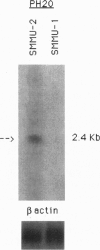
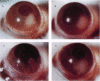
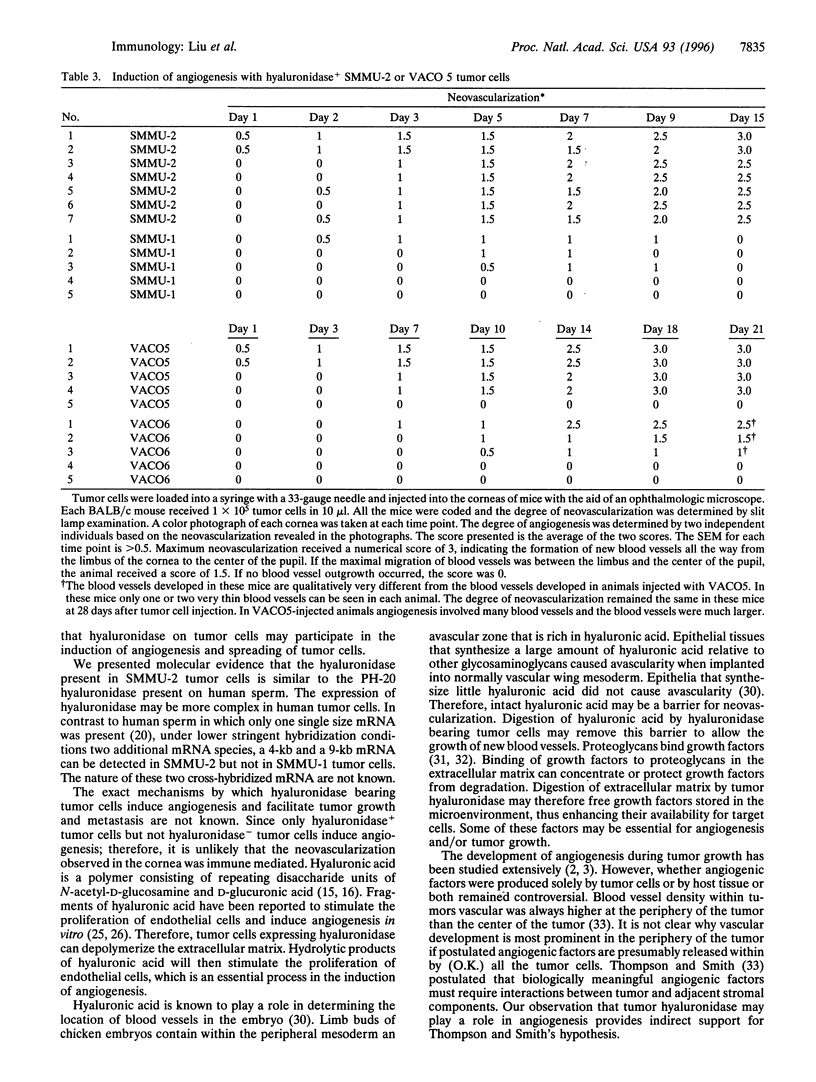
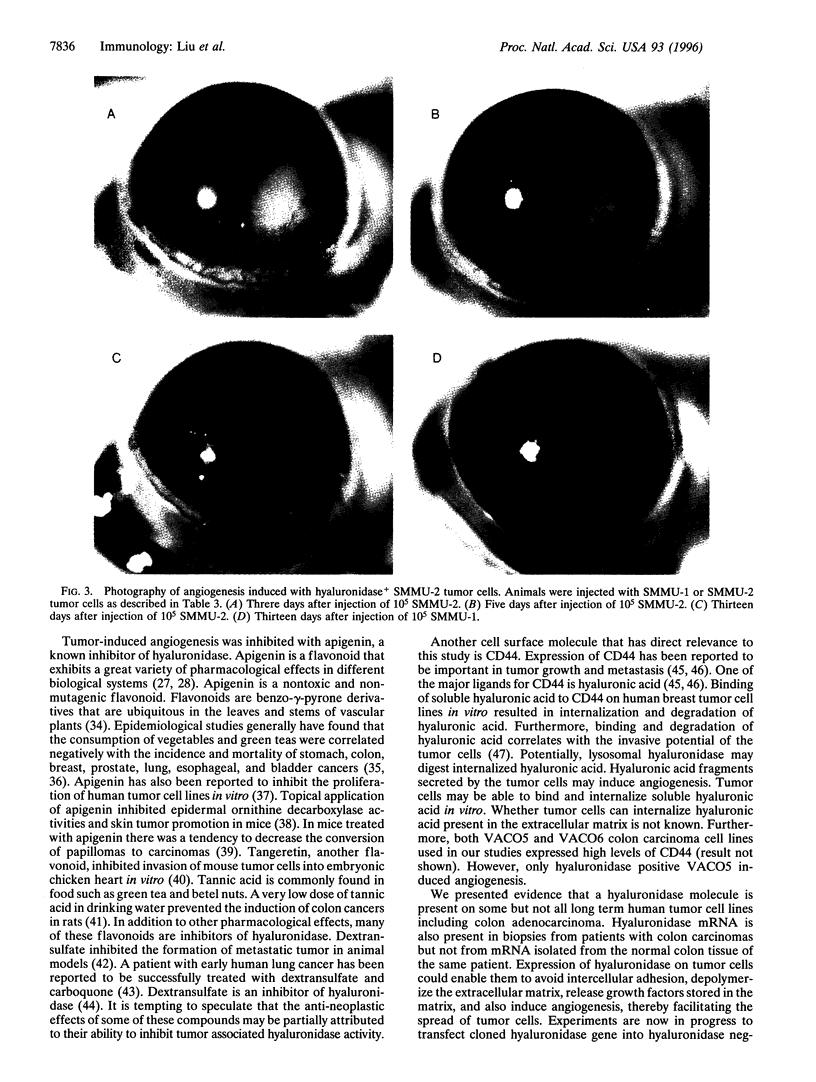
Abstract
Hyaluronic acid is a proteoglycan present in the extracellular matrix and is important for the maintenance of tissue architecture. Depolymerization of hyaluronic acid may facilitate tumor invasion. In addition, oligosaccharides of hyaluronic acid have been reported to induce angiogenesis. We report here that a hyaluronidase similar to the one on human sperm is expressed by metastatic human melanoma, colon carcinoma, and glioblastoma cell lines and by tumor biopsies from patients with colorectal carcinomas, but not by tissues from normal colon. Moreover, angiogenesis is induced by hyaluronidase+ tumor cells but not hyaluronidase- tumor cells and can be blocked by an inhibitor of hyaluronidase. Tumor cells thus use hyaluronidase as one of the "molecular saboteurs" to depolymerize hyaluronic acid to facilitate invasion. As a consequence, breakdown products of hyaluronic acid can further promote tumor establishment by inducing angiogenesis. Hyaluronidase on tumor cells may provide a target for anti-neoplastic drugs.
Full text
PDF
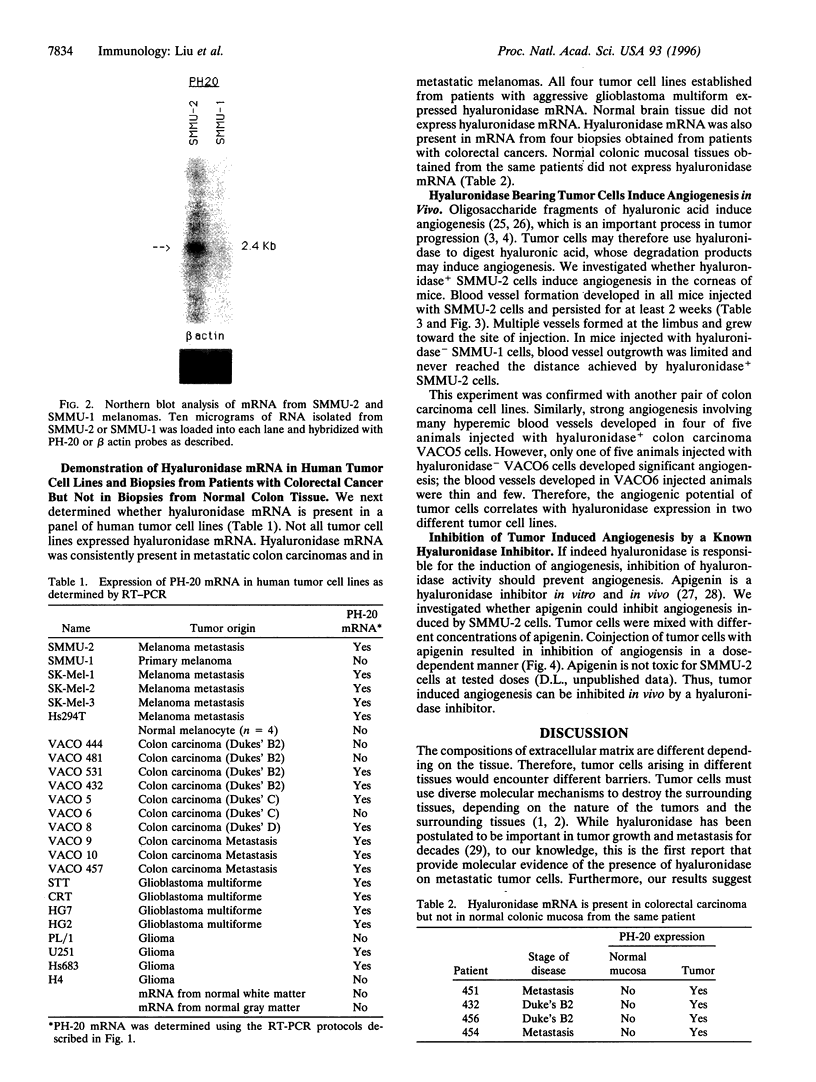

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H. Does fiber-rich food containing animal lignan precursors protect against both colon and breast cancer? An extension of the "fiber hypothesis". Gastroenterology. 1984 Apr;86(4):761–764. [PubMed] [Google Scholar]
- Bracke M. E., Vyncke B. M., Van Larebeke N. A., Bruyneel E. A., De Bruyne G. K., De Pestel G. H., De Coster W. J., Espeel M. F., Mareel M. M. The flavonoid tangeretin inhibits invasion of MO4 mouse cells into embryonic chick heart in vitro. Clin Exp Metastasis. 1989 May-Jun;7(3):283–300. doi: 10.1007/BF01753681. [DOI] [PubMed] [Google Scholar]
- COBBIN L. B., DICKER S. E. Some characteristics of plasma and urine 'hyaluronidase'. J Physiol. 1962 Aug;163:168–174. doi: 10.1113/jphysiol.1962.sp006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981 Sep;41(9 Pt 2):3685–3690. [PubMed] [Google Scholar]
