Abstract
Objective
To determine whether (a) late-life pain predicts growth in older adults’ use of alcohol, and elevated risk of drinking problems; and (b) sociodemographic characteristics moderate these relationships.
Method
Five times over an 8-year interval, N = 5,446 Health and Retirement Study (HRS) participants provided information about their pain and alcohol use. Two-part latent growth modeling and logistic regression were used to analyze these data.
Results
Participants with more pain at baseline had lower initial levels and a faster rate of decline over the next 8 years in alcohol consumption, but they also were at elevated risk of having drinking problems. Income and African American background interacted with pain to predict 8-year change in alcohol consumption and presence of drinking problems.
Discussion
Late-life pain does not predict growth in older adults’ alcohol consumption, but is nonetheless linked to elevated risk of drinking problems, especially among African Americans.
Keywords: pain, alcohol consumption, drinking problems, late-middle-age, older adults
Introduction
As individuals grow older, they are likely to experience more pain (Ferrell, 2000; Gagliese, 2009; Gloth, 2001; Thomas, Peat, Harris, Wilkie, & Croft, 2004). Studies of mixed-age samples have demonstrated positive associations between pain and heavier, more frequent alcohol consumption (Castillo, MacKenzie, Wegener, & Bosse, 2006; Strine & Hootman, 2007; Urquhart et al., 2009), and between more pain and more drinking problems (i.e., negative physical, psychological, and social consequences of alcohol use; Demyttenaere et al., 2007; Dersh, Gatchel, Mayer, Polatin, & Temple, 2006; Lawton & Simpson, 2009). Taken together, these findings suggest that the increased pain that is a hallmark of later life may influence older adults’ use of alcohol. However, almost no research has addressed this question.
Several studies of mixed-age samples report positive associations between a variety of pain characteristics (e.g., location, intensity, chronicity) and larger quantities of and more frequent alcohol consumption (e.g., Armenian, Halabi, & Khlat, 1989; Castillo et al., 2006; Strine & Hootman, 2007; Urquhart et al., 2009; but see Ang, Pelosco, Woolson, Kroenke, & Doebbeling, 2006; Bergman, Herrstrom, Jacobsson, & Petersson, 2002). Moreover, higher pain levels are associated with more self-reported use of alcohol to manage pain (Brennan, Schutte, & Moos, 2005; Riley & King, 2009). The “alcohol self-medication hypothesis” has been cited to explain these findings (Aira, Hatikainen, & Sulkava, 2008; Ilgen, Perron, Czyz, McCammon, & Trafton, 2010). It suggests that individuals in pain consume alcohol in larger quantities, and more often, to dispel pain-related discomfort. However, a recent study by Bobo, Greek, Klepinger, and Herting (2013) provides evidence against this hypothesis. Bobo et al. showed that men who reported being troubled by pain at baseline in late-middle-age were more likely than pain-free peers to subsequently experience a 10-year pattern of declining alcohol use.
Pain also has been shown to be associated with drinking problems (negative physical, psychological, and social consequences of alcohol use). Several investigations have demonstrated links between pain (variously defined as diagnosed pain disorder, self-reported presence of pain, number of bodily pain sites, more severe pain, more pain interference) and presence of substance (drug and alcohol) use disorder diagnoses (e.g., Dersh et al., 2006; Ilgen et al., 2010; Polatin, Kinney, Gatchel, Lillo, & Mayer, 1993; Trafton, Oliva, Horst, Minkel, & Humphreys, 2004), as well as elevated symptoms of alcohol abuse and dependence (Brennan et al., 2005; R. L. Brown, Patterson, Rounds, & Papasouliotis, 1996; Demyttenaere et al., 2007; Dersh et al., 2006; Gureje et al., 2008; Lawton & Simpson, 2009; Von Korff et al., 2005). The “risky lifestyle hypothesis” has been invoked to explain these associations: it suggests that there are positive interconnections among elevated likelihood of painful injuries and illness, risky health behaviors, and substance misuse (Dersh et al., 2006; Gatchel & Dersh, 2002).
Recently, we built upon this earlier, predominately cross-sectional research to show, using a prospective research design, that older adults reporting more numerous painful medical conditions at baseline reduced their alcohol consumption more rapidly over the next 10 years than did older adults without painful medical conditions; they nonetheless had higher levels of drinking problems (Brennan, Schutte, SooHoo, & Moos, 2011). We also extended previous research in this area by showing that the relationship between later-life pain and alcohol use can be moderated by individuals’ sociodemographic characteristics and their social resources. Specifically, we showed that being female, and having more interpersonal social resources, strengthened the association between baseline painful medical conditions and subsequent decline in ethanol consumed. For men more so than women, more numerous painful medical conditions were associated with more drinking problems.
Although the Brennan et al. (2011) study provided useful new information about the relationship between late-life pain and use of alcohol, it had several limitations. Its findings were obtained from an older community sample comprised mainly of drinkers; this is not representative of the overall U.S. population of older adults. Moreover, the sample was almost all Caucasian, precluding analyses of racial background as a moderator of the relationship between later-life pain and alcohol use. Finally, only a single pain item (a count of participants’ recent painful medical conditions) was available in the Brennan et al. (2011) data set. To remedy these limitations, and to further extend research in this area, we sought longitudinal information on older adults’ pain and alcohol use from the Health and Retirement Study (HRS). Longitudinal HRS data are particularly well suited to help us meet these goals because they are nationally representative of older adults’ drinking behavior, reflect close to the proportion of African Americans in the older adult population, and contain repeated measurements of participants’ pain intensity and pain interference, as well as their painful medical conditions. The HRS data also provide an opportunity to expand the set of demographic factors hypothesized to moderate the pain-alcohol linkage to include African American background, marital status, educational attainment, and income, as well as age and gender.
The purpose of this study is to replicate and extend the Brennan et al. (2011) investigation to advance our understanding of the relationship between pain and alcohol use, particularly as it occurs in later life. We address these specific questions: among late-middle-aged individuals (a) Is there a positive, prospective relationship between more baseline pain (number of painful medical conditions, pain severity, and pain interference) and growth in the quantity and frequency of alcohol consumed over a subsequent, 8-year interval?; (b) Is there a positive concurrent association between more pain (number of painful medical conditions, pain severity, and pain interference) and more drinking problems?; and (c) Do age, gender, African American background, marital status, education, and income moderate the prospective relationship between pain and 8-year growth in alcohol consumption? Do they moderate the concurrent relationship between late-life pain and drinking problems?
