Abstract
The oncogene RAS is known to induce genomic instability, leading to cancer development; the underlying mechanism, however, remains poorly understood. To better understand how RAS functions, we measured the activity of the functionally related genes Aurora-A and BRCA2 in ovarian cancer cell lines and tumor samples containing RAS mutations. We found that Aurora-A and BRCA2 inversely controlled RAS-associated genomic instability and ovarian tumorigenesis through regulation of cytokinesis and polyploidization. Over-expression of mutated RAS ablated BRCA2 expresson but induced Aurora-A accumulation at the midbody, leading to abnormal cytokinesis and ultimately chromosomal instability via polyploidy in cancer cells. RAS regulates the expression of Aurora-A and BRCA2 through dysregulated protein expression of farnesyl protein transferase β (FTβ and insulin-like growth factor binding protein 3 (IGFBP-3). Our results suggest that the imbalance in expression of Aurora-A and BRCA2 regulates RAS-induced genomic instability and tumorigensis.
Keywords: RAS, Aurora-A, BRCA2, Polyploid Cancer Cells, Cytokinesis, Genomic instability
Introduction
RAS signaling induces genomic instability 1, which provokes cancer development in many organs, however, the underlying mechanism remains elusive. Activation of RAS largely depends on its active form without CAAX at the C-terminus (C, Cys; A, usually aliphatic amino acid; X, another amino acid) that is processed by farnesyl transferase (FT) during posttranslational modification of RAS proteins 2. Thus, various inhibitors of farnesyl protein transferase activity, designed to prevent the farnesylation of RAS, have been developed to treat RAS-associated cancers 3. The insulin-like growth factor binding protein 3 (IGFBP-3) was shown to block RAS cleavage and thus to inhibit farnesyl protein transferase in lung carcinoma and head and neck squamous cell carcinoma 4. These reports suggest that FT and IGFBP-3 may be involved in regulating RAS-induced genomic instability and tumor development.
Genomic instability is largely classified into two types: microsatellite instability, which is associated with a mutator phenotype, and chromosome instability, which is associated with gross chromosomal abnormalities 5. The centrosome is believed to play an important role in maintaining chromosome stability by aiding in the formation of bipolar spindles during cell division 6, thereby ensuring equal segregation of duplicated chromosomes into two daughter cells. While multipolar mitotic spindles are usually resulted from various centrosome abnormalities such as amplification in cancer cells, which leads to unequal distribution of chromosomes and results in aneuploidy or polyploidy of daughter cells 7. The serine/threonine kinase Aurora-A (AURKA) plays a critical role in maintenance of genetic stability through regulation of centrosome separation, bipolar spindle assembly, and chromosome segregation 8, 9; at the same time, however, amplification of Aurora-A increases the number of centrosomes and mutiplolar spindles, which have been observed in numerous human cancers 10, 11. Recent studies have shown that Aurora-A is required for RAS-mediated oncogenic transformation of oral cancer 12 and bladder cancer 13. Thus, Aurora-A may be associated with genomic instability in RAS-induced tumorigenesis.
The breast cancer susceptibility gene 2 (BRCA2) is a tumor suppressor gene that is known to be involved in maintaining genomic stability in different cancers 14. Although BRCA2 is rarely mutated in sporadic cancers such as ovarian and breast cancers, the transcription or expression of BRCA2 is repressed in these tumor tissues 15. Loss of BRCA2 either by mutation or transcriptional and post-transcriptional aberrations is associated with cancer genomic instability 16. Recently, a study revealed that a heterozygous germline mutation of BRCA2 can promote pancreatic ductal adenocarcinomas driven by Kras (G12D) mutation 17, while another report showed that BRCA2 in HCT116 (a colon cancer cell line) can be suppressed by activated KRAS in 3D culture 18. In addition, studies have shown that BRCA2 mutation is associated with Aurora-A amplification in breast cancer 19 and that BRCA2 may suppress polyploidy by stabilizing Aurora-A 20. We have shown recently that Aurora-A can suppress BRCA2 expression in ovarian cancer 21. The above evidence suggests that Aurora-A and BRCA2 likely function to synergistically regulate RAS-induced genomic instability and tumorigenesis, although the underlying mechanism remains unclear.
To improve our understanding how RAS regulates the genomic instability, we designed a study to investigate the function of Aurora-A and BRCA2 in relation to RAS activation. Because the RAS/RAF mutation accounts for 30–40% of low-grade serous and borderline ovarian cancer cases 22, we mainly conducted the study in ovarian cancer cell lines and human ovarian tumor tissues with RAS mutations. Our results provide insight into how RAS/RAF mutations induce genomic instability and tumorigenesis.
Materials and Methods
Plasmids, siRNAs
We used pBabe/Aurora-A/puromycin 23 and pBabe/U6/Aurora-A shRNA (targeting 5′-GUCUUGUGUCCUUCAAAUU-3′ of Aurora-A mRNA) (puromycin or neomycin) 21 to deliver Aurora-A into immortalized ovarian epithelial cell lines T29 and T80 and Aurora-A shRNA into RAS-transformed cell lines T29H, T80H, and ovarian cancer cell line HEY. A plasmid (PCINBRCA2) containing a full-length BRCA2 cDNA was used to deliver BRCA2 into RAS-transformed cells and Capan-1 cells (a pancreatic cancer cell line) using a previously described method 24. Clones were selected after confirmation of BRCA2 expression by Western blotting. The retroviral expression plasmid IGFBP-3 (pBabe/IGFBP-3/puromycin) was generated with a pair of primers (sense: 5′-ATGGATCCatgcagcgggcgcgacccacgctc-3′, bold cases are BamHI site, and antisense: 5′-CAGAATTCctacttgctctgcatgctgtagc-3′, italic cases are EcoRI site) using a template of an adenoviral expression vector containing IGFBP-3 cDNA (a kind gift from Dr. Ho-Young Lee). pBabe/U6/IGFBP-3 shRNA/puromycin was generated to target IGFBP-3 mRNA at 403–422nt (5′-ggaaatgctagtgagtcgga-3′) using the protocols described in our previous publication 25. The control vectors were empty plasmids (pBabe/puromycin or PCIN) or constructed by directly inserting GFP shRNA into pBabe/U6/puromycin or neomycin vectors 26. Retrovirus production and target cell infection were performed with our well-established method 25. FTβ siRNA (#sc-35417) and control siRNA(#sc-37007) were purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA). FTI-276 (#F9553) was purchased from Sigma Aldrich (St. Louis, MO).
