Abstract
The dystonias comprise a group of syndromes characterized by prolonged involuntary muscle contractions resulting in repetitive movements and abnormal postures. Primary dystonia has been associated with over 14 different genotypes, most of which follow an autosomal dominant inheritance pattern with reduced penetrance. Independent of etiology, the disease is characterized by extensive variability in disease phenotype and clinical severity. Recent neuroimaging studies investigating this phenomenon in manifesting and non-manifesting genetic carriers of dystonia have discovered microstructural integrity differences in the cerebello-thalamo-cortical tract in both groups related to disease penetrance. Further study suggests these differences to be specific to subrolandic white matter regions somatotopically related to clinical phenotype. Clinical severity was correlated to the degree of microstructural change. These findings suggest a mechanism for the penetrance and clinical variability observed in dystonia and may represent a novel therapeutic target for patients with refractory limb symptoms.
Keywords: Dystonia, Cerebellum, Basal ganglia, Thalamus, DYT1 dystonia, DYT6 dystonia, Phenotype, Penetrance
Introduction
Dystonia is the third most prevalent movement disorder after essential tremor and Parkinson’s disease [1]. Clinically, the dystonias comprise a group of syndromes characterized by prolonged involuntary muscle contractions resulting in repetitive movements and abnormal postures [1]. Classification can be done on the basis of symptom distribution, disease onset age, and underlying etiology. Dystonia can be focal, affecting a single muscle group (i.e., dystonias such as writer’s cramp, blepharospasm, or torticollis), or generalized, with the involvement of multiple muscle groups throughout the body [2-4]. Symptoms may first appear anytime from childhood to adulthood, with earlier onset generally associated with a genetic etiology [5, 6].
From both clinical and research perspectives it is probably most useful to classify the disease on the basis of etiology. Primary dystonia is defined by the absence of associated disease and lack of a defined cause [7], whereas secondary dystonia develops secondary to an event, such as medications or neurological lesions. Primary dystonia can occur sporadically, or be inherited. Over 14 different genes have been identified to be associated with dystonia, with the majority following an autosomal dominant pattern with incomplete penetrance [1]. In pure primary dystonia, where torsion dystonia (with or without tremor) is the only symptom, the most common mutation, DYT1, has a clinical penetrance rate of 30% in carriers [8], whereas in DYT6, another associated mutation, symptoms occur in about 60% of its carriers [9, 10]. Clinically, both mutations are associated with early onset dystonia, either in childhood or adolescence, but both demonstrate high phenotypic variability, ranging from different focal manifestations to generalized symptoms [5].
Despite the genetic advancements in the field of dystonia, the pathophysiology accounting for penetrance and symptom variability remains elusive. The DYT1 mutation results in a GAG deletion in the coding region for TorsinA, a member of the AAA+ superfamily of ATPases, but this protein’s precise function is unknown, though it appears important for cellular compartments and synaptic vesicle machinery [5, 11]. It still remains to be confirmed if this mutation results in a loss of function or gain of function [5, 12]. The pathophysiology of the DYT6 mutation is not well defined either. Multiple mutations in this gene have been associated to DYT6 dystonia in the THAP1 gene, including different frameshift, missense and nonsense mutations [5, 13]. Different mutations are associated with different clinical presentations [14-16]. The THAP1 gene encodes for a protein with DNA-binding properties, believed to regulate gene transcription [5, 17]. Cellular studies have shown that THAP1 may normally bind to the TOR1A promoter downregulating TorsinA expression. Its mutated form, on the other hand, is unable to repress this transcription [18, 19]. Although this study provides a potential link between DYT1 and DYT6 dystonias, the relationship between the molecular pathology and anatomical basis of disease remains to be shown.
Historically, dystonia has been viewed as a disorder involving the basal ganglia and the thalamus [20-25]. This view is strengthened by clinical observations; lesions in the basal ganglia can trigger symptomatic dystonia, and the globus pallidus internus is a common target for therapeutic intervention [26, 27]. Nonetheless, post-mortem neuropathological studies, often limited by small sample sizes, have led to inconclusive results with no obvious single neurodegenerative process [28, 29]. Imaging studies, on the other hand, have shown abnormalities in various brain regions [30-33]. However, motor control is subserved by pathways linking the basal ganglia and the cerebellum to cortical areas. Indeed, it appears increasingly apparent from clinical data and animal models that cerebellar pathways may play a role in the pathophysiology of dystonia [20, 27, 31, 34-41]. In this review, we summarize recent neuroimaging findings in primary dystonia, focusing on lesions in cerebellar projections, and describing how this work has led to a compelling explanation for the variability in dystonia penetrance and phenotype, possibly opening up new avenues for treatment.