Method
Sample
Our sample is drawn from the HRS. Since 1992 HRS has obtained biennial panel and longitudinal data on the health and well-being of nationally representative samples of adults older than age 50. HRS initially comprised two data collections HRS (1992, 1994, and 1996) and the Study of Asset and Health Dynamics (AHEAD; 1993 and 1995). In 1998, HRS combined these two cohorts with additional subsamples to create a complete panel of participants representing all individuals older than 50 years in the United States (Hauser & Willis, 2005; Juster & Suzman, 1995).
Due to differences in the HRS and AHEAD surveys, and cross-wave changes in survey design, several HRS health items (e.g., painful medical conditions, alcohol consumption) did not become completely commensurate across waves until 1996. Accordingly, we chose 1996 as the baseline assessment point for this study. We selected from the overall 1996 HRS sample participants age 55 to 65, and obtained these individuals’ 1998, 2000, 2002, and 2004 HRS data, resulting in an 8-year, 5-wave HRS longitudinal sample parallel to the 10-year community sample whose pain and drinking behavior we reported on earlier (Brennan et al., 2011).
At HRS inception in 1992, a total of 12,652 individuals were recruited and interviewed (for details, see Hauser & Willis, 2005; Leacock, 2006). Of these individuals, a total of 9,474 survived to and were aged 55 to 65 by the 1996 data collection; follow-up data were successfully obtained for 8,635 (91%) of these individuals. Follow-up rates for surviving members of this N = 8,635 cohort at the 1998, 2000, 2002, and 2004 data collections were, respectively, 96%, 93%, 93%, and 88%. These percentages closely mirror follow-up rates found in the overall HRS cohort initially interviewed in 1992 (Health and Retirement Study, 2010).
By 2004 a total of 7,596 participants had survived and 6,121 of them (81%) had provided complete interviews for all five waves of data collection. We removed from this sample 675 participants whose data were provided at one or more data collection points by a proxy informant, because HRS proxy informants do not provide key health information about participants, such as their alcohol consumption, depressive symptoms, cognitive status, and other subjective states (Health and Retirement Study, 2010). Thus, our final longitudinal sample size was N = 5,446.
This sample comprised 3,240 women (59%) and 2,206 men (41%). At baseline, participants’ average age was 60 years. About 82% of the sample was Caucasian, 15% was African American, and 3% had other racial backgrounds. Most (74%) participants were married; on average, participants had 12 years education.
Measures
HRS health measures are adapted from established U.S. national health surveys, such as the National Health Interview Survey and the National Health and Nutrition Examination Survey (for details see Fisher, Faul, Weir, & Wallace, 2005).
Demographic Factors
These were assessed at baseline HRS participation and included age, gender (0 = male; 1 = female), African American background (0 = no; 1 = yes), marital status (0 = unmarried; 1 = married), education in years, and income, in categorical intervals (1 = US$0–US$10,000 to 10 = US$90,000+).
Pain
We assessed number of painful medical conditions by counting participants’ affirmative answers to whether they had experienced chest, joint, headache, or back pain during the interval since their last HRS assessment. Pain severity tapped how severe a participant’s pain was at the time of interview (range = 0–3; from “no pain” to “severe pain”). Pain interference was a dichotomous variable (0 = no; 1 = yes) that indicated whether a participant’s pain made it difficult for him or her to perform usual activities, such as household chores or work.
Drinking Behavior
Alcohol Consumption
Amount of alcohol is the number of drinks per day consumed by participants, on days they drank, during the last 3 months. Frequency of alcohol consumption is the number of days per week that alcohol was consumed, during the last 3 months. Individuals who answered “no” to the question, “Do you ever drink alcoholic beverages …?” were assigned a “0” for amount of alcohol and frequency of alcohol consumption.
Drinking Problems
Drinking problems were assessed only at participants’ initial HRS interview, using the CAGE instrument (Ewing, 1984; Mayfield, McLeod, & Hall, 1974). CAGE items tap participants’ responses (0 = no; 1 = yes) to four questions: “Have you ever felt that you should cut down on drinking?,” “Have people ever annoyed you by criticizing your drinking?,” “Have you ever felt bad or guilty about drinking,?” “Have you ever taken a drink first thing in the morning (‘eyeopener’) to steady your nerves or get rid of a hangover?” From participants’ answers to CAGE items, we created a dichotomous variable (0 = no; 1 = yes) indicating presence of one or more drinking problems. CAGE is a valid screening tool for detection of alcohol problems (Buchsbaum, Buchanan, Centor, Schnoll, & Lawton, 1991; Mayfield et al., 1994) and distinguishes well between individuals with and without drinking problems (Chan, Pristach, & Welte, 1994; McIntosh, Leigh, & Baldwin, 1994). However, use of the term “ever” in the HRS CAGE measure renders ambiguous whether an HRS participant’s drinking problems are active or remitted, and if remitted, recency of remission. Moreover, because CAGE assessment occurs only at participants’ initial HRS interview, it is not possible to measure change over time in their drinking problems.
Illness Severity and Medication Use
Following previous research (Brennan & Greenbaum, 2005; Brennan, Kagay, & Geppert, 2000; Joseph, Atkinson, & Ganzini, 1995), we used a count of baseline diagnosed medical conditions, exclusive of painful medical conditions, that included hypertension, diabetes, chronic lung disease, heart problems, stroke, and emotional or nervous problems (mean = .92; SD = 1.0) to assess participants’ illness severity. Number of medications was a count of medications taken by participants at baseline to treat hypertension, diabetes, chronic lung disease, heart problems, arthritis, and emotional or nervous problems (mean = .82; SD = 1.1).