Cell culture and tumor formation
T29, T29H, T80, and T80H cells have been described previously 27. Ovarian cancer cell lines HOC-7, SKOV3 and HEY and pancreatic cancer cell line Capan-1 were cultured with EMEM or DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. T29 cells transformed by KRASV12 (T29K) was described previously 27. To generate tumor growth in vivo, we subcutaneously injected 5 × 106 T29H/BRCA2, T80H/BRCA2, T29/Aurora-A, T80/Aurora-A, T29H/Aurora-A shRNA (Aurora-Ai), T80H/Aurora-Ai cells or control cells expressing empty vectors or GFP shRNA (GFPi) into 4- to 6-week-old BALB/c nu/nu mice (U.S. National Cancer Institute’s Frederick Cancer Research Facility) following protocols approved by the institutional committee of MD Anderson Cancer Center for animal experiments. For T29H and T80H cells transfected with BRCA2, one of three clones from each cell line with high BRCA2 expression was used to conduct tumor formation assays. Each cell line was injected into 2 sites in 8 mice, for a total of 16 injections. Tumor burden was assessed and recorded using methods described previously 28.
Western blotting
For all Western blots, we analyzed samples with a total of 40-μg proteins from whole-cell lysates using the protocol described in our previous publication 21. The primary antibody used to detect Aurora-A (cat. #GTX13824) was obtained from GeneTex (Irvine, CA), while the antibody used to detect BRCA2 (cat. #MAB2476) was from R&D Systems (Minneapolis, MN). Antibodies against RAS either targeting N-terminus (#sc-166691) or C-terminus (#sc-521, KRAS; #sc-520, HRAS) and antibodies against IGFBP-3 (#sc-9028), FTα (#sc-487), and FTβ (# sc-137) were purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA). β-Actin (cat. #A2228, Sigma Aldrich, St. Louis, MO) was used as a loading control. T29/Vector and T29/Aurora-A cells were treated with proteosome inhibitor MG-132 (Sigma) at the concentration of 10 μM and analyzed for the expression of Aurora-A and BRCA2 by Western blotting. The intensity of protein bands was quantified with ImageJ software downloaded from NIH website (http://imagej.nih.gov/ij/).
Immunofluorescence
Immunofluorescence staining was performed according to a published protocol 21. Primary antibodies against Aurora-A and BRCA2 were obtained from GeneTex and R&D Systems, respectively. DNA dye To-Pro-3 was obtained from Molecular Probes (Carlsbad, CA). In brief, cells were cultured in chamber slides (Nalge Nunc International, Rochester, NY) for 24 h, fed with fresh medium to increase the number of mitotic cells for 8–16 h, and then fixed (with PBS-buffered paraformaldehyde solution: 3% paraformaldehyde, PBS, pH 7.4, 2% sucrose) and permeabilized (with a buffer containing 0.5% Triton X-100,20 mM HEPES, pH 7.4, 50 mM NaCl, 3 mM MgCl2, 300 mM sucrose). Slides were blocked by a 2-h incubation with 20% fetal bovine serum (FBS) and 2% goat serum in PBS, and then the slides were incubated with primary antibody at 4°C overnight. Afterward, the cells were incubated with fluorescein isothiocyanate (FITC)–conjugated secondary antibody against mouse IgG or Texas red–conjugated antibody against rabbit IgG (Jackson ImmunoResearch Laboratory, West Grove, PA) for 30 min. Stained cells were examined and photographed with an Olympus FV500 confocal fluorescence microscope.
Cell cycle and cytogenetic analysis
Cells were applied for cell cycle analysis by flow cytometry according to our previsouly published method 21. T29/vector, T29/Aurora-A, T80/vector, T80/Aurora-A, T29H/GFPi, T29H/Aurora-Ai, T80H/vector, T80H/BRCA2 cells were cultured for 24 and collected for chromosome preparation using standard procedures 29. Briefly, cells were exposed to Colcemid(0.04 μg/mL) for 1 h, subjected to hypotonic treatment(0.075 M KCl for 20–25 min at room temperature), and fixed in a mixture of methanol and acetic acid. Slides were stained with Giemsa stain and examined for structural and numerical abnormalities in the chromosomes. A minimum of 30 metaphase spreads were analyzed for each cell line, and representative spreads were captured using a Genetiscan imaging system. The proportions were compared using chi-squared analysis of Fisher exact test. The assay was repeatedly performed by Molecular Cytogenetics Core Facility personnel in the Department of Genetics at The University of Texas MD Anderson Cancer Center.
Immunostaining of Aurora-A and BRCA2
Ovarian tumor tissues from 22 patients diagnosed with low-grade serous carcinoma or borderline tumor were analyzed by immunohistochemical staining for expression of Aurora-A and BRCA2. KRAS/BRAF mutations with either G12V or V600E were identified from tissue genomic DNA of all cases by PCR amplification with specific primers targeting the coding regions of RAS-G12V and RAF-V600E, followed by sequencing of the DNA fragment. Ten cases were confirmed with RAS/RAF mutations, while the remaining cases lacked KRAS/BRAF mutations. The use of tissue blocks and chart reviews were approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. Slides were treated and stained using the method published before 21. The primary antibody against Aurora-A (GTX13824, monoclonal antibody, Genetax) or BRCA2 (MAB2476, monoclonal antibody, R&D Systems) was applied at a dilution of 1:200 or 1:100 at 4°C in a humidified chamber.
Evaluation of staining intensity and expression percentage for BRCA2 and Aurora-A was scored. using the following criterias: Tissues with <5% of cells positive for BRCA2 or Aurora-A were given a score of 0, those with 5% – 20% positive cells were scored as 1, those with 20% – 40% positive cells were scored as 2, those with 50% – 70% positive cells were scored as 3, and those with 70% – 100% were scored as 4. The expression correlation of BRCA2 and Aurora-A was analyzed by Pearson’s correlation using SPSS16.0 software.
Cell treatment with FTβ siRNA or FTI-276
To transfect HEY and T29K cells with FTβ siRNA, 5 × 105 cells per well in 6-well plates were used for FTβ siRNA and control siRNA transfection using the manufacturer’s protocol from Santa Cruz Biotech Inc. (Santa Cruz, CA). The transfection medium was replaced with fresh growth medium 12 h later, and the cells were kept in culture for additional 24, 48, and 72 h and harvested to detect FTβ, Aurora-A, and BRCA2 expression. A similar cell number was used for treatment either with FTI-276 for 24 h and the cells were analyzed for expression of the above-listed proteins.
Results
RAS-induced transformation enhances Aurora-A expression but represses BRCA2 expression
To better understand how RAS promotes genomic instability, we measured the expression of BRCA2 and Aurora-A in RAS-transformed human ovarian surface epithelial cell lines previously developed in our lab 27. While the expression of BRCA2 was markedly lower in RAS-transformed cells than in control cells (Figure 1A), the expression of Aurora-A was dramatically increased in these cells, suggesting that RAS suppresses the expression of BRCA2 but increases the expression of Aurora-A. Next, we determined whether BRCA2 can regulate the expression of Aurora-A or RAS by transfecting RAS-transformed ovarian epithelial cell lines (T29H and T80H) with a vector expressing BRCA2 24. Selected stable clones with ectopic expression of BRCA2 showed a marked decrease of Aurora-A and RAS (Figure 1B), indicating that BRCA2 suppresses Aurora-A and RAS expression. To determine whether Aurora-A can suppress the expression of BRCA2, we delivered Aurora-A cDNA into immortalized non-tumorigenic T29 and T80 cells (Aurora-A; Figure 1C–D) or silenced Aurora-A expression in T29H and T80H cells with Aurora-A–specific short hairpin RNA (shRNA) (Aurora-Ai; Figure 1C–D). Ectopic expression of Aurora-A suppressed BRCA2 expression, but did not stimulate RAS expression in T29 and T80 cells compared with in vector-transfected control cells, and knockdown of Aurora-A restored the BRCA2 level and reduced RAS expression in T29H and T80H cells, suggesting that Aurora-A also negatively regulates the expression of BRCA2. We infer from these results that RAS-driven malignancy is modulated by Aurora-A and BRCA2.
