Abstract
The objective of this review is to summarise the published evidence that supports the existence of amyopathic dermatomyositis (ADM) and its clinical significance including risk for rapidly progressive, fatal interstitial lung disease (ILD) and possible risk for internal malignancy. By establishing such inherent risks, we hope to establish the importance of formally recognising ADM as a subset of dermatomyositis (DM). Population-based epidemiologic studies have suggested that amyopathic DM might account for 20% of the total population of dermatomyositis (DM) patients (1). Patients presenting with ADM have been reported by investigators of multiple nationalities to be at risk for rapidly progressive, potentially fatal ILD (2-5). In addition, a new autoantibody, anti-CADM-140, has been reported to be a risk factor for the development of interstitial lung disease in CADM patients (6-9). It has been argued that ADM patients may be at increased risk of developing internal malignancy compared to the general population, though its rate in comparison to classic DM (CDM) needs further study (1, 10-12). In our population, 41% of CADM patients were previously classified as LE or UCTD. We conclude that ADM is a real entity that makes up a significant portion of the DM disease. It is important to formally recognise amyopathic DM as a subset of DM as it carries increased risk of ILD and possibly malignancy. Without appropriate disease classification, the opportunity for ILD and malignancy screening may be missed.
Keywords: amyopathic dermatomyositis, interstitial lung diseases, connective tissue disease
Introduction
Dermatomyositis (DM) has traditionally been viewed as a disease that must affect the muscles before a definitive diagnosis can be made, as reflected in the criteria set forth by Bohan and Peter in 1975 (Table I) (13, 14). Since that time, growing attention has been given to a group of patients who have the skin manifestations of DM without any clinical or laboratory evidence of muscle involvement. Prior to the 1990s, these patients were referred to by dermatologists as having “dermatomyositis sine myositis,” an entity that virtually did not exist in the published literature in that era. In the early 1990s, two dermatologists, Euwer and Sontheimer, brought attention to this subphenotype of DM by reporting a small series of patients as having amyopathic DM (ADM), a term that had been previously coined by Carl Pearson, an American rheumatologist (15, 16).
Table I.
Bohan and Peter criteria of DM.
| 1. Proximal muscle weakness: usually symmetrical |
| 2. Elevated serum muscle enzymes: CK, aldolase |
| 3. Electromyographic abnormalities |
| 4. Muscle biopsy findings typical of PM or DM: necrosis, phagocytosis, regeneration, inflammation |
| 5. Dermatological features of DM, Gottron's sign or papules, or heliotrope rash Definite DM requires four criteria (including rash) and definite PM requires four criteria (without rash). Probable disease comprises three criteria (including rash) for DM and three criteria (without rash) for PM. Possible disease requires two criteria (including rash) for DM and two criteria (without rash) for PM. Adapted from Sultan, et al. (24). |
Over the past two decades, a body of published evidence has appeared arguing that ADM is more than a dermatologic oddity. Preliminary population-based data suggest that ADM might account for 20% of all DM patients (1). Patients presenting with ADM as their entry into the IIM spectrum have been reported from investigators of multiple nationalities to be at risk for rapidly progressive, potentially fatal interstitial lung disease (ILD) (20-27). A new IIM autoantibody, anti-CADM-140, has been reported to be enriched in clinically ADM patients at risk for ILD (6-9). In addition, it has been argued that ADM patients may be at increased risk of developing internal malignancy compared to the general population, though its rate in comparison to Classic DM (CDM) needs further study (1, 10-12). Lastly, it is more difficult to diagnose ADM without it being explicitly incorporated within the DM criteria. In our population, 41% of clinically amyopathic DM (CADM) patients were previously misdiagnosed. If ADM is not recognised within the DM spectrum and patients are misclassified, appropriate screening and treatment for ILD and malignancy may not be performed.
