Abstract
Objective
Clostridium difficile is a gram-positive, anaerobic, spore-forming bacillus. Usually it does not cause disease unless a patient who is colonized with toxin-producing strains has been treated with antibiotics, particularly those that change the anaerobic flora of the large intestine.
Methods
We investigated in a prospective study intestinal colonization of C. difficile and its toxins in children with malignancy that used different antibiotics and cytotoxic drugs.
Findings
One hundred fifty-two patients were included in this prospective study. Stool samples were obtained within the first 48 hours after admission and cultured for C. difficile; cytopathic effect of C. difficile was detected on HELA cells, also ELISA test was performed for detection of toxins A and B. 25% of patients had positive culture for C. difficile; 36/38 (92%) revealed positive cytopathic effect on HELA cells. No significant relation was found between age, gender, history of antibiotic consumption and C. difficile positive culture and cytopathic effect on HELA cells. The only relation was seen between cotrimoxazol usage and cytopathic effect on HELA cells (P=0.03).
Conclusion
Although the rate of C. difficile colonization (25.6%) and toxigenic strains (23.7%) in admitted children in hematologic ward is high, the rate of ELISA positive test for toxin A+B was not correspond with culture and cytopatic effect on HELA cell. With respect to sensitivity and specificity of ELISA test, possibility for existence of toxin C with cytopathic effect is high in this type of patients.
Keywords: Colonization, Clostridium Difficile, Cancer, Cytopathic Effect
Introduction
Clostridium difficile (C. difficile) is a gram-positive, anaerobic, spore-forming bacillus. Usually it does not cause disease unless a patient who is colonized with toxin-producing strains has been treated with antibiotics, particularly those that change the anaerobic flora of the large intestine[1].
Hospitals and nursing facilities could be a source of C. difficile organisms and spores[2]. In addition to antibiotic therapy other risk factors include recent receipt of cancer chemotherapy[3] and antiviral therapy[4]. Children with cancer may be at a particular risk for developing C. difficile infection because of frequent use of antibiotics and cytotoxic drugs.
Although studies have been done on the epidemiology and acquisition of C. difficile in adults with hematological malignancies, little information is available on C. difficile infection in children with cancer. We investigated intestinal colonization of C. difficile and its toxins in children with malignancy as a part of prospective study.
Subjects and Methods
During 19 months (March 2008 to September 2009), 152 children with various types of malignancy admitted to hematologic ward at Mofid Children's Hospital were evaluated. Patients with documented malignancy admitted for starting chemotherapy and also those admitted because of complications of chemotherapy such as fever and neutropenia are included in this study. Patients who were admitted in hematologic ward for other reasons such as investigation for cause of pancytopenia or anemia and also patients with suspecte, but not documented malignancy, are not included in the study.
Stool samples were obtained within the first 48 hours after admission and cultured for C. difficile; cytopathic effect of C. difficile was detected on HELA cells and also ELISA test was performed to detect toxins A and B. Samples were inoculated directly on plates containing C. difficile agar (Mast Diagnostic, Germany), defibrinated horse blood, cycloserine, and fructose. The cultures were incubated for three consecutive days. The process was done automatically (ANOXOMAT). Positive specimens were searched for cythopatic effect on HELA cells. At the same time the samples were analyzed for toxins A and B by ELISA A+B kits (Novitec; 703096-w5574).
Data were analyzed by SPSS 11.5. Chi-square and Fisher's tests were used for qualitative variables.
Findings
C. difficile and its toxins were isolated from the stools of 152 hospitalized children. Seventy (46%) patients were girls and 82 (54%) boys. Ninety-two cases had history of prior hospitalization one week ago and 44 cases had been admitted to the hospital between 1 to 4 weeks ago, while the rest had not been hospitalized during the 4 weeks prior to this admission.
Thirty-eight (25%) cases, had positive cultures for C. difficile; 36/38 (92%) revealed positive cytopathic effect on HELA cells and only in 1 (0.7%) case positive ELISA (A+B) test showed the presence of toxin. ELISA was negative in all negative cultures. Although the highest (15/41, 36.6%) colonization rate was seen in age 5-10 years, we found no significant relation between age groups either in the rate of C. difficile colonization (P=0.3) or in a positive cytopatic effect (P=0.3). We did not find any correlation between gender and colonization (P=0.9) or gender and cytopathic effect (P=0.9) (Table 1).
Table 1.
