Abstract
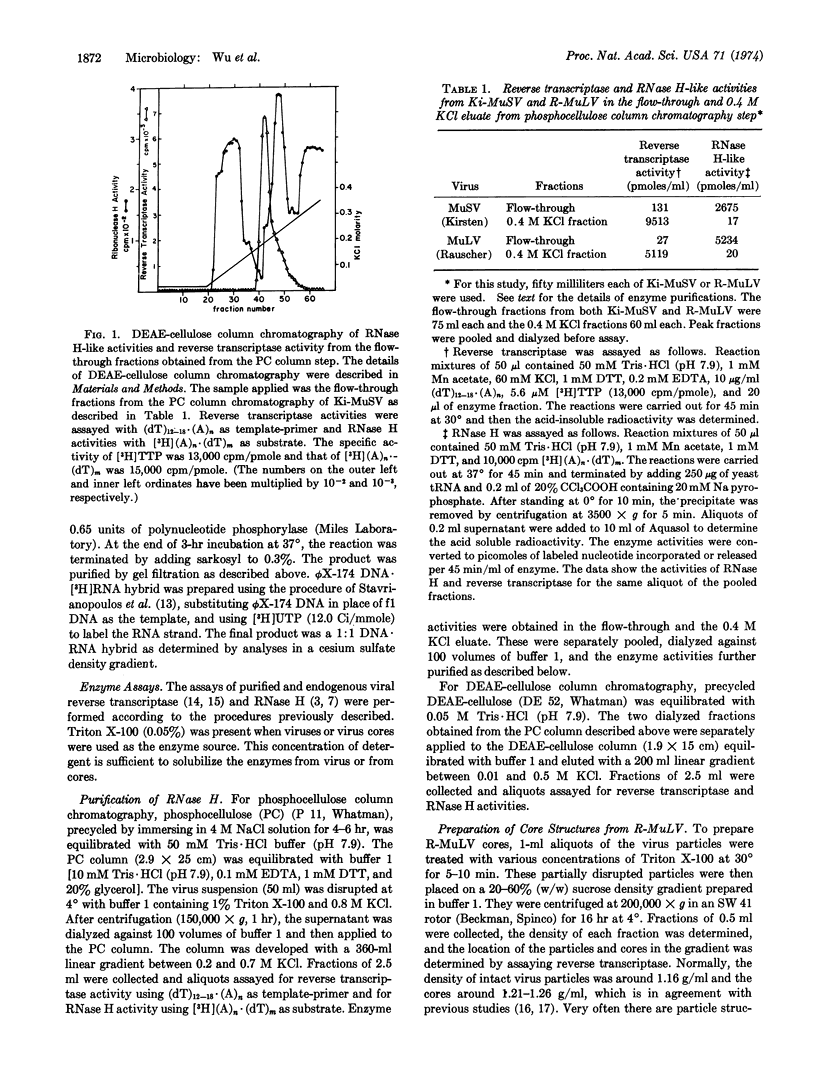
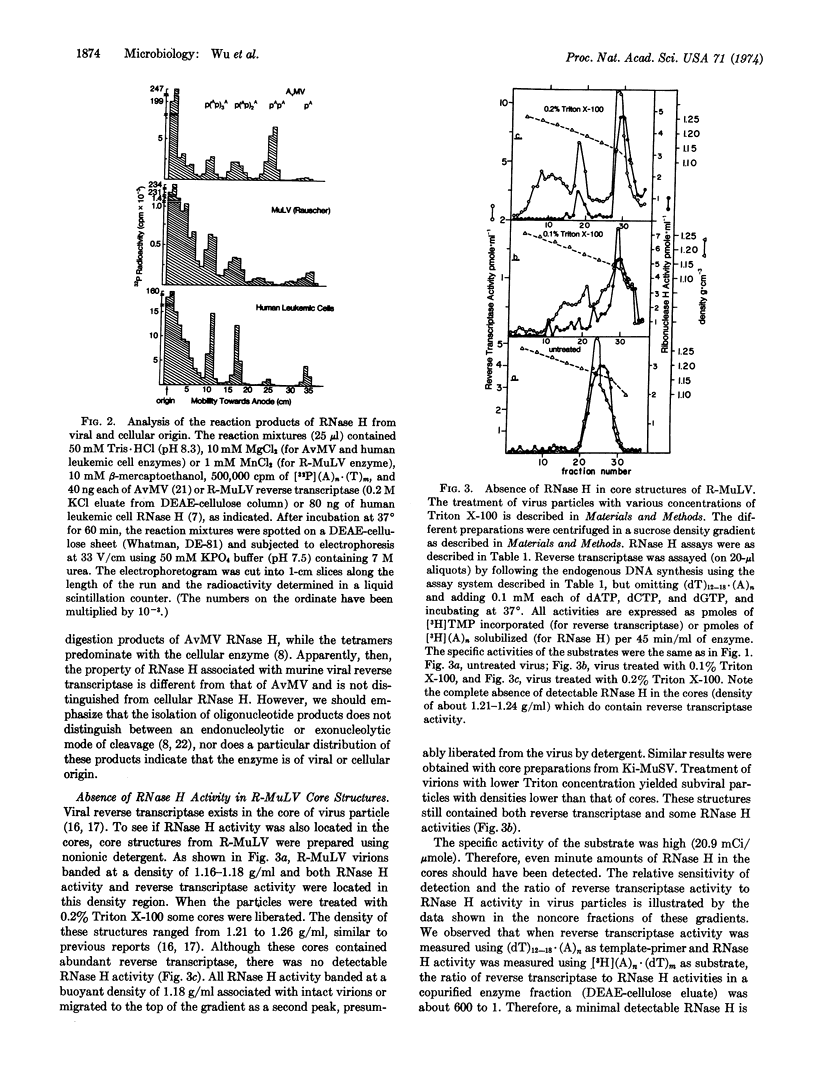
Ribonuclease H (RNA·DNA-hybrid ribonucleotidohydrolase, EC 3.1.4.34) has been reported to copurify with reverse transcriptase (RNA directed DNA polymerase) of RNA tumor viruses. In addition, viral specific ribonuclease H and reverse transcriptase of avian type-C viruses are thought to be part of the same polypeptide. In this report we show that a fraction of the ribonuclease H activity from Rauscher murine leukemia and Kirsten murine sarcoma viruses was separated from reverse transcriptase by anion exchange chromatography while the remaining portion co-purified with the viral polymerase. The amount of this co-purified nuclease activity was about 4- to 8-fold lower than the activity found in avian myeloblastosis virus (with respect to the ratio of ribonuclease H to reverse transcriptase) and this nuclease activity can only be detected by using labeled substrate of high specific radioactivity. However, a complete separation of ribonuclease H activity from reverse transcriptase was obtained by purifying core structures of the virus by sucrose density gradient centrifugation. While reverse transcriptase was present in the cores, there was no detectable ribonuclease H. Furthermore, a specific antibody against Rauscher leukemia virus reverse transcriptase did not inhibit any virion associated ribonuclease H activity. Our results suggest that in these virions these two enzyme activities reside in two separate molecules and probably in two different compartments of the virus. These findings emphasize a basic difference between the avian and murine type-C virus DNA polymerases.
Keywords: anion exchange chromatography, core structure, antibody to reverse transcriptase
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Gallo R. C. Purification, characterization, and comparison of the DNA polymerases from two primate RNA tumor viruses. J Virol. 1973 Sep;12(3):431–439. doi: 10.1128/jvi.12.3.431-439.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Comparison of Rous sarcoma virus-specific deoxyribonucleic acid polymerases in virions of Rous sarcoma virus and in Rous sarcoma virus-infected chicken cells. J Virol. 1971 May;7(5):625–634. doi: 10.1128/jvi.7.5.625-634.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Todaro G. J., Zeve V., Scolnick E. M., Aaronson S. A. Separation of RNA-dependent DNA polymerase activity from the murine leukaemia virion. Nature. 1970 Oct 31;228(5270):435–438. doi: 10.1038/228435a0. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. Ribonuclease H: a ubiquitous activity in virions of ribonucleic acid tumor viruses. J Virol. 1972 Dec;10(6):1136–1142. doi: 10.1128/jvi.10.6.1136-1142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkern R. C., Cantoni G. L. Studies on a calf thymus ribonuclease specific for ribonucleic acid-deoxyribonucleic acid hybrids. Biochemistry. 1973 Jun 19;12(13):2389–2395. doi: 10.1021/bi00737a004. [DOI] [PubMed] [Google Scholar]