Definitions
As Sontheimer highlights in his article entitled “Would a new name hasten the acceptance of amyopathic dermatomyositis…”, the nomenclature of DM can be confusing (17). Classic DM (CDM) includes both the hallmark skin manifestations of DM, proximal muscle weakness, and laboratory data supporting muscle inflammation (5). Amyopathic DM (ADM) is a subset of DM patients characterised by biopsy-confirmed hallmark cutaneous manifestations of classic DM occurring for 6 months or longer with no clinical evidence of proximal muscle weakness and no serum muscle enzyme abnormalities (12). Hypomyopathic DM (HDM) differs from ADM in that although these patients do not exhibit any clinical evidence of muscle weakness, they may have sub-clinical evidence of muscle involvement on laboratory, electrophysiologic, and/or radiologic evaluation (12, 17). Clinically Amyopathic DM (CADM) encompasses both the amyopathic and hypomyopathic DM groups (12, 17). Though the focus of this review is ADM, much of the literature refers to CADM, so this distinction must be kept in mind.
Nomenclature used for the purposes of this review can be seen in Table II.
Table II.
Definitions of terms used to describe DM.
| Term | Definition |
|---|---|
| Amyopathic DM (ADM) | A subset of DM patients characterised by biopsy-confirmed hallmark cutaneous manifestations of classical DM occurring for 6 months or longer with no clinical evidence of proximal muscle weakness and no serum muscle enzyme abnormalities. If more extensive muscle testing is carried out, the results should be within normal limits (if such results are positive or abnormal, the patient can be classified as having “hypomyopathic dermatomyositis” [see below]). |
| Classic DM (CDM) | Patients having the hallmark cutaneous manifestations of DM, proximal muscle weakness, and laboratory evidence of muscle inflammation that is characteristic of DM. |
| Hypomyopathic DM (HDM) | A designation for patients with cutaneous DM and no clinical evidence of muscle disease (i.e. weakness) for 6 months or longer, who during evaluation are found to have subclinical evidence of myositis upon laboratory (e.g. muscle enzymes), electrophysiological, and/or radiological evaluation. |
| Clinically amyopathic DM (CADM) | A term that includes both amyopathic DM and/or hypomyopathic DM patients (i.e. clinically amyopathic DM = amyopathic DM + hypomyopathic DM). The CADM designation has been coined to emphasise the fact that the predominant clinical problem is skin disease. |
Adapted from Gerami et al. (12).
The DM spectrum versus current criteria
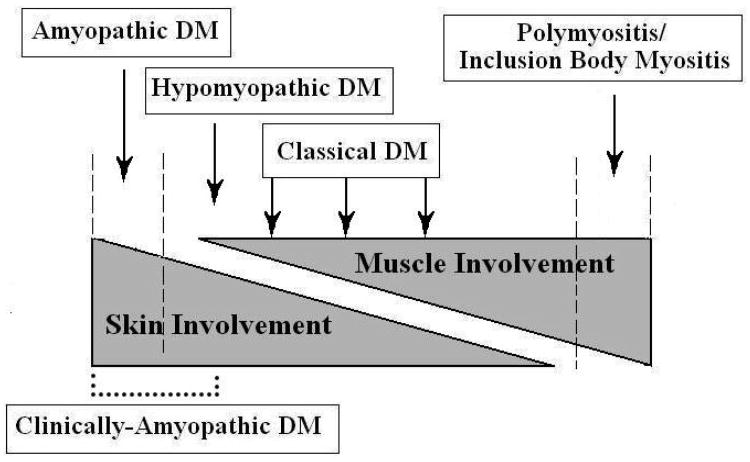
Euwer and Sontheimer pioneered the concept of viewing inflammatory myopathies as a spectrum, with muscle and skin involvement occurring to varying degrees (Fig. 1) (16, 18). ADM is on the pure skin portion of the spectrum, while HDM is slightly closer to the muscle side. CDM is in the middle of the spectrum and polymyositis (PM), with a different pathologic process, have purely muscle disease. A similar range of cutaneous and systemic manifestations has worked well to characterise the varied presentations seen in lupus erythematosus (LE) and scleroderma (18, 19). Of note, some argue due to substantial differences between PM and DM in the areas of pathogenesis, histology, and clinical features that it should not be on the spectrum (20-22). Alternatively, the entity dermatomyositis sine dermatitis has been proposed as pure muscle DM, though little literature exists to support this variant (23).
Fig. 1.