Summary of Analyses
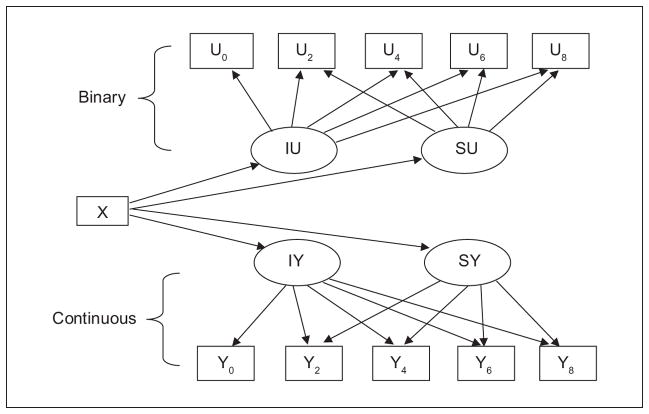
We used SPSS 18.0 and Mplus 5.12 (Muthén & Muthén, 1998–2010) software to analyze the data. We first conducted descriptive statistical analyses. Next, we conducted two-part latent growth modeling (Brown, Catalano, Fleming, Haggerty, & Abbott, 2005; Olsen & Schafer, 2001; Petras, Nieuwbeerta, & Piquaro, 2010) to describe participants’ 8-year alcohol consumption trajectories, and to determine the prospective effects of baseline pain characteristics on these trajectories. Two-part latent growth modeling is the most appropriate method to use to analyze longitudinal HRS alcohol consumption data, because these data contain a preponderance of zeros attributable to the substantial number of HRS participants who are permanent or temporary nondrinkers at various points in their later-life drinking careers. Because two-part latent growth modeling entails simultaneous estimation of both the binary (yes/no) and the continuous (if yes, how much) parts of latent growth trajectories, it permits extraction of maximal information about longitudinal use of alcohol by the older participants in the HRS (Brown et al., 2005; Olsen & Schafer, 2001; Petras et al., 2010) . Figure 1 summarizes the two-part latent growth model examined in this study. Participants’ original measured alcohol consumption, obtained at baseline assessment, then 2, 4, 6, and 8 years later, are processed by Mplus software (Muthén & Muthén, 1998–2010) to generate two sets of indicators: binary indicators (shown in boxes U0-U8), reflecting likelihood of consuming alcohol (no/yes), and continuous indicators (shown in boxes Y0-Y8), indicating quantity of alcohol consumed, if alcohol was consumed. Mplus generates indicator values using rules: (a) If an original measured variable is missing, both the binary and continuous indicators of the variable are assigned missing; (b) If an original measured variable is greater than zero, the new binary indicator is coded “1,” and the new continuous indicator retains its original measured value; (c) If an original measured variable is zero, the new binary indicator is coded “0,” and the new continuous indicator is assigned missing (Muthén & Muthén, 1998–2010).
Figure 1.
Two-part conditional latent linear growth model.
Binary and continuous indicators in the model are linked to latent growth parameters IU, SU, IY, and SY (shown in ellipses, Figure 1), using the time metric of years since baseline assessment (0, 2, 4, 6, and 8), controlling for baseline age, as well as gender, race, illness severity, and number of medications. In the binary part of the model, the latent variable “IU” represents “threshold,” the estimated cut point value of the probability that alcohol was consumed and “SU” represents “slope,” the estimated growth rate in the likelihood of alcohol being consumed. In the continuous part of the model, the latent variable labeled “IY” represents “intercept,” the estimated average initial level of alcohol consumption among participants who consumed alcohol, and “SY” indicates “slope,” the estimated average linear growth rate of alcohol consumption, among individuals who consumed alcohol. Also of interest for interpreting drinking growth trajectories, but, for simplicity, not shown in Figure 1, is the variance in each of the IU, IY, SU, and SY growth parameters. Measured variable “X” in Figure 1 represents baseline pain variables hypothesized to influence the level and shape of the binary and continuous parts of participants’ 8-year drinking trajectories.
Following previous research (Brown et al., 2005; Petras et al., 2010), we first estimated unconditional mean, linear, and quadratic two-part growth models of participants’ alcohol consumption to determine each model’s overall fit to the data. Chi-square difference tests confirmed that, for both amount of alcohol consumed and frequency of alcohol consumption, unconditional linear models best fit the data. Accordingly, participants’ linear growth trajectories are the focus of this study.
Next, we conducted two-part latent growth models that incorporated baseline pain variables (number of painful medical conditions, pain severity, pain interference) as predictors of binary and continuous latent growth model parameters. Each baseline pain predictor was centered on its group mean (Kraemer & Blasey, 2004), and each model controlled statistically for age, gender, race, illness severity, and number of medications.
We then conducted logistic regression analyses to determine effects of participants’ baseline pain characteristics (number of painful medical conditions, pain severity, and pain interference) on baseline presence of drinking problems (yes/no). Each of these logistic regression models controlled statistically for age, gender, race, illness severity, and number of medications.
Finally, to determine moderating effects of age, gender, African American background, marital status, education, and income on relationships between pain and alcohol use, we constructed centered interaction terms to enter as predictors into models with drinking behavior as outcome. In separate two-part latent growth and logistic regression models examining each moderating effect, demographic variables not contributing to the interactive term, illness severity, and medication use were first entered as covariates, followed by centered main effects, then centered interaction terms.
Results
Pain and Drinking Characteristics
At baseline, more than half (57%) of the sample reported that they had experienced one or more painful medical conditions (Table 1). The most frequent sources of pain were joints (43%) and back (30%), followed by head (10%) and chest (5%). Among individuals reporting pain, average pain severity was moderate (mean = 1.6; range = 1–3), and most (66%) of them reported that pain interfered significantly with their usual activities.
Table 1.