Gender and the rate of colonization and cytopatic effect in HELA cells
| Sex | C. Defficle culture | Cytopatic effect | ||
|---|---|---|---|---|
| positive | negative | positive | negative | |
| Male | 26 (32%) | 56 (68%) | 24 (29%) | 58 (71%) |
| Female | 13 (19%) | 57 (81%) | 12 (17%) | 58 (83%) |
C. Defficle: Clostridium Defficle
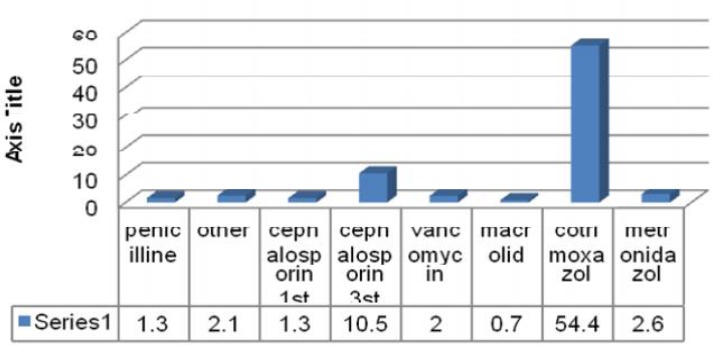
Eighty-four (55.3%) children had received antibiotics one week before admission, 77 (50.7%) cases had been given oral antibiotics. Seven types of antibiotics had been used in patients; the most common was cotrimoxazol (Fig. 1). We found no significant correlation between the use of antibiotics and the rate of C. difficle colonization (P=0.3) or cytopathic effect on HELA cells (P=0.1). The only relation was seen between cotrimoxazol usage and cytopathic effect on HELA cells (P=0.03).
Fig. 1.
The kind and percentage of antibiotics usage
Discussion
The first step for developing C. difficile colitis is colonization with toxicogenic type. Total rate of colonization with C. difficile in this study was 25%, 92% of which were toxicogenic. Paola Gianfirilli et al examined stool of 15 patients with leukemia for the presence of C. difficile and its toxins. Four out of 15 cases were positive for C. difficile and only one of them was toxicogenic; they reported a low rate of C. difficile colonization in leukemic children who were on maintenance chemotherapy[5].
Although study on this type of patients for detection of C. difficile colonization rate waslimited, studies on normal population showed varying colonization rates in different areas.
In immunocompetent population host factors like age, diet, and immune response play an important role in determining whether the C. difficile causes asymptomatic carrier status or active disease. It seems that different levels of colonization in different studies is largely dependent on these factors[6]. As Rexach et al showed, this difference in colonization rate is linked to differences in geography and environment[7]. The environment and circumstances in which children contact with each other is more likely to acquire opportunistic organisms accordingly. Another study reported that 20-36% of pediatric cancer patients were carriers of C. difficile and younger children appeared to be more frequently infected[8].
In a literature review we did not find any data about relation of age and C. difficile colonization rate in cancer patients but in compared with other studies[6, 7] done in immune competent children we did not find any relationship between colonization rate and age.
Although theoretically chemotherapy could change the pattern of flora of gastrointestinal tract, our finding shows no difference between colonization rate in cancer patients whether they had received chemotherapy or not. Murabata et al reported chemotherapy as a risk factor for developing C. difficile associated diarrhea and that it is not a rare infection in these patients[9]. We did not look for C. difficile associated diarrhea, so we could not compare our results with theirs. Oskarditter et al suggest that children with malignant disorders have a higher rate of infection with the organism even before chemotherapy or antimicrobial treatment[8]. Our different result may be due to the fact that we studied asymptomatic patients.
Armin et al found that 45.2% of immunocompetent children admitted to the hospital were colonized with C. difficile at the time of admission and 8.8% of the strains were toxigenic[6]. It was compatible with other studies which reported 48% and 50% colonization rates[10, 11]. Although the colonization rate was lower in this immunocompromised group in comparison with Armin's normal children (25.6% vs. 45.2%), the percentage of toxigenic type is much higher in patients with malignancy than in immunocompetent children (92% vs. 8.8%)[6]. This difference could be due to high rate of antibiotic usage in this high risk group which may clear the sensitive C. difficile from the intestine or change the proportion of toxicogenic to nontoxicogenic organisms. We suppose, as Predrag et al pointed[12] out, non toxicogenic strain was sensitive to antibiotics and thus toxicogenic strains increased in these cases. Differences in rates of colonization and toxicogenic type arise also when studies were conducted in different time periods, in different geographical areas and included different population groups, symptomatic or asymptomatic patients[12]. Although antibiotic usage is a risk factor for C. difficile associated colitis and some researchers believe that antibiotics alter the normal flora of the bowel, thus rendering the host susceptible to colonization by C. difficile and C. difficile associated colitis[13], in consistence with our previous study[6], we did not find any relationship between colonization and previous antibiotic usage. Our findings were compatible with other studies that found no correlation between C. difficile colonization and antibiotic usage in immunocompetent children [6, 10, 14, 15].
