Abstract
Objective(s)
We have previously shown that Fas expression inversely correlates with the metastatic potential of osteosarcoma (OS) to the lung. FasL is constitutively expressed in the lung microenvironment and eliminates Fas+ osteosarcoma cells leaving Fas− cells to form metastases. Absence of FasL in the lung epithelium or blocking the Fas-signaling pathway interfered with this clearance mechanism allowing Fas+ cells to remain and form lung metastases. We also demonstrated that while the majority of patient OS lung metastases were Fas−, 10-20% of the lesions contain Fas+ cells, suggesting that these cells were not sensitive to FasL-induced apoptosis. The expression of c-FLIP, an inhibitor of the Fas pathway, has been associated with tumor development, progression, and resistance to chemotherapy. We therefore evaluated the expression of c-FLIP in OS patient tumor specimens and human xenograft lung metastases.
Methods
Osteosarcoma patient tissues, which included both primary and metastatic lesions, were evaluated for the expression of c-FLIP. In addition, tumors from human osteosarcoma xenografts were examined for c-FLIP expression.
Results
c-FLIP expression was significantly higher in the lung metastases than in the primary tumors.
Conclusion(s)
c-FLIP may play an important role in the metastatic potential of osteosarcoma to the lung. Inhibition of c-FLIP may be a future therapeutic target.
Keywords: Osteosarcoma, pulmonary metastasis, c-FLIP, Fas/FasL pathway
INTRODUCTION
The most common site of metastasis in patients with osteosarcoma (OS) is the lung. Approximately 90% of patients already have microscopic spread to the lung at the time of diagnosis (1). The molecular events that contribute to the metastatic potential of OS include aberrations in ezrin, annexin 2 and the C-X-C chemokine receptor type 4/stromal cell-derived factor-1 (CXCR4/SDF-1) pathway (2-5). Our laboratory has shown that the Fas signaling pathway plays an important role in the ability of OS cells to metastasize to the lung (6, 7). Fas (CD95) is a death receptor found on a variety of normal and neoplastic cells and is involved in the induction of apoptosis upon interaction with Fas ligand (FasL) (8, 9). Since FasL is constitutively expressed on lung epithelial cells, cells that express Fas are eliminated as a result of their interaction with FasL (9). We have previously shown that Fas expression is inversely correlated with the metastatic potential of OS and that the lung microenvironment is responsible for the clearance of Fas+ OS cells from the lung (7, 9). Loss of Fas expression, absence of FasL from the lung epithelium and blockage of the Fas signaling pathway resulted in the survival of Fas+ OS cells and formation of Fas+ OS lung metastases.
We have also demonstrated that the majority of OS lung metastases from patients were Fas-, whereas the primary tumors contained both Fas- and Fas+ cells (10). However, 25-30% of the lung metastases contained a significant number of Fas+ cells. Since the downstream mediators of the Fas pathway are also key players in the initiation of the apoptotic cascade, this raises the possibility that Fas-associated proteins that block Fas signaling may contribute to the metastatic potential of OS cells.
c-FLIP, the structural homologue of procaspase-8, binds to the Fas-associated death domain (FADD) at the death-inducing signaling complex (DISC) and inhibits FasL-mediated apoptosis (11). c-FLIP expression has recently been associated with tumorigenesis in several types of cancers (12, 13). For example, c-FLIP has been identified to be overexpressed in approximately 60% of patients with non-Hodgkin’s lymphoma. In this study, expression was found to correlate with tumor progression and poor patient outcome (13). Furthermore, a study in patients with bladder urothelial carcinomas demonstrated that 81% were positive for c-FLIP and that c-FLIP expression was correlated with poor survival (12). Several studies have also demonstrated increased expression of c-FLIP in colon carcinomas, which are resistant to Fas-mediated apoptosis (12, 14). c-FLIP expression has also been associated with drug resistance. For example, in one study, high levels of c-FLIP expression were correlated with resistance of colon carcinoma to chemotherapy in colon carcinoma. Pharmacologic downregulation of c-FLIP restored sensitivity to FasL-induced cell death and enhanced chemotherapy-induced apoptosis (15). Expression of c-FLIP conferred resistance to anti-cancer drugs with various mechanisms of action, including doxorubicin, etoposide, cytosine arabinoside, daunorubicin, chlorambucil and cisplatin (16).