The Idiopathic Inflammatory Dermatomyopathy Spectrum ADM lies on the pure skin involvement side of the Spectrum, while CDM lies in the middle with both skin and muscle involvement. Reprint from Sontheimer (17) with permission from Elsevier™ pending.
The spectrum concept is in stark contrast to the Bohan and Peter Criteria (Table If) (13, 14). Using these criteria, the clinician cannot make the diagnosis of DM without muscle involvement. Peter and Bohan also created a myositis classification scheme that has sub-categories; there is currently no sub-category for ADM (3). Many proposed revisions have been made to make the criteria more exclusive to prevent misclassification of other myopathies, but few argue for a more inclusive approach (24).
However, ADM was included in the classification system proposed by Amato (25). In 2004, the International Myositis Assessment and Clinical Studies (IMACS), composed of neurologists and rheumatologists, agreed upon this classification to further define criteria for inclusion in randomised controlled trials (25). This criterion includes a subcategory for ADM, among several others (Table III). This classification was based solely on expert opinion and precisely how many individual criteria are needed to make the ADM diagnosis was not clarified. In a clinical setting, the physician may not submit the patient for muscle testing or electromyography (EMG) if weakness is not detected on physical exam.
Table III.
Characteristics of ADM.
| Sontheimer (17) | Amato (9) |
|---|---|
|
|
Exclusion criteria:
|
The criteria for qualifying for the ADM subcategory was initially proposed by Euwer and Sontheimer (16). They proposed that ADM include one to two of the pathognomonic skin features in association with one or more characteristic signs. In addition, a skin biopsy consistent with a diagnosis of DM and an absence of clinical muscle disease for two years is needed (Table III) (16). An exception was made so that a patient can be labeled as ADM at initial presentation without the 2-year waiting period, with the caveat that they may change categories if muscle involvement develops. Later, Sontheimer refined the ADM criteria in an effort to exclude drug induced myositis and partially treated CDM (Table III) (17). Additionally, the asymptomatic muscle period was shortened to 6 months, reflecting the rapidity with which new muscle symptoms often develop in patients presenting initially with skin disease only and allowing for earlier screening for ILD and malignancy. The exclusion criteria of not being on an immunosuppressive, though conceptually sound, may need further subcategorisation for clinical studies because many patients are given immunosuppressive treatment for their skin disease.
Although solutions might include adding ADM as a subcategory of the Bohan and Peter criteria or conceptualising ADM as a separate disease entity by creating its own criteria, ideally it would be placed in the context of newly revised criteria. These criteria should be based on statistical analyses with expertise from dermatologists, rheumatologists, and neurologists.
Epidemiology
The prevalence of ADM within the DM population is not insignificant. The only population-based study used the Rochester database of residents in Olmsted, Minnesota. That study identified 29 DM patients, 21% of whom had CADM (1). Additional studies with larger populations would certainly be of interest. Previous estimations of CADM varied from 2–18%, though none of these studies took a population-based approach and each had referral bias (13, 16, 26-30). A sense of the amount of ADM in the breakdown of CADM was provided by the review of the literature, which identified 291 cases of CADM reported through May of 2004. Seventy percent of these cases were classified as ADM, though reporting bias may exist (16).
The number of ADM patients may also reflect subspecialty referral bias. A study at a large tertiary medical centre demonstrated the disparity of ADM cases seen between rheumatology and dermatology departments (11). It showed that 4% of DM cases seen by rheumatology were classified as ADM, in contrast to 40% of the DM patients seen by dermatology. At this centre, 29% of total DM patients were classified as ADM. One possible explanation of this difference is that patients with only a rash are more likely to see a dermatologist than a rheumatologist. Another is that dermatologists have more familiarity with ADM and therefore make the diagnosis more often. Such differences call attention to the importance of including both specialties in formulating the diagnostic criteria. Inclusion of ADM within the spectrum of DM may facilitate appropriate diagnosis.
Characteristics of ADM are similar to CDM
ADM and CDM affect similar groups with identical skin findings, suggesting that they fall within the same spectrum. It has been shown that 70–86% of CADM patients are Caucasian (1, 11, 12). CADM is diagnosed at an average age of 44–50 years old and affects predominantly women (1, 11, 12, 31). Wide ranges of demographics have been published given the different populations studied, but it can be concluded that CADM affects predominantly Caucasian middle-aged women. CDM affects the same demographic (11, 13, 14, 27). Several studies have shown no difference in skin manifestations between the CADM and CDM groups (11, 16, 32).