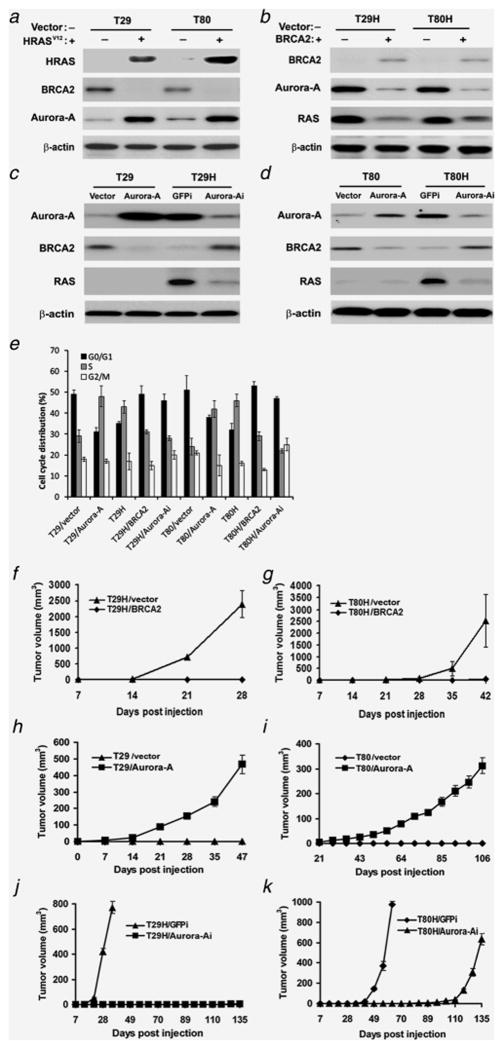
Figure 1. Protein expression status detected by immunoblotting and tumor growth curve in nude mice.
A. RAS transformation (+) induced concurrent suppression of BRCA2 and amplification of Aurora-A in immortalized cells (T29 and T80). HRAS was detected by antibody against C-terminus (#sc-520). B. Ectopic expression of BRCA2 (+) inhibited Aurora-A and RAS expression in RAS-transformed cells. C-D. Overexpression of Aurora-A decreased BRCA2 expression in immortalized cells (T29 and T80), and knockdown of Aurora-A by specific shRNA (Aurora-Ai) increased BRCA2 expression and decreased RAS level in RAS-transformed cells (T29H and T80H). Vector- or GFPi-treated cells were used as relative controls. E. Cell cycle distribution detected by flow cytomtry. F–G. The mean tumor sizes in mice receiving vector control cells (T29H/vector and T80H/vector) or BRCA2-transfected cells transformed with HRASV12 (T29H/BRCA2 or T80H/BRCA2) are shown. The data suggest that tumor formation was completely blocked by the introduction of wild-type BRCA2 in RAS-transformed cells. H–I. Tumor formation in mice was induced by the introduction of Aurora-A in immortalized cells (T29/Aurora-A and T80/Aurora-A) compared to vector controls (T29/vector and T80/vector). J–K. Knockdown of Aurora-A in RAS-transformed cells (T29H and T80H) cells markedly hampered or delayed tumor growth. Data were collected in three independent experiments. Error bars = 95% confidence intervals.
Aurora-A and BRCA2 regulates cell cycle progression and tumor growth of RAS-transformed cells
Since Aurora-A and BRCA2 participate in cell cycle regulation which controls ovarian tumorigenesis, we detected the cell cycle distribution by flow cytometry (Figure 1E). Introduction of RAS or Aurora-A in immortalized ovarian surface epithelial cells promoted cell cycle progression by increasing cell population in S phase and downregulating cell population in G0/G1 phase as compared with these in control cells. In constrast, overexpression of BRCA2 or knockdown of Aurora-A in RAS-transformed cells promoted cell arrest at G0/G1 phase and reduced cells in S phase as compared with these in control cells.
To test whether Aurora-A and BRCA2 affect ovarian tumor growth, we injected RAS-transformed T29H or T80H cells overexpressing BRCA2 into nude mice and compared tumor growth to that in mice receiving vector control cells. No tumors were observed in any of the mice injected with BRCA2-transfected T29H and T80H cells, while all the mice injected with vector control cells experienced rapid tumor growth within 4–7 weeks (Figure 1F–G), indicating that the expression of BRCA2 completely blocked tumor formation of the RAS-transformed cells. In addition, when ectopic Aurora-A expression was induced in immortalized nontumorigenic T29 and T80 cells, subcutaneous tumor growth resulted (Figure 1H–I), whereas shRNA-induced knockdown of Aurora-A in RAS-transformed cells reduced or delayed tumor growth, compared with tumor growth in control cells expressing GFPi (Figure 1J–K). Taken together, the above data demonstrated that Aurora-A and BRCA2 play opposite roles in RAS-associated tumor formation in vivo.
Unbalanced expression of Aurora-A and BRCA2 in cancer cells and tissues with RAS/RAF mutations
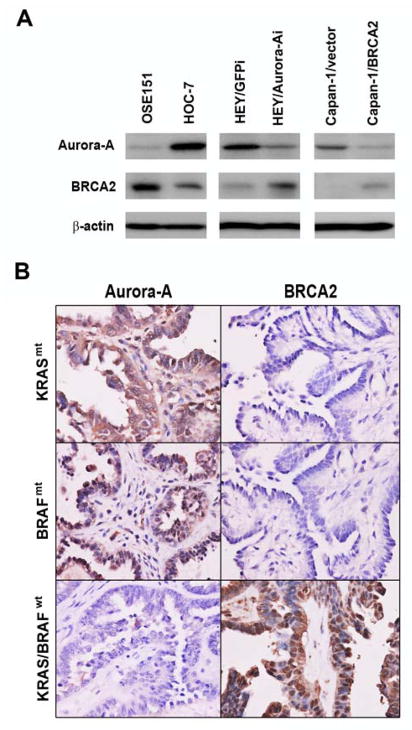
Since the above results were derived from RAS-transformed ovarian surface epithelial cells, we set out to confirm the results in a panel of cells including normal ovarian surface epithelial (OSE) cells, ovarian cancer cells, and pancreatic cancer cells harboring KRAS mutations. We detected higher expression of BRCA2 and lower expression of Aurora-A in OSE 151 cells (Figure 2A), a normal ovarian surface epithelial (OSE) cell line described in our previous report 25, but lower BRCA2 and higher Aurora-A in the ovarian cancer cell lines HOC-7 and HEY with confirmed mutations in KRAS (SFigure 1) and in the pancreatic cancer cell line CAPAN-1, which has a reported KRAS mutation and a truncated BRCA2 mutation (Figure 2A). Furthermore, knockdown of Aurora-A by shRNA in HEY cells and introduction of BRCA2 in CAPAN-1 cells resulted in decreased Aurora-A expression and increased BRCA2 expression (Figure 2A).