The relation between cotrimoxazole usage and colonization with toxigenic strains was significant in our subjects. Cotrimoxazole was the most common antibiotic that was used; it is quite probable that this antibiotic suppressed the growth of non-toxigenic strains. Although children with malignancy colonized with toxicogenic type of C. difficile are at higher risk for developing clostridium associated diarrhea, for documentation of this hypothesis we need another prospective study with long time follow up of colonized cases.
This study showed incompatibility between ELISA test and HELA cell culture (1 case versus 36 cases). As we know the sensitivity and specificity of ELISA test for toxin detection is 100%, so we assume that another toxin such as binary toxin might have been responsible for the cytopathic effect on HELA cells.
Our research had some limitations: although our patients had malignancy, they were on different stages of immune suppression and type of their underline disease was not similar. Also we did not search for binary toxin. We hope further research in this area be done to overcome these limitations.
Conclusion
The rate of colonization with clostridium is not high. However, it is important to realize that a high rate of toxicogenic strains is isolated in patients, especially in cases with cotrimoxazol consumption, and this puts this organism as a notable pathogen in cancer patients with diarrhea.
Acknowledgment
We would like to thanks all staph at pediatrc infectious research center and hematologic ward for kind cooperation.
Conflict of Interest
None
References
- 1.Johnson S, Clabots CR, Linn FV, et al. Nosocomial Clostridium difficile colonization and disease. Lancet. 1990;336(8707):97–100. doi: 10.1016/0140-6736(90)91605-a. [DOI] [PubMed] [Google Scholar]
- 2.Clabots CR, Johnson S, Olson MM, et al. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166(3):561–7. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 3.Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109–13. doi: 10.1093/clinids/17.1.109. [DOI] [PubMed] [Google Scholar]
- 4.Colarian J. Clostridium difficile colitis following antiviral therapy in the acquired immunodeficiency syndrome. Am J Med. 1988;84(6):1081. doi: 10.1016/0002-9343(88)90316-6. [DOI] [PubMed] [Google Scholar]
- 5.Chiesa C, Gianfrilli P, Occhionero M, et al. Clostridium difficile isolation in leukemic children on maintenance cancer chemotherapy. Clin Pediatr (Phila) 1985;24(5):252–5. doi: 10.1177/000992288502400502. [DOI] [PubMed] [Google Scholar]
- 6.Armin SH, Babaie D, Karimi A, et al. Toxigenic Clostridium difficile colonization in children. J Pediatr Inf Dis. 2009;4(4):375–8. [Google Scholar]
- 7.Rexach CE, Tang-Feldman YJ, Cantrell MC, et al. Epidemiologic surveillance of clostridium difficile diarrhea in a freestanding pediatric hospital and a pediatric hospital at a university medical center. Diagn Microbiol Infect Dis. 2006;56(2):109–14. doi: 10.1016/j.diagmicrobio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Oskarsdottir S, Mellander L, Marky I, et al. Clostridium difficile in children with malignant disease. Pediatr Hematol Oncol. 1991;8(3):269–72. doi: 10.3109/08880019109033462. [DOI] [PubMed] [Google Scholar]
- 9.Murabata M, Kato H, Yano H, et al. Intestinal colonization and nosocomial spread of Clostridium difficile in pediatric cancer patients under long-term hospitalization. Kansenshogaku Zasshi. 2008;82(5):419–26. doi: 10.11150/kansenshogakuzasshi1970.82.419. [DOI] [PubMed] [Google Scholar]
- 10.Malamou-Ladas H, O'Farrell S, Nash JQ, et al. Isolation of Clostridium difficile from patients and the environment of hospital wards. J Clin Pathol. 1983;36(1):88–92. doi: 10.1136/jcp.36.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuki S, Ozaki E, Shozu M, et al. Colonization by Clostridium difficile of neonates in a hospital, and infants and children in three day-care facilities of Kanazawa, Japan. Int Microbiol. 2005;8(1):43–8. [PubMed] [Google Scholar]
- 12.Predrag S, Branislava K, Miodrag S, et al. Clinical importance and representation of toxigenic and non-toxigenic Clostridium difficile cultivated from stool samples of hospitalized patients. Braz J Microbiol. 2012;43(1):215–23. doi: 10.1590/S1517-838220120001000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcfarland LV, Brandmarker SA, Guandalini S. Pediatric clostridium difficile: a phantom menace or clinical real. J Pediatr Gastroentrol Nutr. 2000;31(3):220–31. doi: 10.1097/00005176-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Merida V, Moerman J, Colaert J, et al. Significance of clostridium difficile and its cytotoxin in children. Eur J Pediatr. 1986;144(5):494–6. doi: 10.1007/BF00441746. [DOI] [PubMed] [Google Scholar]