Pain and Drinking Characteristics of HRS Late-Middle-Aged Adults (N = 5,446).
| Pain Characteristics | Baseline
|
|
|---|---|---|
| % or M | (SD) | |
| Overall group | ||
| % 1+ painful medical conditions | 57.0 | |
| Number of painful medical conditions | 0.88 | (0.94) |
| % with joint pain | 42.5 | |
| % with back pain | 30.4 | |
| % with headaches | 10.0 | |
| % with chest pain | 5.1 | |
| Severity of pain | 0.38 | (0.79) |
| Interference by pain (% yes) | 16.1 | |
| Of those who reported pain | ||
| Number of painful medical conditions | 1.76 | (0.95) |
| Severity of pain (range = 1–3) | 1.55 | (0.85) |
| Interference by pain (% yes) | 65.9 | |
| Drinking Characteristics | Baseline | 2 years | 4 years | 6 years | 8 years |
|---|---|---|---|---|---|
| Alcohol consumption | |||||
| Consumed alcohol, last 3 months (% yes) | 53.2 | 50.9 | 49.1 | 48.8 | 47.5 |
| If consumed alcohol, mean number of drinks per day | 1.75 | 1.69 | 1.64 | 1.67 | 1.66 |
| Frequency of alcohol consumption | |||||
| Drank once a week or more, last 3 months (% yes) | 35.8 | 33.1 | 30.6 | 33.3 | 32.1 |
| If drank once a week or more, mean number of days per week | 3.10 | 3.33 | 3.46 | 3.45 | 3.50 |
| Drinking problems | |||||
| One or more drinking problems (% yes) | 22.7 | ||||
| Of those with drinking problems, number of CAGE items affirmed | 1.89 | (0.98) | |||
Just over half (53%) of participants had consumed alcohol in the last 3 months. This percentage declined 2, 4, 6, and 8 years after baseline assessment, to about 51%, 49%, 49%, and 48%, respectively. At baseline, drinkers consumed 1 to 2 drinks (1.75), per drinking occasion, on average. Reflecting the large number of infrequent drinkers in this sample, only 36% of individuals who drank did so more than once a week; of those who consumed alcohol once a week or more often, average frequency of alcohol consumption at baseline was about three times per week; this number increased slightly 2, 4, 6, and 8 years after baseline assessment.
With respect to drinking problems, 23% of participants affirmed 1+ CAGE items, indicating presence of past or current drinking problems. Among these participants, the average CAGE score was about 2.
Two-Part Unconditional Growth Models of Alcohol Consumption and Drinking Frequency
The top of Table 2 displays the results of two-part unconditional (i.e., no predictors) linear latent growth models of amount of alcohol consumed, and frequency of drinking, over the 8-year study interval. The threshold (IU = −.13) for the binary part of the unconditional two-part linear growth model of alcohol consumption corresponds to a predicted 53% probability (i.e., 1−[1/1+exp (−.13)]; p. 441, Muthén & Muthén (1998–2010)) of being a drinker over the 8-year course of the study. As indicated by the slope coefficient for change in likelihood of consuming alcohol (SU), participants’ likelihood of consuming alcohol declined somewhat (SU = −.17, p < .01) over the 8-year interval.
Table 2.
Unconditional and Conditional Linear Latent Growth Models of Alcohol Consumption and Frequency of Drinking.
| Alcohol Consumption
|
Drinking Frequency
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Binary
|
Continuous
|
Binary
|
Continuous
|
|||||
| IU | SU | IY | SY | IU | SU | IY | SY | |
| Unconditional linear growth models | ||||||||
|
| ||||||||
| Initial growth factor mean | −0.13 | −0.17** | 1.02** | −0.03** | 1.96** | −0.09** | 1.09** | 0.06** |
| Variance of growth factor mean | 24.82** | 0.00 | 1.83** | 0.03** | 20.98 | 0.00 | 6.06** | 0.08** |
| Model fit | ||||||||
| Log likelihood | −31, 432.6 | −28, 792.9 | ||||||
| BIC | 62, 985.6 | 57, 706.4 | ||||||
| Conditional linear growth models | IUC | SUC | IYC | SYC | IUC | SUC | IYC | SYC |
|---|---|---|---|---|---|---|---|---|
| Predictors | ||||||||
| Number of painful medical conditions | ||||||||
| Initial growth factor mean | −0.28** | −0.00 | −0.02 | −0.00 | −0.32** | −0.01 | −0.14** | −0.02* |
| Variance of growth factor mean | 21.85** | 0.00 | 1.47** | 0.01** | 17.58** | 0.00 | 5.16** | 0.02** |
| Model fit | ||||||||
| Log likelihood | −30, 894.6 | −28, 411.0 | ||||||
| BIC | 62, 116.1 | 57, 148.9 | ||||||
| Severity of pain | ||||||||
| Initial growth factor mean | −0.46** | 0.00 | −0.01 | −0.01* | −0.43** | 0.00 | −0.10* | −0.02* |
| Variance of growth factor mean | 21.76** | 0.00 | 1.47** | 0.01** | 17.54** | 0.00 | 5.18** | 0.02** |
| Model fit | ||||||||
| Log likelihood | −30, 888.3 | −28, 408.7 | ||||||
| BIC | 62, 103.5 | 57, 144.3 | ||||||
| Pain interference | ||||||||
| Initial growth factor mean | −1.26** | 0.03 | −0.12 | −0.01 | −1.16** | 0.02 | −0.31** | −0.04* |
| Variance of growth factor mean | 21.73** | 0.00 | 1.46** | 0.01** | 17.55** | 0.00 | 5.17** | 0.02** |
| Model fit | ||||||||
| Log likelihood | −30, 878.3 | −28, 406.5 | ||||||
| BIC | 62, 083.4 | 57, 139.9 | ||||||
Notes:
p < .05.
p < .01.
BIC = Bayesian information criterion; IU = threshold for binary part of model, = likelihood of 0 → 1 = 1−[1/1+exp (−threshold value)]; SU = slope for the binary part of model; IY = intercept for continuous part of model; SY = slope for the continuous part of model; IUc = intercept coefficient for binary part of model; SUc = slope coefficient for binary part of model; IYc = intercept coefficient for continuous part of model; SYc = slope coefficient for continuous part of model. SU variance fixed at zero. Conditional growth models control for age, gender, race, illness severity, and number of medications. Residual variances for Alcohol Consumption growth models range = 0.53 to 0.73**; residual variance for Drinking Frequency growth models range = 1.37 to 2.06**.
The continuous part of the model for alcohol consumption indicates that individuals who drank alcohol consumed, on average, just over 1 drink per day on days they drank (IY = 1.02, p < .01). However, there was statistically significant variability (1.83; p < .01) in number of drinks consumed by individuals on days they drank. As indicated by the SY coefficient in this model, participants who drank declined in the number of drinks they consumed, per day, over the course of the study (SY = −.03, p < .01). There was statistically significant variance in this growth parameter (.03, p < .01).