Taken together, these data suggest that overexpression of c-FLIP and inhibition of the Fas/FasL death pathway may play a role in the ability of OS cells to survive in a FasL+ lung microenvironment. To date, c-FLIP expression has not been investigated in OS and OS lung metastasis. We therefore evaluated the expression of c-FLIP in human patient samples and human xenograft OS models. In the present study, we compared the expression of c-FLIP in primary OS tumors and pulmonary OS metastases.
METHODS
Patients
Paraffin-embedded tissue samples from 10 OS patients were initially evaluated. However, since 4 out of 10 patients had matching primary and metastatic sections, only these were included in the analysis. Patient age ranged from 4.5 to 23 years (median 15.3 years). All of the patients received doxorubicin, methotrexate, cisplatin, and ifosfamide prior to resection of the primary tumor (7). Lung metastases were surgically excised at a median of 22 months following diagnosis. The study protocol was approved by the Institutional Review Board at The University of Texas M.D. Anderson Cancer Center.
Cell Lines
The human OS cell lines KRIB and CCH-OS-D were obtained from Dr. Dennis Hughes (Division of Pediatrics, University of Texas MD Anderson Cancer Center), were maintained in complete Dulbecco’s Modified Eagle’s Medium (D-MEM) supplemented with 10% fetal bovine serum, and incubated at 37°C. Both cell lines were STR fingerprinted using the AmpFLSTR Identifier Kit (Applied Biosystems™, Carlsbad, CA) and tested negative for mycoplasma.
Animal Models and Slide Preparation
Eight female nu/nu mice purchased from the National Cancer Institute were injected intratibially with 5 × 105 KRIB cells. The legs were amputated six weeks following injection and tumors were resected. Lungs were resected at the time of or 2 weeks following amputation. CCH-OS-D cells (5 × 105) were injected intratibially into 8 NOD/SCID/IL-2Rγ-deficient mice. Amputation and whole lung resections were performed 6 weeks following injection. Tumors were resected from legs that were amputated. Tissues were formalin-fixed, paraffin-embedded and analyzed using hematoxylin and eosin staining and immunohistochemistry.
Immunohistochemistry
Slides were warmed and de-waxed using xylene, ethanol and phosphate-buffered saline (PBS). Antigen retrieval was achieved by heating slides in 0.1M sodium citrate (pH 6.0) in a microwave for 5 minutes. Slides were then cooled in PBS for 45 minutes, incubated with 3% H2O2 blocking buffer for 12 minutes and then with protein block (94% PBS, 5% normal horse serum, 1% normal goat serum) for 20 minutes. An antibody against c-FLIP (Abbiotec™, San Diego, CA) and a secondary goat anti-rabbit IgG horseradish peroxidase antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were then applied to the slides. Slides were rinsed with PBS-Brij (pH 6.0), incubated with 3,3′-Diaminobenzidine (DAB), and counterstained with hematoxylin. PBS was used for washes between incubations. Slides stained with secondary goat anti-rabbit IgG horseradish peroxidase antibody alone served as the negative controls. Observers were blinded to primary versus metastatic slides as well as to patient identification associated with the slides. Slides were imaged using a light microscope (Leica DMLS, Wetzlar, Germany). Images were captured at 40×, 100× and 400× magnification using a digital output camera (Olympus DP71, Tokyo, Japan) and Olympus DP Controller imaging software (v. 3.3.1.292, 2007).
RESULTS
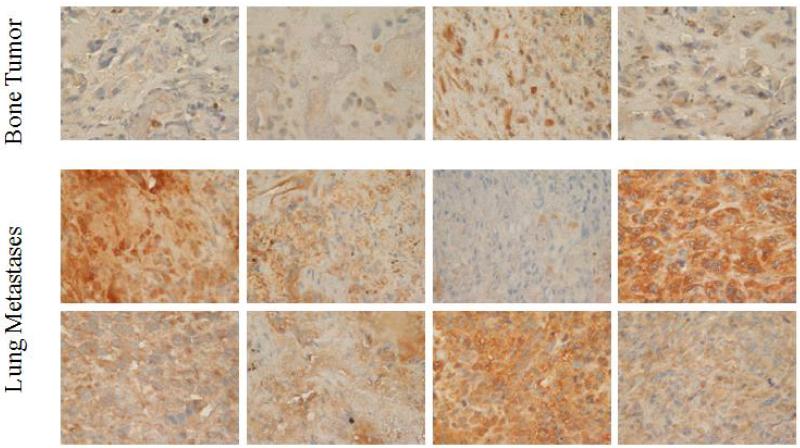
The expression of c-FLIP in osteosarcoma samples was evaluated using immunohistochemistry analysis. Analyzed patient samples consisted of 8 lung metastases and 4 primary tumors. While 8 of 8 lung metastases were positive for c-FLIP expression, only one of the four primary tumors was positive for c-FLIP (Figure 1). Overall, lung metastases showed significantly higher c-FLIP expression than primary tumors.