Figure 2. Inverse expression of Aurora-A and BRCA2 in normal and cancer cells and ovarian tumor tissues with KRAS/BRAF mutations.
A. Aurora-A and BRCA2 expression in normal ovarian surface epithelial cells, ovarian cancer cells and pancreatic cancer cells treated with Aurora-A shRNA or BRCA2 cDNA. B. Representative images from cancer tissues with or without KRAS/BRAF mutations from patients diagnosed with low-grade serous ovarian carcinoma. High Aurora-A expression was correlated with negative expression of BRCA2 (×400) (upper and middle panels). High expression of BRCA2 was correlated with negative detection of Aurora-A in another case (× 400) (bottom panel).
The above results also suggested the possibility that Aurora-A and BRCA2 are negatively regulated in ovarian cancer, particularly in low-grade serous ovarian carcinomas and ovarian borderline tumors with KRAS/BRAF mutations. Thus, we selected tumor tissue samples from 22 cases diagnosed with low-grade serous ovarian carcinoma and borderline tumor with or without identified KRAS/BRAF mutations and detected Aurora-A and BRCA2 expression by immunostaining. We measured high expression of Aurora-A and low expression of BRCA2 in 6 of 10 (60%) samples with RAS/RAF mutations (P = 0.018, two-tailed Pearson’s correlation), but high expression of BRCA2 and low expression of Aurora-A in 8 of 12 (66.7%) samples without KRAS/BRAF mutations (P = 0.023, two-tailed Pearson’s correlation) (Table 2). No statistical differences in Aurora-A and BRCA2 expression were found in samples with low-grade serous carcinoma and in those with borderline tumor or between samples with KRAS mutation and those with BRAF mutation. Representative images are shown in Figure 2B.
Table 2.
Immunohistochemical analysis of low grade serous (LGS) and serous borderline tumor (SBT) with or without RAS/RAF mutations
| Case No. | Diagnosis | KRAS | BRAF | Aurora-A expression (%) | BRCA2 expression (%) |
|---|---|---|---|---|---|
| 1 | LGS | wt | wt | 10 | 90* |
| 2 | LGS | wt | wt | 30 | 55* |
| 3 | LGS | wt | wt | 0 | 20 |
| 4 | LGS | wt | wt | 20 | 70* |
| 5 | LGS | wt | wt | 40 | 50 |
| 6 | LGS | wt | wt | 10 | 80* |
| 7 | LGS | wt | wt | 0 | 90* |
| 8 | LGS | Mt | wt | 80* | 20 |
| 9 | LGS | Mt | wt | 70* | 10 |
| 10 | LGS | wt | Mt | 90* | 5 |
| 11 | LGS | wt | Mt | 40 | 50 |
| 12 | SBT | wt | wt | 0 | 65* |
| 13 | SBT | wt | wt | 15 | 75* |
| 14 | SBT | wt | wt | 5 | 85* |
| 15 | SBT | wt | wt | 30 | 40 |
| 16 | SBT | wt | wt | 40 | 20 |
| 17 | SBT | Mt | wt | 75* | 10 |
| 18 | SBT | Mt | wt | 80* | 30 |
| 19 | SBT | Mt | wt | 50 | 40 |
| 20 | SBT | wt | Mt | 55 | 60 |
| 21 | SBT | wt | Mt | 30 | 50 |
| 22 | SBT | wt | Mt | 65* | 10 |
Cases with statistical significance(P < 0.05)
Farnesyl protein transferase β (FTβ) and insulin-like growth factor binding protein 3 (IGFBP-3) mediate the regulation of Aurora-A and BRCA2 in RAS-associated cancer cells
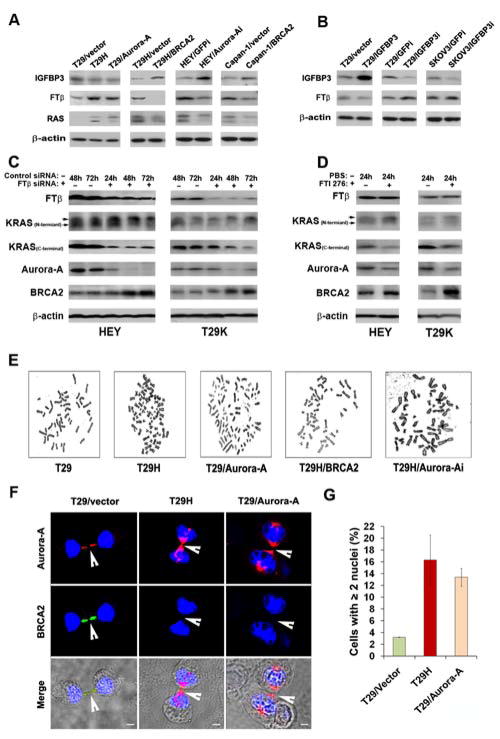
Since IGFBP-3 was reported to inhibit farnesyl protein transferase, which thereby blocks RAS cleavage 4. We measured the expression of IGFBP-3 and farnesyl protein transferase in a panel of ovarian cell lines. We found that the expression of IGFBP-3 was decreased in T29H and T29/Aurora-A cells compared with vector control cells, but was increased by ectopic introduction of BRCA2 in T29H and Capan-1 cells, and by disruption of Aurora-A in HEY cells compared with in their corresponding control cells (Figure 3A). In contrast, the expression of farnesyl protein transferase β (FTβ (but not FTα, data not shown) was increased in RAS- and Aurora-A-transformed cells (T29H and T29/Aurora-A), but decreased in BRCA2-transfected cells (T29H/BRCA2, Capan-1/BRCA2) and Aurora-A shRNA-treated cells (HEY/Aurora-Ai) compared with in their controls. These changes led to corresponding increases or decreases in RAS farnesylation (Figure 3A). These results suggest that transformation of ovarian epithelial cells by RAS or Aurora-A can inhibit IGFBP-3, leading to an increased expression of FTβ, which may in turn promote RAS farnesylation and ovarian tumorigenesis. They also suggest that the restoration of BRCA2 expression by silencing Aurora-A or introducing BRCA2 induces IGFBP-3 overexpression, which inhibits the activity of FTβ, leading to reduced farnesylation of RAS, which may in turn decrease ovarian tumor formation.
Figure 3. Alteration of signal molecules and detection of chromosomal abnormality and abnormal cytokinesis in RAS-associated cancer cells.