With regard to frequency of alcohol consumption, the binary part of the unconditional two-part linear growth model had a threshold IU = 1.96 (p < .01), which indicates a low probability (12%) of being a drinker who consumed alcohol more often than once a week over the 8-year study interval. Among participants who drank at least weekly, average frequency of drinking was low (about 1 day per week, IY = 1.09, p < .01), but there was considerable variance in this frequency (6.06, p < .01). Among drinkers, frequency of drinking grew over the course of 8 years (SY = .06, p < .01), with variance of .08 (p < .01).
Baseline Pain Predictors of Change in Alcohol Consumption and Drinking Frequency
Conditional (i.e., with pain predictors) two-part linear growth models of alcohol consumption and drinking frequency are shown beneath the unconditional models, in Table 2. The conditional model of 8-year change in alcohol consumption shows that having more numerous painful medical conditions near baseline assessment was associated with lower likelihood of consuming alcohol (IUc = −.28, p < .01); however, more numerous painful medical conditions near baseline had no effect on the 8-year rate of change in participants’ likelihood of consuming alcohol (SUc = .00, not significant).
Effects of baseline pain severity and pain interference followed a similar pattern. That is, more severe pain, and pain interference at baseline were each associated with lower likelihood of consuming alcohol at baseline (IUC = −.46 and −1.26, p < .01 for both, for pain severity and pain interference, respectively). More severe pain at baseline predicted a slightly increased the rate of decline in amount of alcohol consumed over the next 8 years, among participants who drank (SYc = −.01, p < .05).
With respect to drinking frequency, having more numerous painful medical conditions, more severe pain, and more debilitating pain at baseline were each associated with a lowered likelihood of drinking alcohol more often than once a week (IUc = −.32, −.43, and −1.16, respectively, all p < .01). Among individuals who drank at least weekly, all of the baseline pain predictors were associated with lower frequency of drinking (IYcs = −.14, −.10, and −.31, respectively, all p < .01), and all of them contributed to a faster rate of decline over the 8-year interval in weekly frequency of alcohol consumption (SYc = −.02, −.02, and −.04, respectively, all p < .05).
Associations Between Baseline Pain and Drinking Problems
We could not model growth in HRS participants’ drinking problems, because drinking problem data are obtained from HRS participants only once, at their baseline assessments. Accordingly, we conducted logistic regressions to determine associations between baseline pain variables and baseline likelihood of drinking problems, adjusted for age, gender, race, illness severity, and number of medications. As shown in Table 3, these analyses revealed that more numerous painful medical conditions, more severe pain, and more debilitating pain were each associated with higher likelihood of drinking problems (B = .17, .15, and .39, respectively, all p < .01). As indicated by odds ratios, having more numerous painful medical conditions, and experiencing more severe pain, each moderately raised the likelihood of having drinking problems, about 15% to 20%. Having pain debilitating enough to interfere with everyday activities raised the likelihood of drinking problems almost 50%.
Table 3.
Concurrent Association Baseline Pain Characteristics With Drinking Problems.
| Outcome
|
|||
|---|---|---|---|
| Baseline drinking problems | |||
|
| |||
| B | Odds ratio | Confidence interval | |
| Predictors | |||
| Number of painful medical conditions | .17** | 1.18 | [1.11, 1.26] |
| Severity of pain | .15** | 1.16 | [1.08, 1.25] |
| Pain interference | .39** | 1.48 | [1.26, 1.73] |
Notes:
p < .01. Logistic regressions control for age, gender, race, illness severity, and number of medications.
Moderating Effects of Demographic Characteristics
Only income and African American background moderated the relationship between pain and drinking behavior (data not shown). Income interacted weakly with pain severity, and with pain interference, to predict change in likelihood of consuming alcohol from 1996 to 2004 (both SUcs = −.03, p < .05). That is, being lower income slightly weakened the relationship between more severe and more debilitating pain at baseline and subsequent reduction in alcohol use.
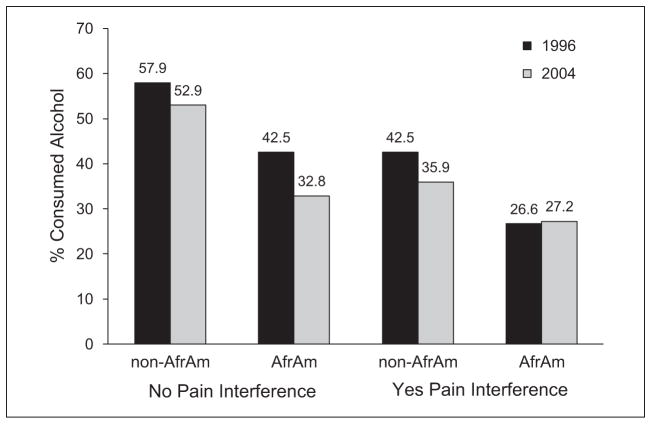
African American background interacted with pain severity to predict change over time in likelihood of consuming alcohol (SUc = .07, p < .05), and to predict change in likelihood of drinking more frequently than once a week (SUc = .07, p < .05). The same pattern obtained for the interaction of African American status with pain interference on 8-year change in likelihood of consuming alcohol, and likelihood of drinking more frequently than once a week (SUc = .15, p < .05 and SUc = .17, p < .01, respectively). Figure 2 helps illustrate these interactions. It shows that, overall, study participants declined in likelihood of drinking from 1996 to 2004, especially those with pain interference. However, whereas drinker prevalence among non-African Americans without baseline pain interference declined from about 43% to about 36% from 1996 to 2004, drinker prevalence among African Americans with pain interference remained about the same (27%) over the same interval.
Figure 2.
Moderating effect of African American background on the influence of pain interference and likelihood of consuming alcohol, 1996 to 2004.