Microstructural Pathway Abnormalities
Primary dystonia is characterized by its absence of brain lesions on routine imaging, which does not preclude the presence of subtle microstructural or functional lesions. Diffusion tensor imaging (DTI), which uses the index of fractional anisotropy (FA) based on the direction of water diffusion, can detect areas of disordered diffusion, thereby indicating microstructural abnormalities. Indeed, such DTI studies have reported abnormalities throughout the brain. Among the hereditary dystonias, previous studies have found abnormalities in the subgyral white matter of the sensorimotor cortex in both manifesting and non-manifesting DYT1 and DYT6 carriers compared to controls [42-44]. In DYT-11 positive myoclonus-dystonia patients, the subthalamic area was found to be abnormal compared to control subjects [45]. Several different studies of primary cervical dystonia have found abnormalities throughout the putamen, pallidum, corpus callosum, prefrontal cortex and thalamus [30, 46-49]. Although these studies have identified regions of abnormalities in patients compared to controls, the majority do not identify genotype or phenotype-specific changes that can account for the characteristic penetrance and phenotypic variability of dystonia. Of note, dorsal pontine microstructural abnormalities in the vicinity of the superior cerebellar peduncle have been shown to be greater in manifesting than non-manifesting carriers [44]. Moreover, the degree of structural integrity loss in this region correlates with abnormally elevated expression of a movement-related metabolic pattern [50]. What then is the evidence that alteration in the cerebello-thalamo-cortical (CbTC) projections is linked to both penetrance and phenotypic variability in primary dystonia?
Cerebellar Connectivity and Penetrance
Previous studies of sporadic dystonias have also demonstrated involvement of the CbTC pathways. Patients with writer’s cramp have decreased gray matter volume in the cerebellum, thalamus, and somatosensory cortex, as well as diffusion abnormalities in fiber tracts connecting somatosensory cortex and subcortical areas [34, 42]. Bonilha et al. found reduced thalamo-prefrontal connections in patients with cervical dystonia [47].
However, by their nature, studies of sporadic dystonia are limited to symptomatic individuals. In contrast, the reduced penetrance in DYT1 and DYT6 dystonias allows for the identification of genotype-specific traits without confounding clinical factors. By combining high-field (3T) DTI and probabilistic tractography, Argyelan and colleagues have identified genotype-specific fiber tract differences between manifesting and non-manifesting DYT1 and DYT6 mutation carriers [51]. With this approach, manifesting and non-manifesting carriers were found to have reduced fiber tract integrity in their cerebellothalamic tract irrespective of clinical status (Fig. 1A, B). Further analysis revealed that cerebellothalamic connectivity in non-manifesting carriers was less reduced than in manifesting carriers, intermediate between manifesting carriers and controls.
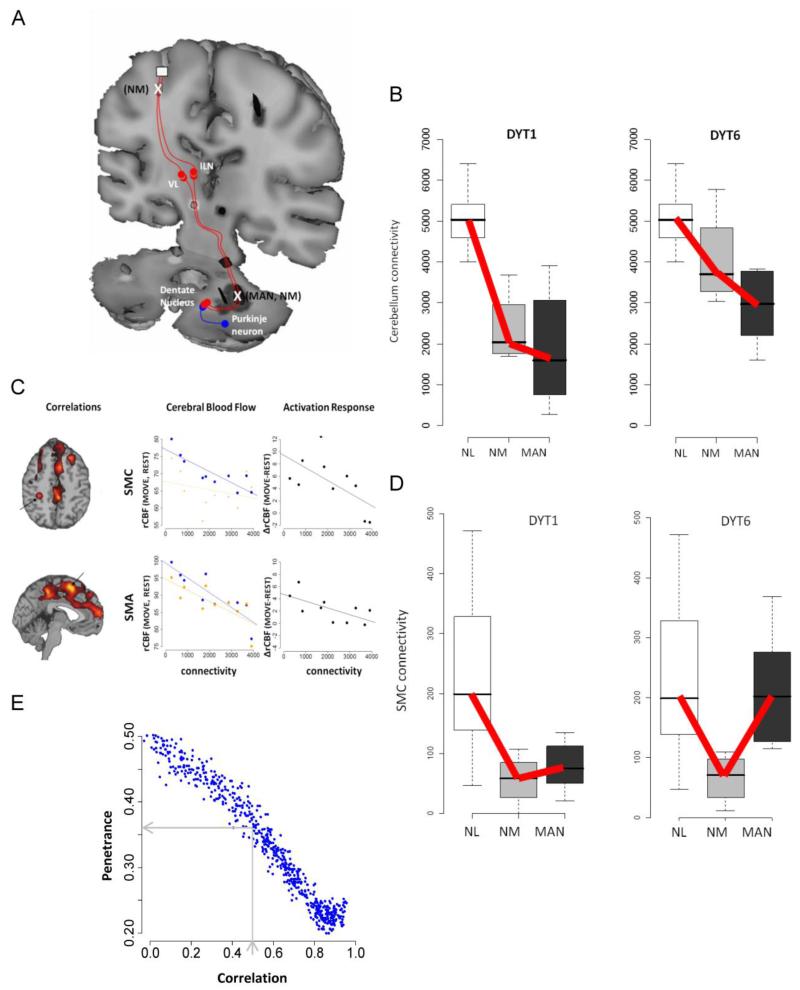
Figure 1. Cerebello-thalamo-cortical connectivity regulates penetrance in dystonia.