A. Transformation of ovarian epithelial cells (T29) by RAS or Aurora-A represses IGFBP-3, but induces FTβ over expression in T29H and T29/Aurora-A cells compared with control cells. However, introduction of BRCA2 cDNA or Aurora-A shRNA into T29H, Capan-1, or HEY cells resulted in increased IGFBP-3 and decreased FTβ, which in turn reduced the farnesylation of RAS. B. Introduction of IGFBP-3 cDNA or IGFBP-3 shRNA suppressed or increased FTβ expression. C. Treatment of cells with FTβ siRNA reduced the expression of FTβ, RAS farnesylation and Aurora-A expression, but elevated BRCA2 protein level. D. Treatment of cells with farnesyl protein transferase inhibitor FTI-276 suppressed KRAS farnesylation and Aurora-A expression, but simultaneously restored BRCA2 level. E. The selected images show that RAS (T29H) or Aurora-A (T29/Aurora-A) transformation led to more polyploid cells than were observed in parental cell lines (T29), but transfection of T29H with BRCA2 or Aurora-A shRNA (Aurora-Ai) reduced cell polyploidy. F. Co-localization of Aurora-A and BRCA2 was detected in the midbody of T29/vector cells during late mitosis, but overexpression of Aurora-A in RAS- or Aurora-A-transformed cells (T29H, T29/Aurora-A) diminished the localization of BRCA2 in the midbody. Blue dye To-Pro-3 indicates nucleus. Scale bars, 5μm. G. Quantification of cells with multiple nuclei in RAS – or Aurora-A–transformed cells. Introduction of RAS or Aurora-A resulted in more cells with multiple nuclei. Error bars = 95% confidence intervals from three independently repeated experiments.
To confirm that IGFBP-3 is able to suppress FTβ expression, we transfected T29 and SKOV3 (an ovarian cancer cell line) cells with either IGFBP-3 cDNA or IGFBP-3 shRNA. As shown in Figure 3B, overexpression of IGFBP-3 reduced FTβ in T29 cells, whereas silencing of IGFBP-3 increased FTβ in T29 and SKOV3 cells compared with in their control cells. Quantification data of FTβ and IGFBP-3 expression with ImageJ software was shown in SFigure 2. These results suggest that IGFBP-3 is involved in regulation of Aurora-A and BRCA2 through FTβ in terms of farnesylation of RAS. To strengthen evidence for this notion, we treated HEY and T29K (KRASV12-transformed T29 cells) with FTβ-specific siRNA or with FTI-276, which specifically inhibits farnesyl protein transferases activity 30. As shown in Figure 3C, treatment with FTβ-specific siRNA reduced the farnesylation of KRAS and Aurora-A expression, which is consistent with a recent report 31, but increased BRCA2 expression compared with control siRNA-treated cells at the same time point. Moreover, treatment of HEY and T29K cells with FTI-276 yielded the same results as those from treatment with FTβ siRNA (Figure 3D). These data suggest that FTβ not only regulates RAS by farnesylation, but also controls the expression of Aurora-A and BRCA2 through a mechanism that may be associated with IGFBP-3.
Aurora-A and BRCA2 regulate chromosomal instability through dysregulated cytokinesis
Amplification of Aurora-A and inactivation of BRCA2 are known to be closely associated with chromosomal instability. By analyzing chromosomal aberrations, as expected, the proportion of polyploid cells was markedly higher in RAS- and Aurora-A–transformed cells than in control cells, and the knockdown of Aurora-A or introduction of BRCA2 in RAS-transformed cells resulted in less polyploidy in the experimental cell lines than in the control lines (Table 1, Figure 3E). In addition, the overall chromosome aberration was increased in cells overexpressing RAS or Aurora-A compared with in control cells; however, the ectopic expression of BRCA2 or silencing of Aurora-A in the transformed cells decreased the overall chromosome aberration. These results demonstrate that dysregulation of Aurora-A and BRCA2 led to chromosomal instability in RAS-transformed cells.
Table 1.
Cytogenetic Analysis of Chromosome Abnormalities in Immortalized Ovarian Epithelial Cells after Overexpression of HRAS or Aurora-A and in HRAS-transformed Cells after Knockdown of Aurora-A or Overexpression of BRCA2
| ID | Cell Linea | Cells with Chromosome Aberrations (%) | Cells with DNA Breaks (%) | Diploid Cells (%) | Polyploid Cells (%) |
|---|---|---|---|---|---|
| 1682 | T29/vector | 6.4b | 6.4 | 81.8c | 6.1b |
| 1683 | T29/Aurora-A | 12.1b(↑) | 6.1 | 64.5c(↓) | 32.3b(↑) |
| 1684 | T80/vector | 2.9c | 2.9 | 93.7c | 0b |
| 1685 | T80/Aurora-A | 6.3c(↑) | 6.3 | 73.5c(↓) | 23.5b(↑) |
| 1688 | T29H/GFPi | 8.3b | 0 | 75c | 16.7b |
| 1689 | T29H/Aurora-Ai | 2.9b(↓) | 2.9 | 85.3c(↑) | 8.8b(↓) |
| 1686 | T80H/vector | 11.4b | 11.4b | 57.1c | 25.7c |
| 1687 | T80H/BRCA2 | 3b(↓) | 3b(↓) | 75.7c(↑) | 18.2c(↓) |
For each cell line, 30–36 cells in metaphase were examined. Increase or decrease in chromosomal aberrance in terms of DNA breaks, diploidy, and polyploidy was indicated as ↑ or ↓, respectively.
p < 0.01.
p < 0.05.
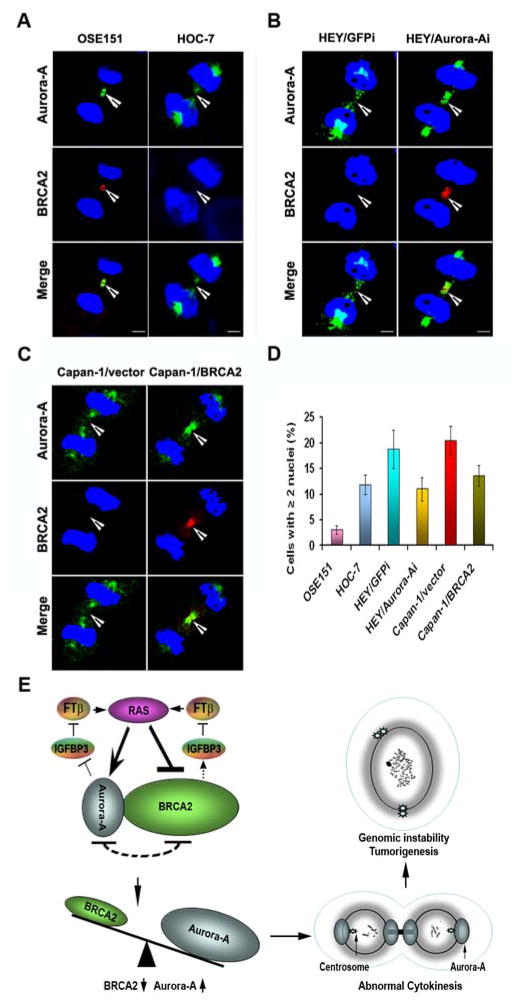
Cytokinesis occurs during the last step of mitosis at which point a cell divides into two daughter cells. Abnormal cytokinesis usually results in cell multinuclearity and eventually induces chromosomal instability. Since Aurora-A and BRCA2 are involved in regulating cytokinesis 32, 33, we examined the expression of both Aurora-A and BRCA2 in the midbody of late mitotic T29, T29H, and T29/Aurora-A cells. In immortalized T29/vector cells, BRCA2 and Aurora-A co-localized at the midbody during the late stage of mitosis (Figure 3F). The transformation of T29 cells by RAS or Aurora-A diminished the localization of BRCA2 and increased the accumulation of Aurora-A in the midbody as compared with T29/vector cells. Counts of cells with two or more nuclei showed that the transformation by RAS or Aurora-A induced at least four times as many as were induced in their control cells (Figure 3G). In normal ovarian epithelial cells (OSE151), Aurora-A and BRCA2 were co-localized at the midbody, while BRCA2 was undetectable in the midbody of mitotic HOC-7, HEY, and Capan-1 cells with KRAS mutations (Figure 4A-C). However, knockdown of Aurora-A or introduction of BRCA2 in HEY or Capan-1 cells restored BRCA2 accumulation in the midbody (Figure 4B-C). Consistent with the results from T29, T29H, and T29/Aurora-A cells, there were fewer OSE151 cells with multinuclearity than HOC-7 cells, and the number of HEY/GFPi and Capan-1/vector cells with multinuclearity was greater than HEY/Aurora-Ai and Capan-1/BRCA2 cells (Figure 4D). These results suggest that RAS mutations can diminish BRCA2 and enhance Aurora-A expression in the midbody during cytokinesis, which results in cell multinuclearity and genomic instability in human ovarian cancer cells as well as pancreatic cancer cells.