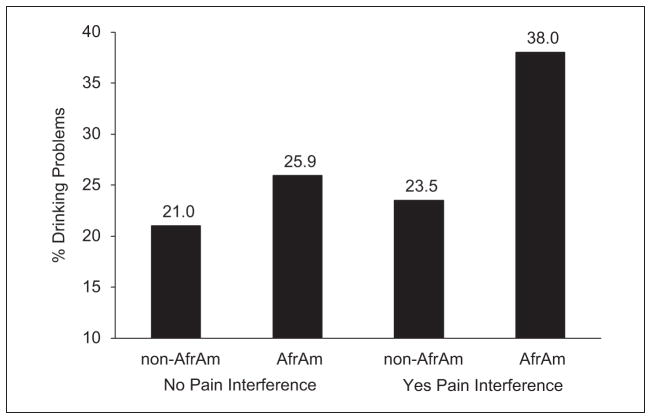
African American background also interacted with number of painful medical conditions (B = .20, p < .05) and with pain interference (B = .57, p < .01) to predict drinking problems. For example (see Figure 3), in the group overall, drinking problems were more prevalent among participants reporting pain interference than among those without pain interference, but whereas pain interference raised the likelihood of drinking problems only slightly for non-African Americans (from about 21% to 24%), it raised it considerably more for African Americans (from about 26% to 38%).
Figure 3.
Moderating effect of African American background on the relationship of pain interference to likelihood of having drinking problems.
Discussion
This study partially replicates and adds to findings from an earlier longitudinal investigation (Brennan et al., 2011) that focused on the relationship between late-life pain and drinking behavior. In so doing, it enhances our understanding of the relationship between pain and alcohol use, particularly as it occurs in later life.
The 8-year longitudinal HRS cohort examined here paralleled our earlier 10-year longitudinal community cohort (Brennan et al., 2011) with respect to age at baseline and length of follow-up. Although “baseline” assessments of the two cohorts occurred about 10 years apart, pain characteristics of the two samples were similar. For example, at baseline assessment, 43% of participants in the HRS cohort reported having joint pain; this was the case among 39% of participants in the community cohort. Back pain prevalence also was similar in the two samples, 30% and 36%, respectively. Most important, the longitudinal patterns of alcohol use, and the effects of baseline pain on those patterns, were comparable in the two samples. As expected, reflecting national norms, HRS respondents aged 55 to 65 were less likely to consume alcohol at initial assessment than were members of the 10-year community cohort, and less likely to have drinking problems (23% vs. 35%). However, among drinkers in both samples, average baseline amount and frequency of alcohol consumption were modest, with significant variability around group means, and the longitudinal, within-person pattern of drinking in both groups was generally moderately downward over the course of 8 to 10 years.
In both samples, pain experienced at late-middle-age had similar effects on subsequent within-person patterns of alcohol consumption over the next 8 to 10 years. In both samples, having more numerous painful medical conditions in late-middle-age was associated concurrently with lower likelihood of consuming alcohol and predicted a somewhat faster rate of decline in frequency of alcohol consumption. Extending previous findings, the present study demonstrated that this negative effect of pain encompasses pain severity and pain interference as well as number of painful medical conditions.
Results of these two longitudinal studies of pain and drinking behavior, together with those of Bobo et al. (2013), run counter to the “alcohol self-medication hypothesis” (Aira et al., 2008; Ilgen et al., 2010), which predicts that more pain experienced in late-middle-age might presage growth in amount and frequency of alcohol consumption over the next several years. Bobo and colleagues (2013) suggest that older adults’ elevated analgesic and general medication use may account for the association between pain and reduced use of alcohol in later life. However, we think this is unlikely, because in the present study the prospective effects of pain on subsequent alcohol use remained even after participants’ baseline illness severity and medication use were statistically controlled. Nonetheless, it is possible that 8-year changes in health, medication use, and other health-promoting self-care, intervened between participants’ baseline experience of pain and their subsequent reduction or cessation of alcohol use. Future research should examine this possibility.
Because HRS assesses participants’ drinking problems only at initial interview, we were unable to model pain as a predictor of longitudinal change in HRS participants’ drinking problems. However, consistent again with previous findings (Brennan et al., 2011), we found positive concurrent associations between all three pain characteristics—more numerous painful medical conditions, more severe pain, and more pain interference—and elevated likelihood of drinking problems. Having more numerous painful medical conditions and more severe pain elevated participants’ risk of drinking problems about 15% to 20%, and experiencing pain debilitating enough to interfere with everyday tasks raised the risk almost 50%. These positive associations also are consistent with results of previous cross-sectional research on mixed-age samples (Demyttenaere et al, 2007; Dersh et al., 2006; Lawton & Simpson, 2009), and consonant with the “risky lifestyle” hypothesis, which asserts that painful injuries, risky health behaviors, and substance misuse are causally intertwined (Dersh et al., 2006; Gatchel & Dersh, 2002). However, the cross-sectional nature of the HRS drinking problem data, and the temporal ambiguity of HRS CAGE item wording (i.e., “have you ever …”), limit our ability to interpret associations between pain and drinking problems in the HRS sample. The relationships we report here may reflect the damaging effects of past alcohol misuse on physical, mental, and social well-being in late-middle-age; or, on the other hand, that elevated pain in late-middle-age exacerbates alcohol misuse (e.g., leads to intensification of alcohol cravings). Future longitudinal research should be devoted to examining the direction of causality between late-life pain and drinking problems, and the tenability of the “risky lifestyle” hypothesis to explain this relationship.
Most previous research on pain and alcohol use has been based on simple bivariate models of the relationship between these constructs. However, considerable recent research in the areas of late-life adaptational processes, health disparities, pain, and alcohol use (e.g., Almeida, 2005; Amoako, Richardson-Campbell, & Kennedy-Malone, 2003; Brennan et al., 2005; Diehl, Hay, & Chui, 2012; Green, et al., 2003; Fillingim, King, Ribeiro-Dasilva, Rahim-Williams, & Riley, 2009; Gagliese, 2009; Reyes-Gibby, Aday, Todd, Cleeland, & Anderson, 2007; Robert & Ruel, 2006), suggests that predictive models of pain and alcohol use will be improved by incorporating sociodemographic characteristics, and life context, as factors that moderate the relationship between pain and alcohol use. Our findings lend some credence to this position. Taken together, results of the present study and Brennan et al. (2011) suggest that male gender, lower income, African American background, and having fewer interpersonal social resources, may act as risk factors to diminish the magnitude of the prospective relationship between pain at entry to later life and subsequent decline in alcohol use. Being male, and African American background, may also be risk factors that contribute to a stronger link between late-life pain and drinking problems.