ILD: the American and Asian experiences
Perhaps the most convincing argument for inclusion of ADM in the criteria would be the increased risk of the patient developing ILD compared to patients without DM. Accordingly, the diagnosis of ADM has implications for both the skin and other organ systems. Monganroth et al. showed that 23% of patients with DM had ILD as defined by computed tomography (CT) imaging (33). An additional 25% of DM patients had DLCO abnormalities with normal CT findings, perhaps indicating early disease. This ILD prevalence estimate is at the lower end of the wide range reported for the DM spectrum as a whole (approximately 20%–70%) (34, 35). Importantly, no statistical difference in the prevalence of ILD between the CADM and CDM groups was shown (33). However, CADM, like CDM, has an increased risk of ILD relative to the general population (33). The most common type of ILD in ADM is non-specific interstitial pneumonia, similar to CDM (12, 36).
Most cases of ILD in DM are mild, chronic, and non-progressive with immunosuppressive treatment (37). However, some patients can have a rapidly progressive type of ILD. The rate of rapidly progressive ILD secondary to DM in the Caucasian population has not been established, nor has the percentage of ADM patients with this subtype of ILD. ILD is a significant cause of death in DM, though the precise survival rate has not been established (36, 38, 39). As such, it is important that all DM patients be screened for presence or development of ILD. Morganroth et al. made some excellent ILD screening recommendations (20).
The experience of ILD in ADM has potentially been different in Asia relative to other countries, although studies that allow for direct comparison do not exist. Though Asian studies have not looked at the prevalence of ILD in their ADM patients, they have found an increased prevalence of rapidly progressive ILD in ADM associated with poorer survival outcomes relative to CDM (3, 4). One study showed 50% of CADM patients succumbing to rapidly progressive ILD in one year (5). Another Korean study showed that ADM independently predicted poorer survival in a multivariate analysis (2).
The Japanese have identified an antibody that uniquely identifies ADM and correlates with ILD (6-9). Humaguchi et al. showed that 77% of CADM patients had the anti-CADM-140 antibody and 93% of these patients had ILD (6). Anti-CADM-140, also known as anti-melanoma differentiation associated gene 5 (MDA5), is an antibody for a RNA helicase encoded by the MDA5 gene (40). The function of this gene is in viral immunity suggesting a link between viral infection and development of the disease (41). Patients with this antibody have decreased survival compared to other antibody subgroups because of increased rates of ILD (6). It may be that this antibody specifically targets the lung and skin antigens, leading to such clinical presentations. However, more European and U.S. based studies are needed to determine if the antibody is linked to ILD in other ethnicities. The most common high resolution CT findings in anti-CADM-140 positive ILD was lower consolidation or ground glass appearance, random ground glass pattern, and the absence of intralobular reticular opacities in one series (42). The anti-CADM-140 antibody has also been associated with the presence of skin ulceration (36). CT findings and clinical phenotype may give the clinician insight into potential pulmonary problems (43).
Though corticosteroids are the mainstay of treatment, longitudinal studies showed that immunosuppressives, particularly mycophenolate mofetil (MMF) may normalise pulmonary function tests (PFTs) in DM patients (37, 44). In CADM, immunosuppressives are not necessarily given as treatment for skin, as antimalarials are typically first line therapy (37). This makes identification of underlying ILD particularly important, since patients may not otherwise receive appropriate treatment for their pulmonary disease. Oral glucocorticoids, usually with immunosuppressives, should be considered if pulmonary abnormalities exist.
Malignancy risk in ADM versus CDM is still unknown
It is now recognised that 20% to 25% of patients with adult onset classic DM are at risk for developing malignancy (45). Such risk is especially high in older individuals, and according to other population-based studies, this risk decreases to close to baseline within 3 to 5 years of the onset of CDM (46). In CADM, the risk has been variable across series, with a prevalence of 8 to 28% (1, 10-12).