A tandem-lesion model is proposed to predict penetrance in genetic carriers of primary torsion dystonia. A. Diagram representation of the tandem-lesion model [51]. In this model, serial lesions in the cerebello-thalamo-cortical pathway, represented by an X, determine clinical penetrance of dystonia symptoms in manifesting (MAN) and non-manifesting (NM) DYT1 and DYT6 carriers. B. Connectivity values of the proximal cerebello-thalamo-cortical lesion for control (NL), non-manifesting and manifesting DYT1 and DYT6 carriers. Cerebellothalamic projections were abnormally reduced in genetic carriers relative to control (p<0.01 and p<0.001, Mann-Whitney U-tests). Test of trends reveals a significant decreasing group-wise trend in DYT1 and DYT6 carriers (NL>NM>MAN; p<0.001 and p=0.002 for DYT1 and DYT6, respectively). C. Voxel-wise searches revealed areas with significant correlation between cerebellar connectivity and rCBF values in several clusters during task performance. In cerebral blood flow plots, blue represents movement and orange represents rest. Motor activation responses indicate the difference [Δ= (move-rest)]. Cerebellar connectivity was negatively correlated with motor cortical activation. This correlation was movement specific in the SMC (MOVE: r=−0.85, p=0.002; REST: r=−0.27, p=0.44; MOVE - REST: r=−0.63, p=0.052) and task-independent in the SMA (MOVE: r=−0.87, p=0.001; REST: r=−0.76, p=0.011). D. Connectivity values of the distal cerebello-thalamo-cortical lesion for control, non-manifesting and manifesting DYT1 and DYT6 carriers. Test of trends reveals a different trend (NL> MAN> NM) for this cluster (thalamocortical tract) in DYT1 and DYT6 carriers (p<0.002 and p=0.0025). E. Monte Carlo simulations to predict penetrance using randomly generated connectivity values for proximal and distal cerebello-thalamo-cortical tract. A correlation coefficient of 0.56, based on human DYT1 carrier and control values, predicts a penetrance rate of approximately 36%. A correlation coefficient of 0.9, based on DYT1 KI mouse model and their littermate controls (see text), predicts a penetrance rate of <20%. (Figure 1A: Reprinted with permission from Elsevier: Niethammer M, Carbon M, Argyelan M, and Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42:202-9) [21]. (Figures B-E: Adapted from: Argyelan M, Carbon M, Niethammer M, et al.: Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:9740-7) [51].
The degree of structural abnormality was negatively correlated with regional blood flow (rCBF) values (measured with H215O PET) in the sensorimotor cortex (SMC) and the supplementary motor area (SMA), such that a greater cerebellar tract disruption was correlated with increased motor cortical metabolism (Fig. 1C). Thus, a loss of these cerebellar pathways, developmental or acquired, may lead to dystonia in manifesting subjects via loss of cortical inhibition. Nevertheless, as both manifesting and non-manifesting carriers exhibited reduced cerebellothalamic connectivity, this reduction in fiber integrity is unlikely to be sufficient to explain the reduced penetrance of DYT1 and DYT6 carriers.
Indeed, a further search subsequently revealed an additional tract abnormality, which differentiated between manifesting and non-manifesting DYT1/DYT6 carriers. Non-manifesting individuals were found to have an additional connectivity abnormality in the thalamocortical segment of the CbTC projection to the SMC and the SMA, in tandem to the first lesion. Manifesting individuals, on the other hand, had a connectivity value intermediate to that of control and non-manifesting subjects, suggesting a greater preservation of fibers in manifesting than in non-manifesting individuals (Fig. 1D). This finding supports a potential model for penetrance in dystonia: reduced connectivity in the proximal segment of CbTC projections, present in all carriers, would result in symptom manifestation due to reduced cortical inhibition, unless this transmission was disrupted by a second lesion in the distal segment of the tract, as seen in non-manifesting carriers (Fig. 1A).
Further support for this model of penetrance was gained by Monte Carlo simulation [51]. The difference between the connectivity values in the proximal and distal segments of the CbTC pathways was used as an index of relative integrity. Assigning random values to each segment within the range of measured connectivity values in the DYT1 carriers and normal controls resulted in predicted penetrance of 20-50%, depending on the level of correlation assumed between the two segments. Based on the observed correlation value of 0.56 (as measured in DYT1 and control subjects), these Monte Carlo simulations predicted a penetrance of 36%, which is in accordance with published values of DYT1 penetrance (Fig. 1E) [9, 10].