This study has several noteworthy limitations. Although there is evidence for the reliability and validity of pain and alcohol use self-reports (e.g., Harris, Wilsnack, & Klassen, 1994; Liu et al., 1996; McHorney, Ware, Rogers, Raczek, & Lu, 1992), we caution that longitudinal survey data quality can be marred by participants’ recall bias and other threats to validity (Stone, Shiffman, Atienza, & Nebeling, 2007). In addition, this study addressed effects of a limited number of pain characteristics. Other key pain characteristics, such as pain chronicity, duration, and controllability, may be linked to older adults’ use of alcohol and should be the focus of future investigations. Only about 3% of our HRS sample reported racial backgrounds other than African American or Caucasian, so we were unable to separately examine the moderating effects of Latino, Asian, and other racial backgrounds on the relationship between later-life pain and alcohol use. Future research should focus on this issue. Temporal design characteristics of this study, such as its focus on the prospective effects of pain on long-term alcohol use, and the relatively long intervals between participant assessments, may have prevented us from finding noteworthy relationships between later-life pain and alcohol use. Future studies that use time-varying measures of pain, over shorter (e.g., daily) time intervals are needed to fully describe the interplay between older adults’ experience of pain and their use of alcohol.
Despite these limitations, this study contributes important new information about the relationship between pain and alcohol use in later life, and has potential implications for the prevention and treatment of alcohol misuse by older adults with pain. Although our results overall detract from the idea that late-middle-aged and older adults self-medicate pain with increased alcohol consumption, they do suggest that late-life pain and late-life drinking problems are linked, especially in certain subgroups of older adults, such as men and African Americans. This highlights the need for health care providers to monitor not only older pain patients’ quantity and frequency of alcohol use, but also whether they are experiencing drinking problems—negative physical, psychological, and social consequences associated with alcohol use. Future research should determine whether such monitoring, targeted to specific subgroups of older adults, can be beneficial to help prevent, and promote treatment for, pain-linked drinking problems in later life.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, grant number AA017477, and by Department of Veterans Affairs Health Services Research and Development Services research funds.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The views expressed in this manuscript are those of the authors and do not necessarily represent those of the Department of Veterans Affairs.
Reprints and permissions: sagepub.com/journalsPermissions.nav
References
- Aira M, Hartikainen S, Sulkava R. Drinking alcohol for medicinal purposes by people aged over 75: A community-based interview study. Family Practice. 2008;25:445–449. doi: 10.1093/fampra/cmn065. [DOI] [PubMed] [Google Scholar]
- Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science. 2005;14:64–68. [Google Scholar]
- Amoako EP, Richardson-Campbell L, Kennedy-Malone L. Self-medication with over-the-counter drugs among elderly adults. Journal of Gerontological Nursing. 2003;29:10–15. doi: 10.3928/0098-9134-20030801-05. [DOI] [PubMed] [Google Scholar]
- Ang DC, Peloso PM, Woolson RF, Kroenke K, Doebbeling BN. Predictors of incident chronic widespread pain among veterans following the first Gulf War. Clinical Journal of Pain. 2006;22:554–563. doi: 10.1097/01.ajp.0000208907.42506.21. [DOI] [PubMed] [Google Scholar]
- Armenian HK, Halabi SS, Khlat M. Epidemiology of primary health problems in Beirut. Journal of Epidemiology and Community Health. 1989;43:315–318. doi: 10.1136/jech.43.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman S, Herrstrom P, Jacobsson LT, Petersson IF. Chronic widespread pain: A three year follow-up of pain distribution and risk factors. Journal of Rheumatology. 2002;29:818–825. [PubMed] [Google Scholar]
- Bobo JK, Greek AA, Klepinger DH, Herting JR. Predicting 10-year alcohol use trajectories among men age 50 years and older. American Journal of Geriatric Psychiatry. 2013;21:204–213. doi: 10.1016/j.jagp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Greenbaum MA. Functioning, problem behavior, and health services use among nursing home residents with alcohol-use disorder: Nationwide data from the VA minimum data set. Journal of Studies on Alcohol. 2005;66:395–400. doi: 10.15288/jsa.2005.66.395. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Kagay CR, Geppert JJ. Elderly Medicare inpatients with substance use disorders: Characteristics and predictors of hospital readmissions over a four-year interval. Journal of Studies on Alcohol. 2000;61:891–895. doi: 10.15288/jsa.2000.61.891. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH. Pain and use of alcohol to manage pain: Prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100:777–786. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, SooHoo S, Moos RH. Painful medical conditions and alcohol use: A prospective study among older adults. Pain Medicine. 2011;12:1049–1059. doi: 10.1111/j.1526-4637.2011.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Catalano RF, Fleming CB, Haggerty KP, Abbott RD. Adolescent substance use outcome in the Raising Healthy Children Project: A two-part latent growth curve analysis. Journal of Consulting and Clinical Psychology. 2005;73:699–710. doi: 10.1037/0022-006X.73.4.699. [DOI] [PubMed] [Google Scholar]
- Brown RL, Patterson JJ, Rounds LA, Papasouliotis O. Substance abuse among patients with chronic back pain. Journal of Family Practice. 1996;43:152–160. [PubMed] [Google Scholar]
- Buchsbaum DG, Buchanan RG, Centor RM, Schnoll SH, Lawton MJ. Screening for alcohol abuse using CAGE scores and likelihood ratios. Annals of Internal Medicine. 1991;15:774–777. doi: 10.7326/0003-4819-115-10-774. [DOI] [PubMed] [Google Scholar]
- Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Chan AWK, Pristach EA, Welte J. Detection by the CAGE of alcoholism or heavy drinking in primary care and the general population. Journal of Substance Abuse. 1994;6:123–135. doi: 10.1016/s0899-3289(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Von Korff M. Mental disorders among persons with chronic back or neck pain: Results from the World Mental Health Surveys. Pain. 2007;129:332–342. doi: 10.1016/j.pain.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Dersh J, Gatchel RJ, Mayer T, Polatin P, Temple OR. Prevalence of psychiatric disorders in patients with chronic disabling occupational spinal disorders. Spine. 2006;31:1156–1162. doi: 10.1097/01.brs.0000216441.83135.6f. [DOI] [PubMed] [Google Scholar]
- Diehl M, Hay EL, Chui H. Personal risk and resilience factors in the context of daily stress. Annual Review of Gerontology and Geriatrics. 2012;32:251–274. doi: 10.1891/0198-8794.32.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing JA. Detecting alcoholism: The CAGE questionnaire. Journal of the American Medical Association. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Ferrell B. Pain. In: Abrams WB, Beers MH, Berkow R, editors. Merk manual of geriatrics. 3. Rahway, NJ: Merk Publishing; 2000. pp. 383–396. [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: A review of recent clinical and experimental findings. Journal of Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GG, Faul JD, Weir DR, Wallace RB. Documentation of chronic disease measures in the Health and Retirement Study (HRS/AHEAD) 2005 Retrieved from http://hrsonline.isr.umich.edu/sitedocs/userg/dr-009.pdf.