FIGURE 1.
Expression of c-FLIP in primary bone tumors and lung metastases from OS patients. Immunohistochemistry analysis was used to detect c-FLIP expression. OS lung metastases showed greater c-FLIP expression (lower panel) than primary tumor samples (upper panel).
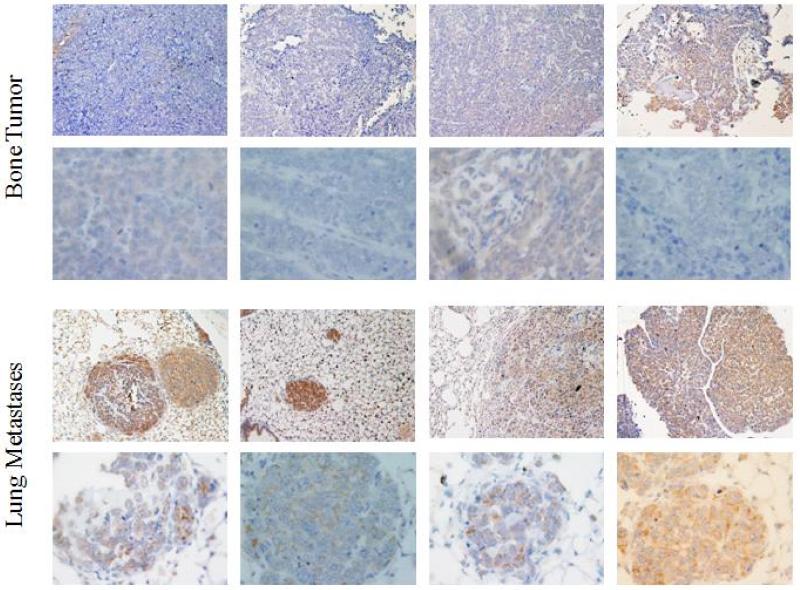
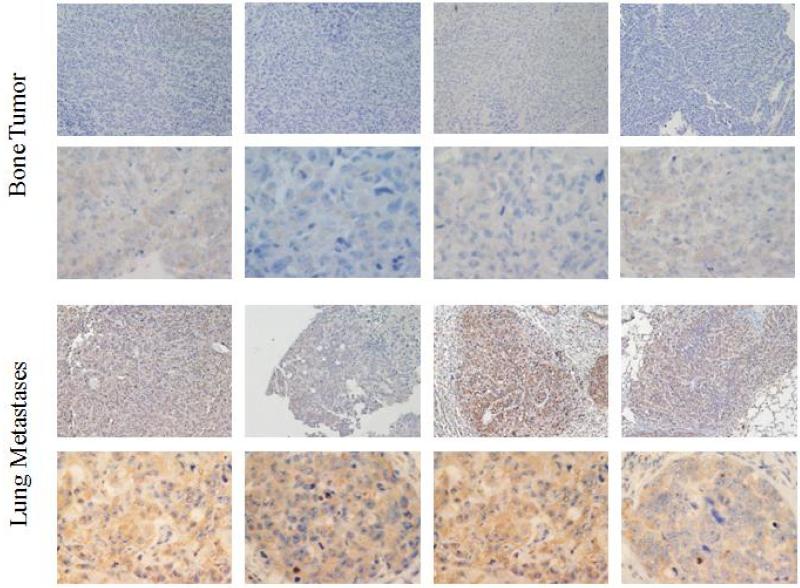
To confirm our findings from patient samples, we evaluated c-FLIP expression in human xenograft mouse models. KRIB and CCH-OS-D cells were injected intratibially. The primary and metastatic tumors were resected and tissue slides were stained for c-FLIP expression. In KRIB xenografts, 8 of 8 lung metastases and 3 of 8 primary tumor samples were c-FLIP positive (Figure 2). Similarly, in CCH-OS-D xenografts, 8 of 8 lung metastases showed c-FLIP positivity compared to the 3 of 8 primary tumor samples (Figure 3). When compared to c-FLIP positive lung metastases, it was observed that the primary tumor contained c-FLIP staining that was diffuse, while the metastases showed defined cellular staining that was more intense. The results from tissues of lung metastases and primary tumors from KRIB and CCH-OS-D xenografts mirrored our patient tumor findings that c-FLIP expression was significantly higher in lung metastases than in primary tumors.