Some studies suggest the malignancy risk in CADM may be lower than that of CDM. In the only population-based study, patients with CADM had a lower risk of internal malignancy than patients with CDM (1). However, this difference was not statistically significant. Sontheimer reported only one case of internal malignancy occurring within 3 years of the appearance of DM skin disease in a cohort of more than 100 patients with CADM (47). Klein et al. showed that 3% of CADM versus 16% of CDM patients had a malignancy, but again this difference was not statistically significant (11). In a report of 28 patients seen in a Singapore dermatology centre, 15% with ADM had malignancy versus 66% of those with CDM and HDM (48).
Other studies argue that the risk of malignancy is the same between ADM and CDM. Whitmore found no difference in the malignancy rates between CADM and CDM (49). Azuma et al. found similar malignancy rates between CADM and CDM (20% versus 24%) and found that the standardised incidence ratio was 13.8 for the combined DM /CADM group over the normal Japanese population (60). Though the difference in malignancy rates between CADM and CDM remains unclear, age appropriate cancer screening is still recommended (47).
Previous classification of patients
It may be common that ADM is often classified as another rheumatologic or dermatologic entity. This may in part be due to the lack of recognition of the subset of DM occurring without muscle involvement. The authors of this review examined their database of over eighty DM patients and found that twenty-two had CADM. Upon review of their medical records, six of the twenty-two CADM patients (27%) were previously classified with either having cutaneous lupus erythematosus (CLE) or systemic lupus erythematosus (SLE) prior to the diagnosis of DM. An additional four of the twenty-two patients (18%) were given a diagnosis of undifferentiated connective tissue disease (UCTD). One patient was given the dual diagnosis of CLE and UCTD. Overall, nine of the twenty-two patients, or 41%, were not given the diagnosis of CADM upon previous evaluation (Table IV). This is in accordance with the findings of Euwer and Sontheimer, who 20 years ago also found that LE was the most common initial diagnosis in CADM patients (16). Inclusion of CADM in the spectrum of DM criteria may facilitate clinical recognition of this subset.
Table IV.
Classification of CADM at previous visit.
| Previous clinical diagnosis | no. of CADM patients with given previous diagnosis/ total no. CADM patients in database (%) |
|---|---|
| CLE or SLE | 6/22 (27%) |
| UCTD | 4/22 (18%) |
| Total not categorised as CADM | 9/22 (41%) |
| Total categorised as CADM | 11/22 (50%) |
UCTD: undifferentiated connective tissue disease.
Based on this information, it is important to highlight some key clinical differences between CLE and ADM that will aid in making a diagnosis, as they cannot be distinguished by routine histology. The main difference is in the skin findings. It has largely been accepted that the Gottron's papules and Gottron's sign, the latter referring to non-papular erythema over extensor joints, are pathognomonic for the disease (see Table V and Fig. 2) (16). Characteristic skin findings include the heliotrope rash (Fig. 3), periungual telangectasias, and sun-exposed violaceous erythema. Compatible characteristics include poikiloderma and bullous lesions. Euwer and Sontheimer propose that ADM should have one or two pathognomonic lesions in association with one or more characteristic lesions (16). It is also common that in CLE the facial erythema will spare the nasolabial folds, whereas this area is often affected in DM patients (51). Pruritus is a more common symptom in DM (16, 52). SLE would have systemic findings that affect the kidney, brain, and blood cell counts. Though the antibodies of LE and ADM may overlap, the CADM-140 antibody is fairly specific for ADM (6, 7). In terms of common antibodies tested in DM patients, approximately 62% of ADM patients have a positive ANA (> 1:80) and 3% have a positive anti-Jo-1 antibody (12).
Table V.
Skin findings of dermatomyositis.
Pathognomonic:
|
Characteristic:
|
Compatible:
|
| Euwer and Sontheimer define amyopathic dermatomyositis as having one or two pathognomonic signs in association with one or more characteristic signs and a compatible skin biopsy specimen. |
Adapted from Euwer and Sontheimer (16).
Fig. 2.
Gottron's papules of the hand. Note raised erythematous papules on knuckle pads and erythema overlying the extensor tendons on the hands.