Figure 4. Analysis of Aurora-A and BRCA2 during cytokinesis.
A–C. Co-localization of Aurora-A and BRCA2 in the midbody of normal ovarian surface epithelial cells (OSE151), ovarian cancer (HOC-7 and HEY) and pancreatic cancer cells (Capan-1) with KRAS mutations. KRAS mutation results in Aurora-A increase and BRCA2 depletion in midbody during cytokinesis, whereas knockdown of Aurora-A in HEY cells or introduction of BRCA2 in Capan-1 cells restored the appearance of BRCA2 in the midbody although the reduced level of Aurora-A was still detectable in HEY/Aurora-A and Capan-1/BRCA2 cells. D. The number of cells with multinuclearity was higher in HOC-7, HEY, and Capan-1 cells than in OSE151 cells, but the decreased multinuclearity was observed in HEY and Capan-1 cells after transfection with Aurora-A shRNA or BRCA2 cDNA. E. A schematic model illustrating that RAS induces unbalanced expression of Aurora-A and BRCA2, which are in turn to regulate IGFBP-3 and FTβ to activate RAS signaling. The accumulation of Aurora-A and the depletion of BRCA2 result in abnormal cytokinesis and cell multinuclearity, which eventually induce genomic instability and tumorigenesis.
Discussion
Using RAS-transformed ovarian surface epithelial cells as a model system in this study, we have identified a negative regulatory loop between Aurora-A and BRCA2, which are downstream targets of RAS. We showed that RAS transformation of ovarian epithelial cells can induce amplification of Aurora-A and simultaneously repress BRCA2 expression, which was also observed in ovarian cancer cell lines and ovarian cancer tissues with RAS/RAF mutations. Aurora-A and BRCA2 oppositely regulated RAS-induced genomic instability in RAS-mutated cells through abnormal cytokinesis. In normal or immortalized ovarian epithelial cells, Aurora-A and BRCA2 are co-localized at the midbody during late mitosis, in which BRCA2 and Aurora-A may control the segregation of two daughter cells through regulation of cytokinesis and prevent the generation of polyploid cells. A model on how Aurora-A and BRCA2 function in RAS mutated cancer is illustrated in Figure 4E. Upon activation by the RAS oncogene, which tips the balance toward Aurora-A, the diminished expression of BRCA2 and the accumulation of Aurora-A in the midbody may hamper the abscission of cleavage furrow to induce polyploidy or aneuploidy, which ultimately results in cellular genomic instability and tumorigenesis.
Cytokinesis is the last important step of cell division where identical eukaryotic daughter cells finally separate. The association of cancer with abnormal cytokinesis has been frequently reported over the past 20 years. The proteins that regulate or participate in abnormal cytokinesis in cancer cells include kinases (such as Aurora-A, Aurora-B, and PLK1), mitotic checkpoint proteins (such as ATM, CHK1, and CHK2), mitotic regulators (such as BRCA1 and centrobin) 34. One of the phenomena induced by abnormal cytokinesis is multinuclearity leading to chromosomal polyploidy or aneuploidy 35, which largely contributes to genomic instability and tumorigenesis 36. Both Aurora-A and BRCA2 are cell cycle regulatory proteins participating in cellular mitosis 37, 38. We have identified in this study that Aurora-A and BRCA2 are two mediators that co-localize at the midbody of late mitotic cells to control the genomic instability of cells which is regulated by mutated RAS oncogene. We and other research groups have found that BRCA2 is involved in regulation of cytokinesis 32, 39, although a recent study reported that BRCA2 may not regulate cytokinesis in Hela cells 40, indicating that the role of BRCA2 in regulation of cytokenesis is sophisticated. It is interesting to note that no RAS or BRCA2 mutations in Hela cells were reported, but the amplification of RAS or Aurora-A has been observed in some literatures 41, 42. Therefore, it is possible that mutated RAS may be essential to defective cytokinesis through altering the expression ratio of Aurora-A and BRCA2.
Our results demonstrated that FTβ and IGFBP-3 plays an important role in mediating the effect to RAS to Aurora-A and BRCA2. The two proteins appear to form an negative regulatory loop to repress the expression of each other; such negative loop plays an important role in regulating the expression of Aurora-A and BRCA2 and the chromosomal instability induced by RAS. However, whether the interaction of FTβ and IGFBP-3 could regulate chromosomal instability without directly involving RAS, Aurora-A, and BRCA2 is unknown, although it has been reported that FTβ-stimulated farnesylation can increase RAS activity, and RAS-induced MAPK activation can lead to resistance of breast cancer cells to IGFBP-3 43. It is known that RAS mutations or Aurora-A amplification can activate the NF-κB which is involved in regulating the expression of FTβ and IGFBP-3 44–46, therefore, it will be very interesting to examine the role of NF-κB in the regulation of FTβ and IGFBP-3 and RAS-mediated transformation.