FIGURE 2.
Expression of c-FLIP in CCH-OS-D primary bone tumors and lung metastases. Immunohistochemistry analysis was used to detect c-FLIP expression. Eight of 8 lung metastases and 3 of 8 primary tumors were positive for c-FLIP. The primary tumors that were positive for c-FLIP displayed diffuse staining, while the lung metastases showed staining that was defined with greater intensity.
FIGURE 3.
Expression of c-FLIP in KRIB primary bone tumors and lung metastases. Immunohistochemistry analysis was used to detect c-FLIP expression. Eight of 8 lung metastases and 3 of 8 primary tumors were positive for c-FLIP. The primary tumors that were positive for c-FLIP displayed diffuse staining, while the lung metastases showed staining that was defined with greater intensity.
DISCUSSION
Despite advances in chemotherapy and surgery, survival rates in patients with OS pulmonary metastases are very poor. In particular, the 5-year survival rate in patients with lung metastases is less than 20% (7). Furthermore, the majority of deaths associated with OS are due to metastatic disease (17). Therefore, understanding the molecular pathway involved in the metastatic process and that permit OS cells to grow in the lung microenvironment is important in the development of novel therapeutic strategies for patients. Investigating the process of metastasis may uncover mechanisms of drug resistance, which in turn can lead to new therapeutic approaches.
We have previously demonstrated the importance of the Fas/FasL pathway and a FasL+ lung microenvironment to the metastatic potential of OS (9). We demonstrated that the majority of metastatic tumors in the lung from patients were Fas−, whereas the primary tumors in the bone contained a mixed population of Fas− and Fas+ cells (10). Although enhanced Fas expression in tumors has been associated with sensitivity to chemotherapy, some studies have shown that the presence of Fas is not always synonymous with Fas pathway functionality and drug sensitivity (18). Alterations in the Fas/FasL pathway, such as downregulation of caspases and upregulation of the anti-apoptotic proteins bcl-2 and c-FLIP, have been reported to correlate with resistance to apoptosis (11, 19, 20).
A large body of evidence indicates that c-FLIP is overexpressed in many human cancer cells. Studies have demonstrated Fas pathway resistance and increased c-FLIP expression in patients with gastric cancer, non-Hodgkin’s lymphoma, bladder urothelial carcinoma and colorectal carcinoma (12, 13, 15, 21, 22). Furthermore, c-FLIP has been shown to be linked with tumor progression, metastasis and chemotherapy resistance (15, 23, 24).
While loss of Fas expression was associated with pulmonary OS metastasis, we also found Fas+ cells in lung metastases obtained from some of the patients, suggesting that these Fas+ cells had evaded FasL-induced cell death in the lung microenvironment (10). Theoretically, this could be mediated by the overexpression of an inhibitor of the Fas signaling pathway, such as c-FLIP. We previously demonstrated that therapeutic agents which downregulated c-FLIP expression in vivo induced tumor cell apoptosis and regression of pulmonary OS metastasis (25). Therefore, c-FLIP may be a candidate for OS therapeutic intervention. In addition, because the Fas/FasL pathway is important in OS lung metastasis, c-FLIP may be a key regulator of tumor progression. Thus, we evaluated the expression of c-FLIP in patients with OS.
We obtained primary and lung metastasis OS samples from patients and analyzed them for c-FLIP expression. While samples from 10 patients were initially obtained, 4 of these patients had matching primary and metastastic sections. Therefore, for the purpose of this study we included only these 4 patients. Obtaining matching samples from patients is a significant challenge; however, a study with a larger cohort is warranted. We observed that all lung metastases from patients were c-FLIP positive, while only 1 patient had a primary tumor that was c-FLIP positive (Figure 1). Furthermore, analysis of human xenograft models demonstrated that all lung metastases were c-FLIP positive. However, only 3 mice with CCH-OS-D and 3 mice with KRIB had a primary tumors with c-FLIP positivity (Figure 2 and Figure 3). Of note, the staining pattern in this sample was observed to be more diffuse and with less intensity than the c-FLIP staining in lung metastases. Overall, this data together with our previously published findings indicate that OS lung metastases have decreased Fas and increased c-FLIP expression. This is consistent with studies of colorectal and bladder urothelial carcinomas, in which samples exhibited low Fas and high c-FLIP expression (12, 21).