- Gagliese L. Pain and aging: The emergence of a new subfield of pain research. Journal of Pain. 2009;10:343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Dersh J. Psychological disorders and chronic pain: Are there cause-and-effect relationships? In: Turk DC, Gatchel RJ, editors. Psychological approaches to pain management: A practitioner’s handbook. New York, NY: Guilford Press; 2002. pp. 30–51. [Google Scholar]
- Gloth FM. Pain management in older adults: Prevention and treatment. Journal of the American Geriatric Society. 2001;49:188–199. doi: 10.1046/j.1532-5415.2001.49041.x. [DOI] [PubMed] [Google Scholar]
- Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Vallerand AH. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Alonso J. The relation between multiple pains and mental disorders: Results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Harris TR, Wilsnack RW, Klassen AD. Reliability of retrospective self-reports of alcohol consumption among women: Data from a U.S. national sample. Journal of Studies on Alcohol. 1994;55:309–314. doi: 10.15288/jsa.1994.55.309. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Willis RJ. Survey design and methodology in the Health and Retirement Study and the Wisconsin Longitudinal Study. In: Waite LJ, editor. Aging, health and public policy: Demographic and economic perspectives. New York, NY: Population Council; 2005. pp. 209–235. [Google Scholar]
- Health and Retirement Study. Sample sizes and response rates. 2010 Retrieved from http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf.
- Ilgen MA, Perron B, Czyz EK, McCammon RJ, Trafton JA. The timing of onset of pain and substance use disorders. American Journal on Addictions. 2010;19:409–415. doi: 10.1111/j.1521-0391.2010.00065.x. [DOI] [PubMed] [Google Scholar]
- Joseph CL, Atkinson RM, Ganzini L. Problem drinking among residents of a VA nursing homes. International Journal of Geriatric Psychiatry. 1995;10:243–248. doi: 10.1002/(sici)1099-1166(199707)12:7<767::aid-gps640>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Juster FT, Suzman R. The Health and Retirement Study: Data quality and early results. Journal of Human Resources. 1995;30(Suppl):S7–S56. [Google Scholar]
- Kraemer HC, Blasey CM. Centring in regression analyses: A strategy to prevent errors in statistical inference. International Journal of Methods in Psychiatric Research. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton J, Simpson J. Predictors of alcohol use among people experiencing chronic pain. Psychology, Health, and Medicine. 2009;14:487–501. doi: 10.1080/13548500902923177. [DOI] [PubMed] [Google Scholar]
- Leacock CP. Getting started with the Health and Retirement Study. 2006 Retrieved from http://hrsonline.isr.umich.edu/sitedocs/dmgt/IntroUserGuide.pdf.
- Liu S, Serdula MK, Byers T, Williamson DF, Mokdad AH, Flanders WD. Reliability of alcohol intake as recalled from 10 years in the past. American Journal of Epidemiology. 1996;143:177–186. doi: 10.1093/oxfordjournals.aje.a008727. [DOI] [PubMed] [Google Scholar]
- Mayfield D, McLeod G, Hall P. The CAGE questionnaire: Validation of a new alcoholism instrument. American Journal of Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Rogers W, Raczek AB, Lu JF. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts: Results from the Medical Outcomes Study. Medical Care. 1992;30:MS253–MS265. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- McIntosh MC, Leigh G, Baldwin N. Screening for hazardous drinking: Using the CAGE and measures of alcohol consumption in family practice. Canadian Family Practice. 1994;40:1546–1553. [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Olsen MK, Schafer JL. A two-part random effects model for semicon-tinuous longitudinal data. Journal of the American Statistical Association. 2001;96:730–745. [Google Scholar]
- Petras H, Nieuwbeerta P, Piquaro AR. Participation and frequency during criminal careers over the life span. Criminology. 2010;48:607–637. [Google Scholar]
- Polatin PB, Kinney RK, Gatchel RJ, Lillo E, Mayer TG. Psychiatric illness and chronic low-back pain. The mind and the spine—which goes first? Spine. 1993;18:66–71. doi: 10.1097/00007632-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Aday L, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: Non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. Journal of Pain. 2007;8:75–84. doi: 10.1016/j.jpain.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, 3rd, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. Journal of Pain. 2009;10:944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert SA, Ruel E. Racial segregation and health disparities between Black and White older adults. The Journals of Gerontology. Series B, Social Sciences. 2006;61:S203–S211. doi: 10.1093/geronb/61.4.s203. [DOI] [PubMed] [Google Scholar]
- Stone A, Shiffman S, Atienza A, Nebeling L. The science of real-time data capture: Self-reports in health research. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis and Rheumatism. 2007;57:656–665. doi: 10.1002/art.22684. [DOI] [PubMed] [Google Scholar]
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: Cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110:361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: Implications for concurrent treatment. Drug and Alcohol Dependence. 2004;73:23–31. doi: 10.1016/j.drugalcdep.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Urquhart DM, Bell R, Cicuttini FM, Cui J, Forbes A, Davis SR. Low back pain and disability in community-based women: Prevalence and associated factors. Menopause. 2009;16:24–29. doi: 10.1097/gme.0b013e31817e5ce0. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: Results from the national comorbidity survey replication. Pain. 2005;113:331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]