Currently, there are no reports to show that Aurora-A can regulate BRCA2, however, as a kinase in cancer cells, over expression of Aurora-A may phosphorylate BRCA2 and result in proteasome-mediated degradation during the late stage of mitosis, leading to abnormal cytokinesis. Moreover, emerging evidences suggest that both Aurora-A and BRCA2 can be regulated by various factors during cell cycle by proteolysis-mediated degradation. Studies have demonstrated that BRCA2 can interact with multiple gene products such as USP11 (a deubiquitinating enzyme) 47, Skp2 (a subunit of the Skp1-Cul1-F-box protein ubiquitin complex) 48, and cancer associated BRAD1 beta 49, leading to its proteasome-mediated ubiquitination and degradation in different cancer cells. Polyubiquitination of Aurora-A by anaphase-promoting complex (APC), or Cdh1 (a WD40 repeat protein) can promote the proteasome-mediated degradation of Aurora-A 50. Thus, we treated T29 and T29/Aurora-A cells with proteosome inhibitor MG-132 at the concentration of 10μM and found that the increased full length of Aurora-A and BRCA2 was accompanied with the decreased degradation of Aurora-A and BRCA2 over a time course of 1, 4, and 8 hours (SFigure 3). However, the detailed regulation of Aurora-A and BRCA2 by proteolysis mediated ubiquitination and degradation in cells with RAS mutations will require additional studies. As RAS/Aurora-A is amplified in multiple epithelial cancers, the molecules we identified in this study should have a general implication in clinical treatment of those cancers.
Supplementary Material
Novelty & Impact Statements.
Imbalanced accumulation of Aurora-A and BRCA2 at the midbody during cytokenesis leads to chromosomal instability via polyploidy in RAS-transformed cancer cells. The regulation of Aurora-A and BRCA2 is mediated through insulin-like growth factor binding protein 3 (IGFBP-3) and farnesyl protein transferase beta (FT-beta) in the presence of mutated RAS. As mutation or amplification of RAS/Aurora-A occurs in multiple epithelial cancers, BRCA2, FT-β and IGFBP-3 are likely essential targets to be considered in clinical treatment of those cancers.
Acknowledgments
G.Y. is supported by Doctoral Fund of Ministry of Education of China (20120071110079), by Shanghai Pujiang Program (11PJ1402200) from Shanghai Municipal Government of China, and by National Nature Science Foundation of China (91129721).
J.L. is supported by an R01 grant from the U.S. National Institutes of Health (1R01CA131183-01A2) and by the Specialized Programs of Research Excellence ovarian cancer research grant (IP50CA83638). This work was also supported in part by a U.S. National Cancer Institute Cancer Center Core grant (CA016672).
References
- 1.Abulaiti A, Fikaris AJ, Tsygankova OM, Meinkoth JL. Ras induces chromosome instability and abrogation of the DNA damage response. Cancer Res. 2006;66:10505–12. doi: 10.1158/0008-5472.CAN-06-2351. [DOI] [PubMed] [Google Scholar]
- 2.Goodman LE, Judd SR, Farnsworth CC, Powers S, Gelb MH, Glomset JA, Tamanoi F. Mutants of Saccharomyces cerevisiae defective in the farnesylation of Ras proteins. Proc Natl Acad Sci U S A. 1990;87:9665–9. doi: 10.1073/pnas.87.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062–74. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 4.Lee HY, Moon H, Chun KH, Chang YS, Hassan K, Ji L, Lotan R, Khuri FR, Hong WK. Effects of insulin-like growth factor binding protein-3 and farnesyltransferase inhibitor SCH66336 on Akt expression and apoptosis in non-small-cell lung cancer cells. J Natl Cancer Inst. 2004;96:1536–48. doi: 10.1093/jnci/djh286. [DOI] [PubMed] [Google Scholar]
- 5.Charames GS, Bapat B. Genomic instability and cancer. Curr Mol Med. 2003;3:589–96. doi: 10.2174/1566524033479456. [DOI] [PubMed] [Google Scholar]
- 6.Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–9. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- 7.Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–51. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- 8.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–93. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, Friess H, Sen S. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–7. [PubMed] [Google Scholar]
- 11.Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, Jiang F, Johnston D, Grossman HB, Ruifrok AC, Katz RL, Brinkley W, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–9. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 12.Tatsuka M, Sato S, Kitajima S, Suto S, Kawai H, Miyauchi M, Ogawa I, Maeda M, Ota T, Takata T. Overexpression of Aurora-A potentiates HRAS-mediated oncogenic transformation and is implicated in oral carcinogenesis. Oncogene. 2005;24:1122–7. doi: 10.1038/sj.onc.1208293. [DOI] [PubMed] [Google Scholar]
- 13.Tseng YS, Tzeng CC, Huang CY, Chen PH, Chiu AW, Hsu PY, Huang GC, Wang YC, Liu HS. Aurora-A overexpression associates with Ha-ras codon-12 mutation and blackfoot disease endemic area in bladder cancer. Cancer Lett. 2006;241:93–101. doi: 10.1016/j.canlet.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 15.Hughes-Davies L, Huntsman D, Ruas M, Fuks F, Bye J, Chin SF, Milner J, Brown LA, Hsu F, Gilks B, Nielsen T, Schulzer M, et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115:523–35. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- 16.Abaji C, Cousineau I, Belmaaza A. BRCA2 regulates homologous recombination in response to DNA damage: implications for genome stability and carcinogenesis. Cancer Res. 2005;65:4117–25. doi: 10.1158/0008-5472.CAN-04-3071. [DOI] [PubMed] [Google Scholar]
- 17.Skoulidis F, Cassidy LD, Pisupati V, Jonasson JG, Bjarnason H, Eyfjord JE, Karreth FA, Lim M, Barber LM, Clatworthy SA, Davies SE, Olive KP, et al. Germline Brca2 heterozygosity promotes Kras(G12D) -driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer cell. 2010;18:499–509. doi: 10.1016/j.ccr.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Tsunoda T, Takashima Y, Fujimoto T, Koyanagi M, Yoshida Y, Doi K, Tanaka Y, Kuroki M, Sasazuki T, Shirasawa S. Three-dimensionally specific inhibition of DNA repair-related genes by activated KRAS in colon crypt model. Neoplasia. 2010;12:397–404. doi: 10.1593/neo.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodvarsdottir SK, Hilmarsdottir H, Birgisdottir V, Steinarsdottir M, Jonasson JG, Eyfjord JE. Aurora-A amplification associated with BRCA2 mutation in breast tumours. Cancer Lett. 2007;248:96–102. doi: 10.1016/j.canlet.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Sagulenko E, Savelyeva L, Ehemann V, Sagulenko V, Hofmann W, Arnold K, Claas A, Scherneck S, Schwab M. Suppression of polyploidy by the BRCA2 protein. Cancer Lett. 2007;257:65–72. doi: 10.1016/j.canlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Chang B, Yang F, Guo X, Cai KQ, Xiao XS, Wang H, Sen S, Hung MC, Mills GB, Chang S, Multani AS, et al. Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res. 2010;16:3171–81. doi: 10.1158/1078-0432.CCR-09-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama N, Nakayama K, Yeasmin S, Ishibashi M, Katagiri A, Iida K, Fukumoto M, Miyazaki K. KRAS or BRAF mutation status is a useful predictor of sensitivity to MEK inhibition in ovarian cancer. Br J Cancer. 2008;99:2020–8. doi: 10.1038/sj.bjc.6604783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 24.Wang SC, Shao R, Pao AY, Zhang S, Hung MC, Su LK. Inhibition of cancer cell growth by BRCA2. Cancer Res. 2002;62:1311–4. [PubMed] [Google Scholar]