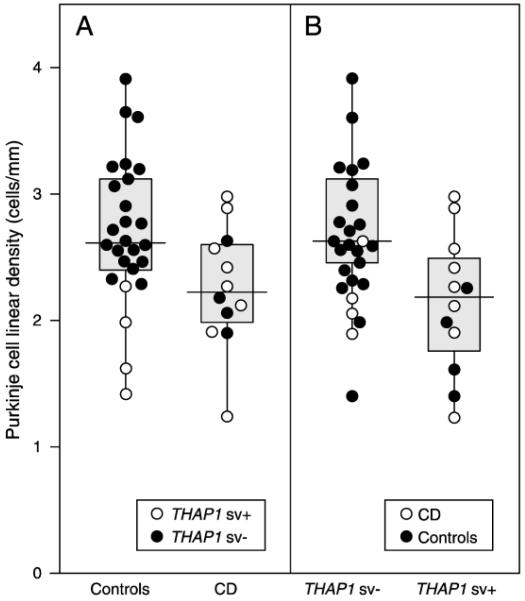
A recent post-mortem neuropathological study of cervical dystonia reported results consistent with the tandem-lesion model. Purkinje cell linear density was reduced in subjects with THAP1 sequence variants independent of clinically reported symptoms [28]. Although the THAP1 sequence variation in the control subjects was an incidental finding, the comparison of the association between Purkinje cell density in subjects with or without THAP1 sequence variation was actually stronger than the comparison of Purkinje cell density versus clinical manifestation (Fig. 2). While it remains to be determined if all the THAP1 variants in this study are indeed pathogenic mutations, the results of this study suggest that genetic-specific differences can result in Purkinje cell reduction in the cerebellum, independent of clinical status.
Figure 2. Cervical dystonia is correlated with a reduced Purkinje cell linear density independent of clinical manifestations.
A. Box and whisker plot and individual data points, comparing cervical dystonia with control subjects. Cervical dystonia (CD) was associated with significantly lower Purkinje cell linear density (p<0.05) B. Cases with THAP1 sequence variation (THAP1 sv+) had lower Purkinje cell linear density counts. Regression analysis showed that Purkinje cell linear density of THAP1 sv+ was significantly lower compared to THAP1 negative cases (THAP1 sv−) (p<0.05). (In A, open and closed circles represent the data for THAP1 positive and negative cases within each group. In B, open and closed circles represent the data for CD and controls within each THAP1 group.) (Reprinted with permission from Elsevier: Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Experimental neurology. 2013;241:95-104) [28**].
Confirmation of the Tandem-Lesion Model in a DYT1 Mouse Model
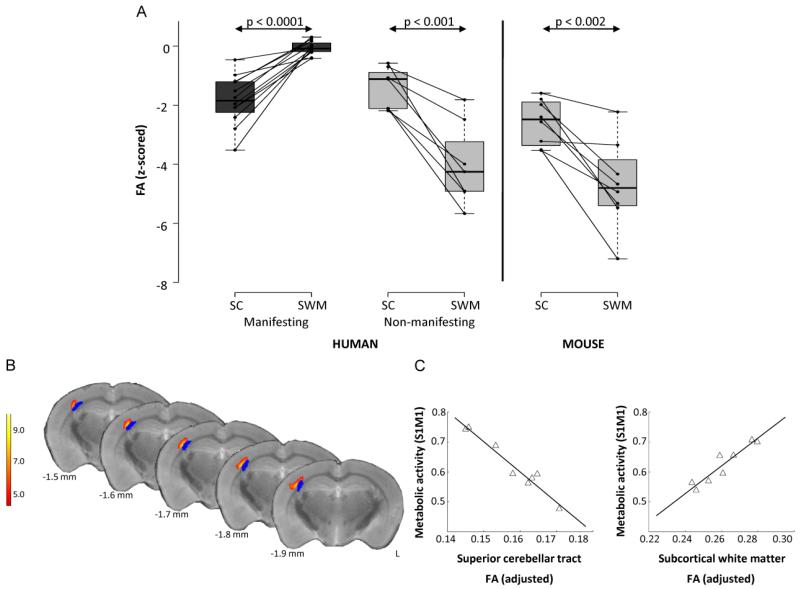
Several animal models of dystonia have implicated the cerebellum in the pathophysiology of dystonia [52-55]. Kainate microinjections into the cerebellar vermis of mice induce dose-dependent dystonic movements [36], and conditional elimination of Purkinje cells in tottering mice is sufficient to cause dystonia [38]. Of note, subclinical lesions in the striatum worsen kainate-induced dystonia, suggesting a functional relationship between the basal ganglia and cerebellum [35]. However, these models do not address the role of lesions in the CbTC pathway observed in the human subjects, prompting a similar multimodal imaging study of PET, high-field MR DTI and tractography using DYT1 knock-in (KI) mice models and their littermate controls [56]. DYT1 KI mice contain the GAG deletion in the endogenous murine torsinA allele, representing a genetic model of human dystonia. However, this animal model does not exhibit a motor phenotype [56]. Similar to the non-penetrant human DYT1 carriers, all individual KI animals exhibited abnormally reduced connectivity in both the cerebellothalamic and thalamocortical segment of the CbTC tract, with a greater reduction seen in the integrity of the distal tract (Fig. 3A) [56]. Additionally, DYT1 KI mice exhibited correlations of metabolic abnormalities and structural abnormalities similar to human studies [51, 56]. The metabolic activity in the right sensorimotor cortex (S1M1) was found to be negatively correlated to the FA values from the superior cerebellar tract (corresponding to the proximal CbTC tract segment), while positively correlated to subcortical white matter FA values (corresponding to the distal CbTC tract segment) (Fig. 3B, C). Moreover, Monte Carlo simulations predicted a low penetrance rate in these mice, owing to significantly higher correlation values between the proximal and distal CbTC lesions in mice compared to humans (0.9 vs. 0.56).