Our results demonstrate for the first time that c-FLIP is overexpressed in OS lung metastases as compared to primary OS tumors. This suggests that upregulation of c-FLIP may contribute to the ability of OS cells to evade FasL in the lung microenvironment. One possibility is that increased expression of c-FLIP may permit both Fas+ and Fas− OS cells to grow in the lung by blocking FasL-induced apoptosis. This would explain our finding of Fas+ cells in some of the patient OS metastatic samples. While the analysis of serial slices of lung from CCH-OS-D and KRIB xenografts revealed few metastatic tumors with both Fas and c-FLIP positivity, the majority of tumors were Fas− (data not shown). We have also previously demonstrated that inhibition of c-FLIP expression by shRNA increased Fas expression in membrane lipid rafts (26). This data suggests a potential relationship between c-FLIP and the regulation of Fas expression. Further studies are required to evaluate the co-localization of Fas and c-FLIP expression within a single tumor cell and to investigate whether c-FLIP may play a role in the regulation of Fas expression.
Overexpression of c-FLIP may be present in a small portion of the cells in the primary tumor or may become upregulated during metastasis from the primary site. Alternatively, factors in the microenvironment at the metastatic site may alter the expression of apoptotic proteins. Since it is well understood that cytokines such as interferon (IFN)-α, IFN-β, and interleukins induce the upregulation of apoptosis-related molecules such as c-FLIP, it is possible that the presence of cytokines in the lung microenvironment may have an impact on c-FLIP expression (27-30). These c-FLIP positive cells can circumvent Fas/FasL-induced cell death and form metastases. However, the mechanism of how c-FLIP is upregulated in OS cells remains to be identified.
In summary, these studies add to the understanding of how modulation of certain proteins in the Fas signaling pathway can contribute to the metastatic potential of OS. Understanding the biology of metastasis and how OS cells evade the FasL+ lung microenvironment may allow the identification of novel therapeutic approaches that target this particular pathway.
ACKNOWLEDGEMENTS
This work was supported by National Cancer Institute grant R01 CA42992 (ESK), National Institutes of Health core grant CA16672 (ESK), and in part by a fellowship award from the American Legion Auxiliary (KR-B). We thank Dr. Nancy Gordon for the patient tissue samples and Dr. Dennis Hughes for the CCH-OS-D cells. This paper includes data that was presented in a dissertation (by KR-B) to The University of Texas MD Anderson Cancer Center and UT Health Graduate School of Biomedical Sciences: Bindal, Krithi R., “The histone deacetylase inhibitor, MS-275, sensitizes metastatic osteosarcoma to FasL-induced cell death: a role for c-FLIP” (2012). UT GSGS Dissertations and Theses. Paper 218.
REFERENCES
- 1.Kaste S, Pratt C, Cain A, et al. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: Imaging features. Cancer. 1999;86:1602–1608. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 3.Wang LL. Biology of osteogenic sarcoma. Cancer J. 2005;11:294–305. doi: 10.1097/00130404-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Gillette J, Chan D, Nielsen-Preiss S. Annexin 2 expression is reduced in human osteosarcoma metastases. J Cell Biochem. 2004;92:820–832. doi: 10.1002/jcb.20117. [DOI] [PubMed] [Google Scholar]
- 5.Perissinotto E, Cavalloni G, Leone F, et al. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res. 2005;11:490–497. [PubMed] [Google Scholar]
- 6.Koshkina NV, Khanna C, Mendoza A, et al. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: The role of the fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res. 2007;5:991–999. doi: 10.1158/1541-7786.MCR-07-0007. [DOI] [PubMed] [Google Scholar]
- 7.Gordon N, Kleinerman ES. The role of Fas/FasL in the metastatic potential of osteosarcoma and targeting this pathway for the treatment of osteosarcoma lung metastases. Cancer Treat Res. 2009;152:497–508. doi: 10.1007/978-1-4419-0284-9_29. [DOI] [PubMed] [Google Scholar]
- 8.Wajant H. The fas signaling pathway: More than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 9.Worth LL, Lafleur EA, Jia SF, et al. Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncol Rep. 2002;9:823–827. [PubMed] [Google Scholar]