- Keller W., Crouch R. Degradation of DNA RNA hybrids by ribonuclease H and DNA polymerases of cellular and viral origin. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3360–3364. doi: 10.1073/pnas.69.11.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Berkower I., Hurwitz J. Mechanism of action of ribonuclease H isolated from avian myeloblastosis virus and Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):466–470. doi: 10.1073/pnas.70.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Ross J., Todaro G. J., Aaronson S. A. Immunological relationships of reverse transcriptases from ribonucleic acid tumor viruses. J Virol. 1972 Jan;9(1):110–115. doi: 10.1128/jvi.9.1.110-115.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. I. PURIFICATION OF THE ENZYME AND CHARACTERIZATION OF THE PHOSPHATASE ACTIVITY. J Biol Chem. 1964 Jan;239:242–250. [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- SINGER M. F., GUSS J. K. The dependence of reactions catalyzed by polynucleotide phosphorylase on oligonucleotides. J Biol Chem. 1962 Jan;237:182–189. [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Karkas J. D., Chargaff E. Mechanism of DNA replication by highly purified DNA polymerase of chicken embryo. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2609–2613. doi: 10.1073/pnas.69.9.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H. DNA polymerase of murine sarcoma-leukemia virus: lack of detectable RNase H and low activity with viral RNA and natural DNA templates. J Virol. 1973 Dec;12(6):1512–1521. doi: 10.1128/jvi.12.6.1512-1521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. F., Mölling K., Bauer H. Ribonuclease H activity present in purified DNA polymerase from avian myeloblastosis virus. Biochem Biophys Res Commun. 1973 Mar 5;51(1):232–240. doi: 10.1016/0006-291x(73)90533-0. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Gallo R. C. RNA-directed DNA polymerase and virus-induced leukemia in mice. Proc Natl Acad Sci U S A. 1973 May;70(5):1298–1302. doi: 10.1073/pnas.70.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Paran M., Gallo R. C. Cordycepin inhibits induction of murine leukovirus production by 5-iodo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3820–3824. doi: 10.1073/pnas.69.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]