Figure 3. Comparison of connectivity values between human and mouse studies and metabolic abnormalities in mutant animals.
A. Regional differences in pathway microstructure: mechanism of penetrance. Left. Fractional anisotropy (FA) measured in the superior cerebellar (SC) pathway and in the subcortical white matter (SWM) subjacent to primary sensorimotor cortex in 12 manifesting (MAN) and eight non-manifesting (NM) genetic carriers of dystonia. For each region, individual subject FA values were z-transformed with respect to the corresponding cluster values from eight age-matched normal control subjects. Each of the two groups of gene carriers exhibited significant FA reductions in cerebellum relative to controls (p<0.001, Student’s t-test). By contrast, manifesting subjects had normal z-transformed values in the SWM, compared to non-manifesting carriers who had reduced values compared to control subjects (p<0.0001, Student’s t-test). Right. Analogous connectivity values in SC and SWM of DYT1 knock-in (KI) mice. Like human NM subjects, DYT1 KI mice had significantly reduced FA values in the SWM (p<0.0001, Student’s t-test). B. Voxel-wise multiple regression analysis revealed a relationship between connectivity values from proximal and distal cerebello-thalamocortical segments to the right sensorimotor cortex (S1M1) (yellow/red). This area was in close proximity to distal tract abnormality (blue). Color scale represents a t-value threshold of 5.89, corresponding to p<0.001. C. Multiple regression analysis examining relationship of proximal and distal pathway tract segments to S1M1 metabolic activity revealed a significant relationship between these measures (p<0.0001) expressed by y=ax1+bx1+c where a=−10.02 (p=0.0002), b=4.60 (p=0.0017), and c=1.01 (p=0.0034). The contribution of each abnormal white matter cluster to the metabolic activity is represented by the leverage plots. (Adapted from: Ulug AM, Vo A, Argyelan M, et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6638-43) [56**].
It is interesting to note that DYT1 KI models revealed additional microstructural abnormalities not previously found in the human studies. In addition to the two previously described tract abnormalities, mutant mice exhibited significantly reduced FA values in bilateral thalamostriatal tracts. Additionally, unilateral abnormalities were detected in several regions identified to be conduit regions of the basal ganglia to the cerebellum. The detection of these additional areas may be due to the higher sensitivity of the scanning modalities in conjunction with the genetic homogeneity of the animals. Furthermore, other studies of DYT1 KI mice have recently reported microstructural abnormalities in the dopaminergic systems, including decreased numbers of tyrosine hydroxylase-positive neurons, reduced dendrites with fewer spines, and a reduced ratio of axo-spinous to axo-dendritic synaptic inputs from glutamatergic and dopaminergic sources [57, 58].
Connectivity and Phenotype
As previously discussed, regions recently implicated from human studies in the pathology of dystonia have included the brainstem, subthalamic nucleus, basal ganglia areas such as putamen and pallidum, as well as cerebellar regions. All these implicated pathways appear to be vastly interconnected and play different roles in coordinating motor movements [30, 45]. The heterogeneity of the etiologies and phenotypes of dystonia examined in these studies may suggest independent pathologies for these different disorders. However, it is intriguing to theorize that regardless of etiology or phenotype, the symptoms of dystonia could be dictated by the overall balance between connectivity along different motor pathways. The tandem-lesion model suggests a mechanism that can explain the partial penetrance of genetic dystonias based on presence of microstructural abnormalities in the brain. Nevertheless, primary dystonia, both genetic and sporadic, exhibits wide variations in phenotype, even in individuals within the same family. Recently, Vo et al. examined the notion that the tandem-lesion model could be applied to account for this phenotype variability observed in primary torsion dystonia [59].
Expanding on the tandem-lesion model, dystonia subjects with different phenotypes were compared using DTI in order to detect microstructural differences associated with specific limb involvement. Tract associations were determined by a voxel-wise comparison of DTI scans, and specific neuroanatomical tracts were identified via tract reconstructions. According to the tandem-lesion model, a penetrant phenotype should be associated with somatotopic-specific alterations in the thalamocortical projections. Specifically, if the thalamocortical lesion results in reduced penetrance, one might expect that this lesion might be present only in fiber tracts relating to the unaffected limbs.
Indeed, subjects with leg dystonia were found to have intact subcortical microstructural integrity (compared to healthy controls) adjacent to the motor cortical leg area while subjects whose dystonia did not involve the leg exhibited significantly reduced connectivity in this region, similar to non-manifesting carriers. Likewise, subjects with dystonia involving the left arm demonstrated intact arm projections subjacent to the motor cortical arm area, while those without involvement of the left arm showed reduced integrity of fibers in this region. Furthermore, these structural changes were phenotype-specific such that an individual with upper limb symptoms had intact projections to the arm region with reduced projections to the leg region. The converse was also true for subjects with leg symptoms, who had intact projections to the leg region and reduced projections to the arm region. In all subjects, these findings were anatomically specific to thalamocortical fibers, without analogous reductions in corticostriatal or corticospinal fiber counts.