- Craft P. S., Harris A. L. Clinical prognostic significance of tumour angiogenesis. Ann Oncol. 1994 Apr;5(4):305–311. doi: 10.1093/oxfordjournals.annonc.a058829. [DOI] [PubMed] [Google Scholar]
- Culty M., Shizari M., Thompson E. W., Underhill C. B. Binding and degradation of hyaluronan by human breast cancer cell lines expressing different forms of CD44: correlation with invasive potential. J Cell Physiol. 1994 Aug;160(2):275–286. doi: 10.1002/jcp.1041600209. [DOI] [PubMed] [Google Scholar]
- Feinberg R. N., Beebe D. C. Hyaluronate in vasculogenesis. Science. 1983 Jun 10;220(4602):1177–1179. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19(0):331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- Gmachl M., Kreil G. Bee venom hyaluronidase is homologous to a membrane protein of mammalian sperm. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3569–3573. doi: 10.1073/pnas.90.8.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Ma J., Wang J., Che X., Narula J., Bigby M., Wu M., Sy M. S. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994 Mar 15;54(6):1561–1565. [PubMed] [Google Scholar]
- Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983 Apr 1;32(7):1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Zöller M., Pals S. T., Ponta H. CD44 splice variants: metastases meet lymphocytes. Immunol Today. 1993 Aug;14(8):395–399. doi: 10.1016/0167-5699(93)90141-7. [DOI] [PubMed] [Google Scholar]
- Hirano T., Oka K., Akiba M. Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75-1. Res Commun Chem Pathol Pharmacol. 1989 Apr;64(1):69–78. [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans and neoplasia. Cancer Metastasis Rev. 1988 Apr;7(1):39–50. doi: 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- Katiyar S. K., Agarwal R., Mukhtar H. Protection against malignant conversion of chemically induced benign skin papillomas to squamous cell carcinomas in SENCAR mice by a polyphenolic fraction isolated from green tea. Cancer Res. 1993 Nov 15;53(22):5409–5412. [PubMed] [Google Scholar]