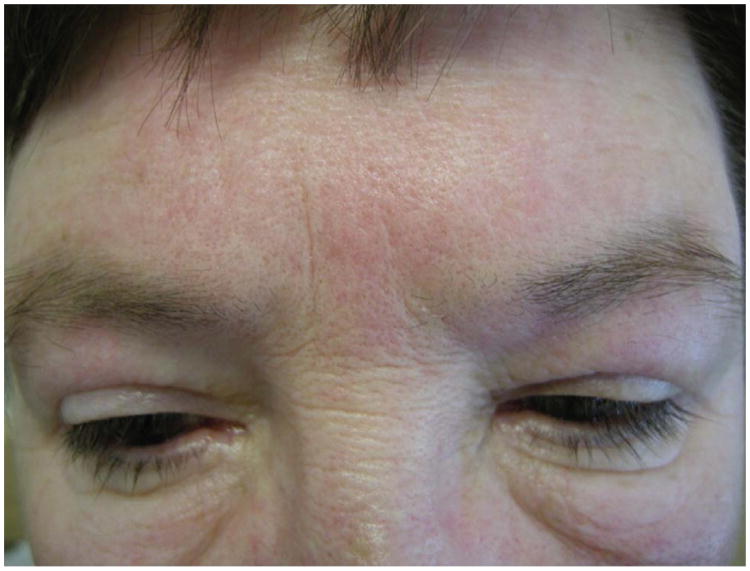
Fig. 3.
Facial erythema and mild heliotrope. Note the erythema along the glabellar area/ lower forehead and mild periorbital erythema. The latter finding is called a heliotrope.
It may also be useful for classification purposes to determine when a patient has transitioned from CADM to classic dermatomyositis. This occurs in approximately 13% of CADM cases (12). The definition of transition of CADM to CDM is the “onset of clinically significant muscle disease (i.e. weakness) [appearing] greater than 6 months after an initial disease presentation as CADM” (12). All cases in one series had a negative creatine kinase (CK) at diagnosis of CADM and later developed positive CKs at the onset of muscle disease, as per definitions of each disease (Table II) (12).
Conclusions and recommendations
Inclusion of ADM in the diagnostic criteria for DM has implications for a patient's diagnosis and subsequent treatment and outcome. 41% of CADM patients may be misclassified at their first visit, most often as CLE, missing the opportunity to screen for potential ILD and malignancy. Inclusion may give these patients access to clinical trials, allowing for the evaluation of more effective treatments for their disease. With validated skin severity measures, such as the CDASI, such clinical trials are now possible (53).
We recommend that a formal interdisciplinary effort be taken by rheumatologists, neurologists, pulmonary medicine specialists, and dermatologists to develop an all inclusive classification framework for the full spectrum of illnesses produced by the idiopathic inflammatory myopathies. The Rheumatologic Dermatology Society (RDS) (http://www.rheumaderm-society.org/) is excited and willing to productively interact with leadership groups in these specialties to develop such a framework. Only by recognising and including all the clinical expressions of this complex autoimmune disease will an understanding of this disease process be achieved.
Footnotes
Competing interests: none declared.