This study also examined the extent to which microstructural changes were correlated to symptom severity. Compared to control subjects, significant hemispheric asymmetries were found in subjects whose symptoms were restricted to the dominant right arm. In this cohort, arm-related FA values were lower in the hemisphere opposite the asymptomatic limb compared to the hemisphere controlling the clinically affected limb, where FA values were in normal range. In subjects with bilateral arm involvement, hemispheric differences were more subtle and may not have been discernible at the smoothing levels used in the data analysis. However, when the right hemispheric cluster identified in the subjects with left arm dystonia was transposed to a mirror region on the left hemisphere, these values were correlated with right arm dystonia severity, demonstrating that a higher score in dystonia severity rating is associated with greater fiber preservation. These data suggest that clinical severity, in addition to clinical distribution, is associated with somatotopic-specific changes in thalamocortical projections.
The proximal cerebellothalamic lesion was also confirmed in DYT1 and DYT6 subjects of this cohort, who exhibited cerebellothalamic FA reductions in a similar region as that of the Argyelan et al. study [51]. The presence of this lesion appeared mainly genotype-related, as subjects with sporadic dystonia, had less consistent reductions in this region when analyzed on an individual level. Overall, 85% (11/13) of genetic carriers were found to have cerebellar FA reductions greater than 1 standard deviation below the mean, whereas only 57% (4/7) of sporadic cases were found to have an FA reduction of this magnitude.
Conclusion
Due to the absence of pathological lesions in post-mortem brains, imaging studies have been great use for the study of dystonia. Recent neuroimaging studies have described an interesting model explaining the variability in disease penetrance and phenotype. Further studies of animal models and patient populations are needed to elucidate a precise connection between the structural-functional aspect of the disease to the molecular pathology and genetic etiology. A mouse model with independent lesions of the two sections of the CbTC pathway would be invaluable, as it would be possible to verify the proposition that penetrance, phenotype and clinical severity are all determined by somatotopically-relevant fiber changes.
Meanwhile, these recent discoveries may be utilized to better guide current treatment protocols. Internal globus pallidus (GPi) deep brain stimulation (DBS) is an effective treatment option for patients with generalized or focal dystonia [60-62]. Although this treatment offers relief for many patients, it is not 100% effective [63-65]. In patients with refractory limb symptoms, for whom GPi DBS does not work, it may be beneficial to selectively target the affected thalamocortical fiber pathways for stimulation [59]. In theory, if the tandem-lesion model holds true, it would be possible to ablate specific fibers for a permanent “cure” of the symptoms.
Acknowledgment
David Eidelberg has received grant support from the National Institute of Neurological Disorders and Stroke (R01 NS 072514), The Bachmann-Strauss Dystonia and Parkinson Foundation, High Q Foundation, and the Dana Foundation.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Renata Lerner has received an MD/PhD candidate stipend from Hofstra North Shore-LIJ School of Medicine.
Martin Niethammer is employed by The Feinstein Institute for Medical Research.
David Eidelberg has board membership with The Bachmann-Strauss Dystonia and Parkinson Foundation, Michael J. Fox Foundation for Parkinson’s Research, Thomas Hartman Foundation for Parkinson’s Research, Inc.; has been a consultant for Pfizer, Inc.; and is employed by The Feinstein Institute for Medical Research. He is coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same; no financial gain. He has received honoraria from The Bachmann-Strauss Dystonia and Parkinson Foundation and Michael J. Fox Foundation for Parkinson’s Research.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Breakefield XO, Blood AJ, Li Y, et al. The pathophysiological basis of dystonias. Nature reviews. Neuroscience. 2008;9:222–34. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 2.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: A consensus update. Movement disorders: official journal of the Movement Disorder Society. 2013;28:863–73. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamm C. Early onset torsion dystonia (Oppenheim’s dystonia) Orphanet journal of rare diseases. 2006;1:48. doi: 10.1186/1750-1172-1-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeth AH. The genetics of primary dystonias and related disorders. Brain: a journal of neurology. 2002;125:695–721. doi: 10.1093/brain/awf090. [DOI] [PubMed] [Google Scholar]
- 5.Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain: a journal of neurology. 2013;136:2017–37. doi: 10.1093/brain/awt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghimi N, Jabbari B, Szekely AM. Primary dystonias and genetic disorders with dystonia as clinical feature of the disease. European journal of paediatric neurology: EJPN: official journal of the European Paediatric Neurology Society. 2013 doi: 10.1016/j.ejpn.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Bressman SB. Dystonia Genotypes, Phenotypes, and Classification. In: Fahn S, Hallett M, DeLong MR, editors. Dystonia 4: Advances in Neurology. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 101–7. [PubMed] [Google Scholar]
- 8.Bressman S. Genetics of dystonia. Journal of neural transmission. Supplementum. 2006:489–95. doi: 10.1007/978-3-211-45295-0_73. [DOI] [PubMed] [Google Scholar]