- Klagsbrun M. The affinity of fibroblast growth factors (FGFs) for heparin; FGF-heparan sulfate interactions in cells and extracellular matrix. Curr Opin Cell Biol. 1990 Oct;2(5):857–863. doi: 10.1016/0955-0674(90)90084-r. [DOI] [PubMed] [Google Scholar]
- Kuppusamy U. R., Das N. P. Inhibitory effects of flavonoids on several venom hyaluronidases. Experientia. 1991 Dec 1;47(11-12):1196–1200. doi: 10.1007/BF01918384. [DOI] [PubMed] [Google Scholar]
- Kuppusamy U. R., Das N. P. Protective effects of tannic acid and related natural compounds on Crotalus adamenteus subcutaneous poisoning in mice. Pharmacol Toxicol. 1993 Apr-May;72(4-5):290–295. doi: 10.1111/j.1600-0773.1993.tb01652.x. [DOI] [PubMed] [Google Scholar]
- Lin Y., Kimmel L. H., Myles D. G., Primakoff P. Molecular cloning of the human and monkey sperm surface protein PH-20. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10071–10075. doi: 10.1073/pnas.90.21.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Di Ferrante D., Di Ferrante N., Nicolson G. L. Heparan sulfate degradation: relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science. 1983 May 6;220(4597):611–613. doi: 10.1126/science.6220468. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Fukaura Y. A very low dose of green tea polyphenols in drinking water prevents N-methyl-N-nitrosourea-induced colon carcinogenesis in F344 rats. Jpn J Cancer Res. 1993 Oct;84(10):1007–1009. doi: 10.1111/j.1349-7006.1993.tb02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Hyatt H., Myles D. G. A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucida. J Cell Biol. 1985 Dec;101(6):2239–2244. doi: 10.1083/jcb.101.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanaiah M., Parthasarathy P. R., Venkaiah B. Isolation and characterization of hyaluronidase from scorpion (Heterometrus fulvipes) venom. Biochem Int. 1990;20(2):301–310. [PubMed] [Google Scholar]