References
- 1.Bendewald MJ, Wetter DA, Li X, Davis MD. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26–30. doi: 10.1001/archdermatol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis and amyopathic dermatomyositis. Rheumatology. 2005;44:1282–6. doi: 10.1093/rheumatology/keh723. [DOI] [PubMed] [Google Scholar]
- 3.Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341–7. doi: 10.1378/chest.08-2740. [DOI] [PubMed] [Google Scholar]
- 4.Suda T, Fujisawa T, Enomoto N, et al. Interstitial lung diseases associated with amyopathic dermatomyositis. Eur Respir J. 2006;28:1005–12. doi: 10.1183/09031936.06.00038806. [DOI] [PubMed] [Google Scholar]
- 5.Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26:1647–54. doi: 10.1007/s10067-007-0562-9. [DOI] [PubMed] [Google Scholar]
- 6.Hamaguchi Y, Kuwana M, Hoshino K, et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol. 2011;147:391–8. doi: 10.1001/archdermatol.2011.52. [DOI] [PubMed] [Google Scholar]
- 7.Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology. 2010;49:1713–9. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–6. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 9.Nagata K, Tomii K, Nanjo S, Kubota M, Tachikawa R, Nishio M. [Four cases of interstitial pneumonia associated with amyopathic dermatomyositis characterized by the anti-CADM-140 antibody] Nihon Kokyuki Gakkai Zasshi. 2011;49:30–6. [PubMed] [Google Scholar]
- 10.Franks AG., JR Skin manifestations of internal disease. Med Clin North Am. 2009;93:1265–82. doi: 10.1016/j.mcna.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Klein RQ, Teal V, Taylor L, Troxel AB, Werth VP. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937–43. doi: 10.1016/j.jaad.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerami P, Schope JM, Mcdonald L, Walling HW, Sontheimer RD. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 14.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 15.Arthritis and Allied Conditions: A Textbook of Rheumatology. 9th. Philadelphia, PA: Lea & Febiger; 1979. [Google Scholar]
- 16.Euwer RL, Sontheimer RD. Amyopathic dermatomyositis (dermatomyositis sine myositis) Presentation of six new cases and review of the literature. J Am Acad Dermatol. 1991;24:959–66. [PubMed] [Google Scholar]
- 17.Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. 2002;46:626–36. doi: 10.1067/mjd.2002.120621. [DOI] [PubMed] [Google Scholar]
- 18.Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4:471–5. doi: 10.1016/s0190-9622(81)80261-7. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. The spectrum of systemic sclerosis--current concepts. Hosp Pract (Hosp Ed) 1981;16:61–7. 70–2. doi: 10.1080/21548331.1981.11946740. [DOI] [PubMed] [Google Scholar]
- 20.Amato AA, Griggs RC. Unicorns, dragons, polymyositis, and other mythological beasts. Neurology. 2003;61:288–9. doi: 10.1212/wnl.61.3.288. [DOI] [PubMed] [Google Scholar]
- 21.Van der Meulen MF, Bronner IM, Hoogendijk JE, et al. Polymyositis: an overdiagnosed entity. Neurology. 2003;61:316–21. doi: 10.1212/wnl.61.3.316. [DOI] [PubMed] [Google Scholar]
- 22.Bronner IM, Linssen WH, Van der Meulen MF, Hoogendijk JE, DE Visser M. Polymyositis: an ongoing discussion about a disease entity. Arch Neurol. 2004;61:132–5. doi: 10.1001/archneur.61.1.132. [DOI] [PubMed] [Google Scholar]
- 23.Leteurtre E, Hachulla E, Janin A, Hatron PY, Brouillard M, Devulder B. Vascular manifestations of dermatomyositis and polymyositis Clinical, capillaroscopic and histological aspects. Rev Med Interne. 1994;15:800–7. doi: 10.1016/s0248-8663(05)82836-x. [DOI] [PubMed] [Google Scholar]
- 24.Sultan SM, Isenberg DA. Re-classifying myositis. Rheumatology. 2010;49:831–3. doi: 10.1093/rheumatology/kep355. [DOI] [PubMed] [Google Scholar]
- 25.Hoogendijk JE, Amato AA, Lecky BR, et al. Neuromuscul Disord; 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis; 10-12 October 2003; Naarden, The Netherlands. 2004. pp. 337–45. [DOI] [PubMed] [Google Scholar]
- 26.El-Azhary RA, Pakzad SY. Amyopathic dermatomyositis: retrospective review of 37 cases. J Am Acad Dermatol. 2002;46:560–5. doi: 10.1067/mjd.2002.120620. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899–920. doi: 10.1016/s0190-9622(98)70263-4. quiz 921-2. [DOI] [PubMed] [Google Scholar]
- 28.Dawkins MA, et al. Dermatomyositis: a dermatology-based case series. J Am Acad Dermatol. 1998;38:397–404. doi: 10.1016/s0190-9622(98)70496-7. [DOI] [PubMed] [Google Scholar]