- 9.Risch NJ, Bressman SB, Senthil G, Ozelius LJ. Intragenic Cis and Trans modification of genetic susceptibility in DYT1 torsion dystonia. American journal of human genetics. 2007;80:1188–93. doi: 10.1086/518427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders-Pullman R, Raymond D, Senthil G, et al. Narrowing the DYT6 dystonia region and evidence for locus heterogeneity in the Amish-Mennonites. American journal of medical genetics. Part A. 2007;143A:2098–105. doi: 10.1002/ajmg.a.31887. [DOI] [PubMed] [Google Scholar]
- 11.Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nature reviews. Neurology. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–32. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Dobricic VS, Kresojevic ND, Svetel MV, et al. Mutation screening of the DYT6/THAP1 gene in Serbian patients with primary dystonia. Journal of neurology. 2013;260:1037–42. doi: 10.1007/s00415-012-6753-6. [DOI] [PubMed] [Google Scholar]
- 14.Houlden H, Schneider SA, Paudel R, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–50. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiromerisiou G, Houlden H, Scarmeas N, et al. THAP1 mutations and dystonia phenotypes: genotype phenotype correlations. Movement disorders: official journal of the Movement Disorder Society. 2012;27:1290–4. doi: 10.1002/mds.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman JR, Lehn AC, Boyle RS, et al. Screening for rare sequence variants in the THAP1 gene in a primary dystonia cohort. Movement disorders: official journal of the Movement Disorder Society. 2013 doi: 10.1002/mds.25479. [DOI] [PubMed] [Google Scholar]
- 17.Lohmann K, Klein C. Genetics of dystonia: What’s known? What’s new? What’s next? Movement disorders: official journal of the Movement Disorder Society. 2013;28:899–905. doi: 10.1002/mds.25536. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser FJ, Osmanoric A, Rakovic A, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1 (DYT6) Annals of neurology. 2010;68:554–9. doi: 10.1002/ana.22157. [DOI] [PubMed] [Google Scholar]
- 19.Gavarini S, Cayrol C, Fuchs T, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Annals of neurology. 2010;68:549–53. doi: 10.1002/ana.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filip P, Lungu OV, Bares M. Dystonia and the cerebellum: a new field of interest in movement disorders? Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2013;124:1269–76. doi: 10.1016/j.clinph.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42:202–9. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berardelli A, Rothwell JC, Hallett M, et al. The pathophysiology of primary dystonia. Brain: a journal of neurology. 1998;121(Pt 7):1195–212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 23.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain: a journal of neurology. 1985;108(Pt 2):463–83. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 24.Pettigrew LC, Jankovic J. Hemidystonia: a report of 22 patients and a review of the literature. Journal of neurology, neurosurgery, and psychiatry. 1985;48:650–7. doi: 10.1136/jnnp.48.7.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitek JL. Pathophysiology of dystonia: a neuronal model. Movement disorders: official journal of the Movement Disorder Society. 2002;17(Suppl 3):S49–62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- 26.Neychev VK, Gross RE, Lehericy S, et al. The functional neuroanatomy of dystonia. Neurobiology of disease. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadnicka A, Hoffland BS, Bhatia KP, et al. The cerebellum in dystonia - help or hindrance? Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2012;123:65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 28**.Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Experimental neurology. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. This study identifies neuropathological changes such as reduced Purkinje cell linear density in post-mortem human brains and links these changes to THAP1 sequence variations.
- 29.Standaert DG. Update on the pathology of dystonia. Neurobiology of disease. 2011;42:148–51. doi: 10.1016/j.nbd.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blood AJ, Kuster JK, Woodman SC, et al. Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PloS one. 2012;7:e31654. doi: 10.1371/journal.pone.0031654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–9. doi: 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramdhani RA, Simonyan K. Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor and other hyperkinetic movements. 2013;3 doi: 10.7916/D8H70DJ7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehericy S, Tijssen MA, Vidailhet M, et al. The anatomical basis of dystonia: Current view using neuroimaging. Movement disorders: official journal of the Movement Disorder Society. 2013;28:944–57. doi: 10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- 34.Delmaire C, Vidailhet M, Elbaz A, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–80. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 35.Neychev VK, Fan X, Mitev VI, et al. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain: a journal of neurology. 2008;131:2499–509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:7825–33. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Movement disorders: official journal of the Movement Disorder Society. 2003;18:60–9. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- 38*.Raike RS, Pizoli CE, Weisz C, et al. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiology of disease. 2012;49C:200–10. doi: 10.1016/j.nbd.2012.07.019. This study provides evidence of cerebellar dysfunction as the primary cause of dystonia symptoms.
