head KA. Natural approaches to prevention and treatment of infections of the lower urinary tract. Altern Med Rev. 2008;13(3):227–44. [PubMed] [Google Scholar]
- 10.Kontiokari T, Laitinen J, Jarvi L, et al. Dietary factors protecting women from urinary tract infection. Am J Clin Nutr. 2003;77(3):600–4. doi: 10.1093/ajcn/77.3.600. [DOI] [PubMed] [Google Scholar]
- 11.Reid G, Bruce AW, Fraser N, et al. Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol. 2001;30(1):49–52. doi: 10.1111/j.1574-695X.2001.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 12.Khodadad A, Farahmand F, Najafi M, Shoaran M. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr. 2013;23(1):79–84. [PMC free article] [PubMed] [Google Scholar]
- 13.Khodadad A, Sabbaghian M. Role of synbiotics in the treatment of childhood constipation: a double-blind randomized placebo controlled trial. Iran J Pediatr. 2010;20(4):387–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Tabbers MM, Boluyt N, Berger MY, et al. Nonpharmacologic treatments for childhood constipation: systematic review. Pediatrics. 2011;128(4):753–61. doi: 10.1542/peds.2011-0179. [DOI] [PubMed] [Google Scholar]
- 15.Meijer BJ, Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol. 2011;45(Suppl):S139–44. doi: 10.1097/MCG.0b013e31822103f7. [DOI] [PubMed] [Google Scholar]
- 16.Hoesl CE, Altwein JE. The probiotic approach: an alternative treatment option in urology. Eur Urol. 2005;47(3):288–96. doi: 10.1016/j.eururo.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Storm DW, Patel AS, Koff SA, et al. Novel management of urinary tract infections. Curr Opin Urol. 2011;21(4):328–33. doi: 10.1097/MOU.0b013e328346d4ee. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492–5. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebowitz RL, Olbing H, Parkkulainen KV, et al. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr Radiol. 1985;15(2):105–9. doi: 10.1007/BF02388714. [DOI] [PubMed] [Google Scholar]
- 20.Dai B, Liu Y, Jia J, Mei C. Long-term antibiotics for the prevention of recurrent urinary tract infection in children: a systematic review and meta-analysis. Arch Dis Child. 2010;95(7):499–508. doi: 10.1136/adc.2009.173112. [DOI] [PubMed] [Google Scholar]
- 21.Costers M, Van Damme-Lombaerts R, Levtchenko E, et al. Antibiotic prophylaxis for children with primary vesicoureteral reflux: where do we stand today? Adv Urol. 2008:217805. doi: 10.1155/2008/217805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guay DR. Cranberry and urinary tract infections. Drugs. 2009;69(7):775–807. doi: 10.2165/00003495-200969070-00002. [DOI] [PubMed] [Google Scholar]
- 23.Salo J, Uhari M, Helminen M, et al. Cranberry juice for the prevention of recurrences of urinary tract infections in children: a randomized placebo-controlled trial. Clin Infect Dis. 2011;54(3):340–6. doi: 10.1093/cid/cir801. [DOI] [PubMed] [Google Scholar]
- 24.Vendrame S, Guglielmetti S, Riso P, et al. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem. 2011;59(24):12815–20. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- 25.Cadieux PA, Burton J, Devillard E, et al. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J Physiol Pharmacol. 2009;60(Suppl 6):13–8. [PubMed] [Google Scholar]
- 26.Johnston BC, Goldenberg JZ, Vandvik PO, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;11:CD004827. doi: 10.1002/14651858.CD004827.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Reid G, Chan RC, Bruce AW, et al. Prevention of urinary tract infection in rats with an indigenous Lactobacillus casei strain. Infect Immun. 1985;49(2):320–4. doi: 10.1128/iai.49.2.320-324.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan RC, Reid G, Irvin RT, et al. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985;47(1):84–9. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osset J, Bartolome RM, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485–91. doi: 10.1086/318070. [DOI] [PubMed] [Google Scholar]
- 30.Bruce AW, Reid G. Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can J Microbiol. 1988;34(3):339–43. doi: 10.1139/m88-062. [DOI] [PubMed] [Google Scholar]
- 31.Uehara S, Monden K, Nomoto K, et al. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int J Antimicrob Agents. 2006;28(Suppl 1):S30–4. doi: 10.1016/j.ijantimicag.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Storm DW, Koff SA, Horvath DJ, et al. In vitro analysis of the bactericidal activity of Escherichia coli Nissle 1917 against pediatric uropathogens. J Urol. 2011;186(4 Suppl):1678–83. doi: 10.1016/j.juro.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Shim YH, Cho SJ, et al. Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatr Nephrol. 2007;22(9):1315–20. doi: 10.1007/s00467-007-0507-1. [DOI] [PubMed] [Google Scholar]