- 29.Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23–7. doi: 10.1001/archderm.138.1.23. [DOI] [PubMed] [Google Scholar]
- 30.Yu B. A clinical analysis of cutaneous type dermatomyositis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1994;16:394–6. [PubMed] [Google Scholar]
- 31.Zhang ZL, Lai AY, Xu D, Chen YH, Tang FL. Clinical analysis of 29 patients with clinically amyopathic dermatomyositis. Zhonghua Yi Xue Za Zhi. 2007;87:1345–7. [PubMed] [Google Scholar]
- 32.Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol. 1999;11:475–82. [PubMed] [Google Scholar]
- 33.Morganroth PA, Kreider ME, Okawa J, Taylor L, Werth VP. Interstitial lung disease in classic and skin-predominant dermatomyositis: a retrospective study with screening recommendations. Arch Dermatol. 2010;146:729–38. doi: 10.1001/archdermatol.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marie I, Hachulla E, Chérin P, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum. 2002;47:614–22. doi: 10.1002/art.10794. [DOI] [PubMed] [Google Scholar]
- 35.Cao H, Parikh TN, Zheng J. Amyopathic dermatomyositis or dermatomyositis-like skin disease: retrospective review of 16 cases with amyopathic dermatomyositis. Clin Rheumatol. 2009;28:979–84. doi: 10.1007/s10067-009-1152-9. [DOI] [PubMed] [Google Scholar]
- 36.Cottin V, Thivolet-Béjui F, Reynaud-Gaubert M, et al. Interstitial lung disease in amyopathic dermatomyositis, dermatomyositis and polymyositis. Eur Respir J. 2003;22:245–50. doi: 10.1183/09031936.03.00026703. [DOI] [PubMed] [Google Scholar]
- 37.Fathi M, Vikgren J, Boijsen M, et al. Interstitial lung disease in polymyositis and dermatomyositis: longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 2008;59:677–85. doi: 10.1002/art.23571. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado F, Patel RR, Iyer VN, Yi ES, Ryu JH. Are respiratory complications common causes of death in inflammatory myopathies? An autopsy study. Respirology. 2011;17:455–60. doi: 10.1111/j.1440-1843.2011.02103.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamasaki Y, Yamada H, Ohkubo M, et al. Longterm survival and associated risk factors in patients with adult-onset idiopathic inflammatory myopathies and amyopathic dermatomyositis: experience in a single institute in Japan. J Rheumatol. 2011;38:1636–43. doi: 10.3899/jrheum.101002. [DOI] [PubMed] [Google Scholar]
- 40.Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193–200. doi: 10.1002/art.24621. [DOI] [PubMed] [Google Scholar]
- 41.Sato S, Kuwana M. Clinically amyopathic dermatomyositis. Curr Opin Rheumatol. 2010;22:639–43. doi: 10.1097/BOR.0b013e32833f1987. [DOI] [PubMed] [Google Scholar]
- 42.Tanizawa K, Handa T, Nakashima R, et al. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir Med. 2011;105:1380–7. doi: 10.1016/j.rmed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): A retrospective study. J Am Acad Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. 2010;138:1464–74. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 45.Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13:208–15. doi: 10.1007/s11926-011-0169-7. [DOI] [PubMed] [Google Scholar]
- 46.Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 47.Sontheimer RD. Clinically amyopathic dermatomyositis: what can we now tell our patients? Arch Dermatol. 2010;146:76–80. doi: 10.1001/archdermatol.2009.323. [DOI] [PubMed] [Google Scholar]
- 48.Ang P, Sugeng MW, Chua SH. Classical and amyopathic dermatomyositis seen at the National Skin Centre of Singapore: a 3-year retrospective review of their clinical characteristics and association with malignancy. Ann Acad Med Singapore. 2000;29:219–23. [PubMed] [Google Scholar]
- 49.Whitmore SE, Watson R, Rosenshein NB, Provost TT. Dermatomyositis sine myositis: association with malignancy. J Rheumatol. 1996;23:101–5. [PubMed] [Google Scholar]
- 50.Azuma K, Yamada H, Ohkubo M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21:178–83. doi: 10.1007/s10165-010-0362-y. [DOI] [PubMed] [Google Scholar]
- 51.Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374–7. doi: 10.1159/000158637. [DOI] [PubMed] [Google Scholar]
- 52.Goreshi R, Chock M, Foering K, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65:1107–16. doi: 10.1016/j.jaad.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein RQ, Bangert CA, Costner M, et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol. 2008;159:887–94. doi: 10.1111/j.1365-2133.2008.08711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]