- Rooney P., Kumar S., Ponting J., Wang M. The role of hyaluronan in tumour neovascularization (review). Int J Cancer. 1995 Mar 3;60(5):632–636. doi: 10.1002/ijc.2910600511. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Liu D., Schiavone R., Ma J., Mori H., Guo Y. Interactions between CD44 and hyaluronic acid: their role in tumor growth and metastasis. Curr Top Microbiol Immunol. 1996;213(Pt 3):129–153. doi: 10.1007/978-3-642-80071-9_9. [DOI] [PubMed] [Google Scholar]
- Thompson W. D., Shiach K. J., Fraser R. A., McIntosh L. C., Simpson J. G. Tumours acquire their vasculature by vessel incorporation, not vessel ingrowth. J Pathol. 1987 Apr;151(4):323–332. doi: 10.1002/path.1711510413. [DOI] [PubMed] [Google Scholar]
- Toole B. P. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990 Oct;2(5):839–844. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- Tsubura E., Yamashita T., Kobayashi M., Higuchi Y. Effect of sulfated polysaccharides on blood-borne pulmonary metastasis in rats. Gan. 1976 Dec;67(6):849–856. [PubMed] [Google Scholar]
- Tu A. T., Hendon R. R. Characterization of lizard venom hyaluronidase and evidence for its action as a spreading factor. Comp Biochem Physiol B. 1983;76(2):377–383. doi: 10.1016/0305-0491(83)90086-x. [DOI] [PubMed] [Google Scholar]
- Wei H., Tye L., Bresnick E., Birt D. F. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990 Feb 1;50(3):499–502. [PubMed] [Google Scholar]
- West D. C., Hampson I. N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985 Jun 14;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Willson J. K., Bittner G. N., Oberley T. D., Meisner L. F., Weese J. L. Cell culture of human colon adenomas and carcinomas. Cancer Res. 1987 May 15;47(10):2704–2713. [PubMed] [Google Scholar]
- Zimmermann K., Preinl G., Ludwig H., Greulich K. O. Inhibition of hyaluronidase by dextran sulfate and its possible application in anticancer treatment. J Cancer Res Clin Oncol. 1983;105(2):189–190. doi: 10.1007/BF00406931. [DOI] [PMC free article] [PubMed] [Google Scholar]