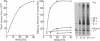Abstract
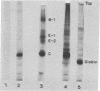
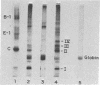
Extracts from Krebs II ascites cells and rabbit reticulocytes effectively synthesize viral proteins with Sindbis viral mRNA isolated from Sindbis-infected BHK cells. The major product is identical to Sindbis capsid protein on the basis of its electrophoretic mobility in sodium dodecyl sulfate-acrylamide gels and two-dimensional tryptic-peptide fingerprints. Various amounts of several additional discrete polypeptides are formed, depending on the components of the cell-free extracts. One of these polypeptides may be a prematurely terminated part of the viral-capsid protein, while another is larger in molecular weight than capsid protein but contains the capsid tryptic peptides. Several of the proteins formed in vitro also are detected in extracts of Sindbis-infected BHK cells labeled with [35S]methionine.
The three proteins found in Sindbis virions are postulated to originate by proteolytic cleavage from a larger molecular weight polypeptide precursor that is translated from a polycistronic mRNA presumed to contain a single site for initiation of protein synthesis. The two in vitro systems appear to translate this polycistronic viral mRNA to yield specific viral capsid although no evidence was found for post-translational proteolysis. Other mechanisms for production of the capsid protein in the cell-free extracts are considered, and some of these may function in the viral-infected cell where unusually large amounts of viral capsid proteins are frequently detected.
Keywords: reticulocyte, ascites, polycistronic, sodium dodecyl sulfate-gels, baby hamster kidney (BHK) cells
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Yau P. M., Herbert E., Zucker W. V. Involvement of hemin, a stimulatory fraction from ribosomes and a protein synthesis inhibitor in the regulation of hemoglobin synthesis. J Mol Biol. 1972 Jan 28;63(2):247–264. doi: 10.1016/0022-2836(72)90373-7. [DOI] [PubMed] [Google Scholar]
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSOOK H., FISCHER E. H., KEIGHLEY G. Factors affecting protein synthesis in vitro in rabbit reticulocytes. J Biol Chem. 1957 Dec;229(2):1059–1070. [PubMed] [Google Scholar]
- Bellamy A. R., Shapiro L., August J. T., Joklik W. K. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. doi: 10.1016/0022-2836(67)90177-5. [DOI] [PubMed] [Google Scholar]
- Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus mRNA. 3. Discrete polypeptides translated from a monocistronic messenger in vitro. Arch Biochem Biophys. 1972 Dec;153(2):706–713. doi: 10.1016/0003-9861(72)90389-x. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. E. Lipid composition of Sindbis virus. Virology. 1971 Dec;46(3):711–720. doi: 10.1016/0042-6822(71)90073-0. [DOI] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Kerr I. M., Martin E. M. Synthesis of capsid and noncapsid viral proteins in response to encephalomyocarditis virus ribonucleic acid in animal cell-free systems. J Virol. 1971 Oct;8(4):491–499. doi: 10.1128/jvi.8.4.491-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei W. D., 3rd, Roy D., Konigsberg W., Lengyel P. Translation of reovirus messenger ribonucleic acids synthesized in vitro into reovirus proteins in a mouse L cell extract. Arch Biochem Biophys. 1973 Sep;158(1):266–275. doi: 10.1016/0003-9861(73)90621-8. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W. Cell-free translation of paramyxovirus messenger RNA. J Virol. 1973 Nov;12(5):1020–1027. doi: 10.1128/jvi.12.5.1020-1027.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowshowitz D. Identification of polysomal RNA in BHK cells infected by sindbis virus. J Virol. 1973 Apr;11(4):535–543. doi: 10.1128/jvi.11.4.535-543.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M. The isolation and purification of rapidly labeled polysome-bound ribonucleic acid on polythymidylate cellulose. J Biol Chem. 1972 May 25;247(10):3365–3367. [PubMed] [Google Scholar]
- Nichols J. L. Nucleotide sequence from the polypeptide chain termination region of the coat protein cistron in bacteriophage R17 RNA. Nature. 1970 Jan 10;225(5228):147–151. doi: 10.1038/225147a0. [DOI] [PubMed] [Google Scholar]
- Oberg B. F., Shatkin A. J. Initiation of picornavirus protein synthesis in ascites cell extracts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3589–3593. doi: 10.1073/pnas.69.12.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Boyle M. K. Selective inhibition of the synthesis of Sindbis virion proteins by an inhibitor of chymotrypsin. J Virol. 1972 Jan;9(1):187–188. doi: 10.1128/jvi.9.1.187-188.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Mathews M. B. Double-stranded RNA as an inhibitor of protein synthesis and as a substrate for a nuclease in extracts of Krebs II ascites cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):225–229. doi: 10.1073/pnas.70.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemond H., Sreevalsan T. Viral RNAs associated with ribosomes in Sindbis virus-infected HeLa cells. J Virol. 1973 Mar;11(3):399–415. doi: 10.1128/jvi.11.3.399-415.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Kinetics of incorporation of structural proteins into Sindbis virions. J Virol. 1969 Apr;3(4):369–375. doi: 10.1128/jvi.3.4.369-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: chemical composition, biological activity, and mode of interference. J Virol. 1973 Oct;12(4):862–871. doi: 10.1128/jvi.12.4.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]