- 39.Fan X, Hughes KE, Jinnah HA, Hess EJ. Selective and sustained alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor activation in cerebellum induces dystonia in mice. The Journal of pharmacology and experimental therapeutics. 2012;340:733–41. doi: 10.1124/jpet.111.190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Sharma N, LeDoux MS. The DYT1 carrier state increases energy demand in the olivocerebellar network. Neuroscience. 2011;177:183–94. doi: 10.1016/j.neuroscience.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zoons E, Booij J, Nederveen AJ, et al. Structural, functional and molecular imaging of the brain in primary focal dystonia--a review. Neuroimage. 2011;56:1011–20. doi: 10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 42.Delmaire C, Vidailhet M, Wassermann D, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Archives of neurology. 2009;66:502–8. doi: 10.1001/archneurol.2009.8. [DOI] [PubMed] [Google Scholar]
- 43.Carbon M, Kingsley PB, Su S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Annals of neurology. 2004;56:283–6. doi: 10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- 44.Carbon M, Kingsley PB, Tang C, et al. Microstructural white matter changes in primary torsion dystonia. Movement disorders: official journal of the Movement Disorder Society. 2008;23:234–9. doi: 10.1002/mds.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Meer JN, Beukers RJ, van der Salm SM, et al. White matter abnormalities in gene-positive myoclonus-dystonia. Movement disorders: official journal of the Movement Disorder Society. 2012;27:1666–72. doi: 10.1002/mds.25128. [DOI] [PubMed] [Google Scholar]
- 46.Fabbrini G, Pantano P, Totaro P, et al. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2008;15:185–9. doi: 10.1111/j.1468-1331.2007.02034.x. [DOI] [PubMed] [Google Scholar]
- 47.Bonilha L, de Vries PM, Hurd MW, et al. Disrupted thalamic prefrontal pathways in patients with idiopathic dystonia. Parkinsonism & related disorders. 2009;15:64–7. doi: 10.1016/j.parkreldis.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Colosimo C, Pantano P, Calistri V, et al. Diffusion tensor imaging in primary cervical dystonia. Journal of neurology, neurosurgery, and psychiatry. 2005;76:1591–3. doi: 10.1136/jnnp.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonilha L, de Vries PM, Vincent DJ, et al. Structural white matter abnormalities in patients with idiopathic dystonia. Movement disorders: official journal of the Movement Disorder Society. 2007;22:1110–6. doi: 10.1002/mds.21295. [DOI] [PubMed] [Google Scholar]
- 50.Carbon M, Ghilardi MF, Argyelan M, et al. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–54. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Argyelan M, Carbon M, Niethammer M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:9740–7. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell DB, Hess EJ. L-type calcium channels contribute to the tottering mouse dystonic episodes. Molecular pharmacology. 1999;55:23–31. doi: 10.1124/mol.55.1.23. [DOI] [PubMed] [Google Scholar]
- 53.Erickson MA, Haburcak M, Smukler L, Dunlap K. Altered functional expression of Purkinje cell calcium channels precedes motor dysfunction in tottering mice. Neuroscience. 2007;150:547–55. doi: 10.1016/j.neuroscience.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Experimental neurology. 1993;120:302–10. doi: 10.1006/exnr.1993.1064. [DOI] [PubMed] [Google Scholar]
- 55.Wilson BK, Hess EJ. Animal models for dystonia. Movement disorders: official journal of the Movement Disorder Society. 2013;28:982–9. doi: 10.1002/mds.25526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Ulug AM, Vo A, Argyelan M, et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6638–43. doi: 10.1073/pnas.1016445108. This study demonstrates evidence of cerebellothalamocortical dysfunction in DYT1 KI mice, similar to that as observed in human studies.
- 57*.Song CH, Fan X, Exeter CJ, et al. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiology of disease. 2012;48:66–78. doi: 10.1016/j.nbd.2012.05.009. This study identifies microstructural abnormalities in the striatum of DYT1 KI mouse models, providing a correlate to changes observed in imaging studies.
- 58.Song CH, Bernhard D, Bolarinwa C, et al. Subtle microstructural changes of the striatum in a DYT1 knock-in mouse model of dystonia. Neurobiology of disease. 2013;54:362–71. doi: 10.1016/j.nbd.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vo A, Sako W, Niethammer M, et al. Thalamocortical Connectivity Mediates Phenotypic Variability in Primary Dystonia. J Neurosci. 2013 Submitted. [Google Scholar]
- 60.Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet neurology. 2007;6:223–9. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 61.Hung SW, Hamani C, Lozano AM, et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–9. doi: 10.1212/01.wnl.0000252932.71306.89. [DOI] [PubMed] [Google Scholar]
- 62.Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2008;5:320–30. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Movement disorders: official journal of the Movement Disorder Society. 2013;28:958–67. doi: 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadnicka A, Kimmich O, Pisarek C, et al. Pallidal stimulation for cervical dystonia does not correct abnormal temporal discrimination. Movement disorders: official journal of the Movement Disorder Society. 2013 doi: 10.1002/mds.25581. [DOI] [PubMed] [Google Scholar]
- 65.Toda H, Hamani C, Lozano A. Deep brain stimulation in the treatment of dyskinesia and dystonia. Neurosurgical focus. 2004;17:E2. doi: 10.3171/foc.2004.17.1.2. [DOI] [PubMed] [Google Scholar]