- 10.Gordon N, Arndt CA, Hawkins DS, et al. Fas expression in lung metastasis from osteosarcoma patients. J Pediatr Hematol Oncol. 2005;27:611–615. doi: 10.1097/01.mph.0000188112.42576.df. [DOI] [PubMed] [Google Scholar]
- 11.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 12.Korkolopoulou P, Goudopoulou A, Voutsinas G, et al. c-FLIP expression in bladder urothelial carcinomas: Its role in resistance to fas-mediated apoptosis and clinicopathologic correlations. Urology. 2004;63:1198–1204. doi: 10.1016/j.urology.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Valente G, Manfroi F, Peracchio C, et al. C-FLIP expression correlates with tumour progression and patient outcome in non-hodgkin lymphomas of low grade of malignancy. Br J Haematol. 2006;132:560–570. doi: 10.1111/j.1365-2141.2005.05898.x. [DOI] [PubMed] [Google Scholar]
- 14.Ryu BK, Lee MG, Chi SG, et al. Increased expression of c-FLIP(l) in colonic adenocarcinoma. J Pathol. 2001;194:15–19. doi: 10.1002/path.835. [DOI] [PubMed] [Google Scholar]
- 15.Longley DB, Wilson TR, McEwan M, et al. C-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–848. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 16.Matta H, Eby MT, Gazdar AF, et al. Role of mrit/cflip in protection against chemotherapy-induced apoptosis. Cancer Biol Ther. 2002;1:652–660. doi: 10.4161/cbt.315. [DOI] [PubMed] [Google Scholar]
- 17.Hughes DP. Strategies for the targeted delivery of therapeutics for osteosarcoma. Expert Opin Drug Deliv. 2009;6:1311–1321. doi: 10.1517/17425240903280422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Kim HS, Kim SY, et al. Increased expression of flip, an inhibitor of fas-mediated apoptosis, in stomach cancer. APMIS. 2003;111:309–314. doi: 10.1034/j.1600-0463.2003.1110203.x. [DOI] [PubMed] [Google Scholar]
- 19.Korkolopoulou P, Lazaris A, Konstantinidou AE, et al. Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur Urol. 2002;41:274–283. doi: 10.1016/s0302-2838(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 20.Tamm I, Kornblau SM, Segall H, et al. Expression and prognostic significance of iap-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 21.Korkolopoulou P, Saetta AA, Levidou G, et al. C-flip expression in colorectal carcinomas: Association with Fas/FasL expression and prognostic implications. Histopathology. 2007;51:150–156. doi: 10.1111/j.1365-2559.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- 22.Ryang DY, Joo YE, Chung KM, et al. Expression of c-flip in gastric cancer and its relation to tumor cell proliferation and apoptosis. Korean J Intern Med. 2007;22:263–269. doi: 10.3904/kjim.2007.22.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Xu X, Bai L, et al. Epidermal growth factor receptor-mediated tissue transglutaminase overexpression couples acquired tumor necrosis factor-related apoptosis-inducing ligand resistance and migration through c-flip and mmp-9 proteins in lung cancer cells. J Biol Chem. 2011;286:21164–21172. doi: 10.1074/jbc.M110.207571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galligan L, Longley DB, McEwan M, et al. Chemotherapy and trail-mediated colon cancer cell death: The roles of p53, trail receptors, and c-flip. Mol Cancer Ther. 2005;4:2026–2036. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- 25.Rao-Bindal K, Kleinerman ES. The histone deacetylase inhibitor, MS-275, sensitizes osteosarcoma cells and osteosarcoma lung metastases to FasL-induced cell death by the downregulation c-FLIP [abstract] AACR; Orlando, Florida: Philadelphia (PA): Apr 2-5, 2011. [Google Scholar]
- 26.Rao-Bindal K, Zhou Z, Kleinerman ES. MS-275 senstizes osteosarcoma cells to Fas ligand-induced cell death by increasing the localization of Fas in membrane lipid rafts. Cell Death and Disease. 2012;3:e369. doi: 10.1038/cddis.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn EY, Pan G, Vickers SM, et al. IFN-gamma upregulates apoptosis-related molecules and enhances Fas-mediated apoptosis in human cholangiocarcinoma. Int J Cancer. 2002;100:445–451. doi: 10.1002/ijc.10516. [DOI] [PubMed] [Google Scholar]
- 28.Kanetaka Y, Hayashida M, Hoshika A, et al. Interferon-alpha induces transient upregulation of c-FLIP through NF-kappa B activation. Exp Cell Res. 2008;314:246–254. doi: 10.1016/j.yexcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6:196, 204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- 30.Kothny-Wilkes G, Kulms D, Pöppelmann B, et al. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 1998;273:29247–29253. doi: 10.1074/jbc.273.44.29247. [DOI] [PubMed] [Google Scholar]