- 25.Yang G, Rosen DG, Mercado-Uribe I, Colacino JA, Mills GB, Bast RC, Jr, Zhou C, Liu J. Knockdown of p53 combined with expression of the catalytic subunit of telomerase is sufficient to immortalize primary human ovarian surface epithelial cells. Carcinogenesis. 2007;28:174–82. doi: 10.1093/carcin/bgl115. [DOI] [PubMed] [Google Scholar]
- 26.Yang YX, Miao ZC, Zhang HJ, Wang Y, Gao JX, Feng MF. Establishment and characterization of a human telomerase catalytic subunit-transduced fetal bone marrow-derived osteoblastic cell line. Differentiation. 2007;75:24–34. doi: 10.1111/j.1432-0436.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC, Mills GB, Bast RC., Jr A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–63. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, Cai KQ, Thompson-Lanza JA, Bast RC, Jr, Liu J. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J Biol Chem. 2004;279:4339–45. doi: 10.1074/jbc.M311153200. [DOI] [PubMed] [Google Scholar]
- 29.Akli S, Zheng PJ, Multani AS, Wingate HF, Pathak S, Zhang N, Tucker SL, Chang S, Keyomarsi K. Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res. 2004;64:3198–208. doi: 10.1158/0008-5472.can-03-3672. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Groffen J, Heisterkamp N. Resistance to farnesyltransferase inhibitors in Bcr/Abl-positive lymphoblastic leukemia by increased expression of a novel ABC transporter homolog ATP11a. Blood. 2005;106:1355–61. doi: 10.1182/blood-2004-09-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biran A, Brownstein M, Haklai R, Kloog Y. Downregulation of survivin and aurora A by histone deacetylase and RAS inhibitors: a new drug combination for cancer therapy. Int J Cancer. 2011;128:691–701. doi: 10.1002/ijc.25367. [DOI] [PubMed] [Google Scholar]
- 32.Daniels MJ, Wang Y, Lee M, Venkitaraman AR. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science. 2004;306:876–9. doi: 10.1126/science.1102574. [DOI] [PubMed] [Google Scholar]
- 33.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–95. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 34.Sagona AP, Stenmark H. Cytokinesis and cancer. FEBS Lett. 2010;584:2652–61. doi: 10.1016/j.febslet.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 35.Lacroix B, Maddox AS. Cytokinesis, ploidy and aneuploidy. J Pathol. 2012;226:338–51. doi: 10.1002/path.3013. [DOI] [PubMed] [Google Scholar]
- 36.Emdad L, Sarkar D, Su ZZ, Fisher PB. Emerging roles of centrosomal amplification and genomic instability in cancer. Front Biosci. 2005;10:728–42. doi: 10.2741/1567. [DOI] [PubMed] [Google Scholar]
- 37.Lee M, Daniels MJ, Garnett MJ, Venkitaraman AR. A mitotic function for the high-mobility group protein HMG20b regulated by its interaction with the BRC repeats of the BRCA2 tumor suppressor. Oncogene. 2011;30:3360–9. doi: 10.1038/onc.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–23. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 39.Jonsdottir AB, Vreeswijk MP, Wolterbeek R, Devilee P, Tanke HJ, Eyfjord JE, Szuhai K. BRCA2 heterozygosity delays cytokinesis in primary human fibroblasts. Cell Oncol. 2009;31:191–201. doi: 10.3233/CLO-2009-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lekomtsev S, Guizetti J, Pozniakovsky A, Gerlich DW, Petronczki M. Evidence that the tumor-suppressor protein BRCA2 does not regulate cytokinesis in human cells. J Cell Sci. 2010;123:1395–400. doi: 10.1242/jcs.068015. [DOI] [PubMed] [Google Scholar]
- 41.Omerovic J, Hammond DE, Clague MJ, Prior IA. Ras isoform abundance and signalling in human cancer cell lines. Oncogene. 2008;27:2754–62. doi: 10.1038/sj.onc.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wesierska-Gadek J, Kramer MP, Schmid G. A combined treatment of HeLa cells with the farnesyl protein transferase inhibitor L-744,832 and cisplatin significantly increases the therapeutic effect as compared to cisplatin monotherapy. J Cell Biochem. 2008;104:189–201. doi: 10.1002/jcb.21612. [DOI] [PubMed] [Google Scholar]
- 43.Martin JL, Baxter RC. Oncogenic ras causes resistance to the growth inhibitor insulin-like growth factor binding protein-3 (IGFBP-3) in breast cancer cells. J Biol Chem. 1999;274:16407–11. doi: 10.1074/jbc.274.23.16407. [DOI] [PubMed] [Google Scholar]
- 44.Williams AC, Smartt H, AMHZ, Macfarlane M, Paraskeva C, Collard TJ. Insulin-like growth factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-kappaB. Cell Death Differ. 2007;14:137–45. doi: 10.1038/sj.cdd.4401919. [DOI] [PubMed] [Google Scholar]
- 45.Han J, Jogie-Brahim S, Harada A, Oh Y. Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-kappaB signaling. Cancer Lett. 2011;307:200–10. doi: 10.1016/j.canlet.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Briassouli P, Chan F, Savage K, Reis-Filho JS, Linardopoulos S. Aurora-A regulation of nuclear factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer Res. 2007;67:1689–95. doi: 10.1158/0008-5472.CAN-06-2272. [DOI] [PubMed] [Google Scholar]
- 47.Schoenfeld AR, Apgar S, Dolios G, Wang R, Aaronson SA. BRCA2 is ubiquitinated in vivo and interacts with USP11, a deubiquitinating enzyme that exhibits prosurvival function in the cellular response to DNA damage. Mol Cell Biol. 2004;24:7444–55. doi: 10.1128/MCB.24.17.7444-7455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moro L, Arbini AA, Marra E, Greco M. Up-regulation of Skp2 after prostate cancer cell adhesion to basement membranes results in BRCA2 degradation and cell proliferation. J Biol Chem. 2006;281:22100–7. doi: 10.1074/jbc.M604636200. [DOI] [PubMed] [Google Scholar]
- 49.Ryser S, Dizin E, Jefford CE, Delaval B, Gagos S, Christodoulidou A, Krause KH, Birnbaum D, Irminger-Finger I. Distinct roles of BARD1 isoforms in mitosis: full-length BARD1 mediates Aurora B degradation, cancer-associated BARD1beta scaffolds Aurora B and BRCA2. Cancer Res. 2009;69:1125–34. doi: 10.1158/0008-5472.CAN-08-2134. [DOI] [PubMed] [Google Scholar]
- 50.Honda K, Mihara H, Kato Y, Yamaguchi A, Tanaka H, Yasuda H, Furukawa K, Urano T. Degradation of human Aurora2 protein kinase by the anaphase-promoting complex-ubiquitin-proteasome pathway. Oncogene. 2000;19:2812–9. doi: 10.1038/sj.onc.1203609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.