Abstract
BACKGROUND
Blood for transfusion is stored for up to 42 days. Older blood develops lesions and accumulates potentially injurious substances. Some studies report increasing toxicity as blood ages. We assessed the safety of transfused older versus newer stored blood.
STUDY DESIGN AND METHODS
PubMed, Scopus and Embase were searched using terms new and old and red blood cell and storage through May 6, 2011 for observational and randomized controlled studies comparing outcomes using transfused blood having longer and shorter storage times. Death was the outcome of interest.
RESULTS
Twenty-one studies were identified, predominantly in cardiac surgery (n=6) and trauma (n=6) patients, including 409,966 patients. A test for heterogeneity of these studies’ results was not significant for mortality (I2=3.7%, p=0.41). Older blood was associated with a significantly increased risk of death [odds ratio (OR) 1.16; 95% confidence interval (CI) (1.07, 1.24)]. Using available mortality data, 97 (63, 199; 95% CI) patients need to be treated with only new blood to save one life. Subgroup analysis of these trials indicated the increased risk was not restricted to a particular type of patient, size of trial, or amount of blood transfused.
CONCLUSION
Based on available data, use of older stored blood is associated with a significantly increased risk of death.
INTRODUCTION
In 1818, the British obstetrician James Blundell performed the first successful blood transfusion using small volumes of freshly drawn blood.1 Since that time, transfusion of red blood cells (RBCs) has become one of the most important therapies in clinical medicine. The World Health Organization reported that 80.7 million units of blood were collected in 167 countries surveyed between 2004 and 2005.2 Approximately 15 million units of whole blood are collected in the United States each year.3 In order to maintain adequate inventories of RBCs of the appropriate type where it is needed, blood must now be processed, shipped, and stored at refrigerated temperature.
For the past 50 years, the primary aim of blood storage research has been to extend the RBC shelf life in order to maximize availability of a relatively scarce and perishable product. Modified storage solutions, containers, and processing procedures have doubled the shelf life to the current six weeks.4-6 This effort has proved successful. In the last US national survey, less than five percent (and less than one quarter of one percent of group O) of RBCs outdated, and fewer than five percent of hospitals surveyed reported canceling elective surgery for even one day because compatible blood was unavailable.7
Less attention has been paid to maintaining and assessing the physiological efficacy of refrigerated RBCs. For licensure in the US, a variety of in vitro assays to evaluate RBC structure and composition has become customary, but the Food and Drug Administration’s (FDA’s) “gold standard” requires that at least 75% of the cells remain in the circulation 24h after infusion, and that hemolysis not exceed 1% at the end of the approved storage period.8 Though 6-week-old blood meets the current FDA criteria, this “old” blood may not be as safe and effective as blood stored for shorter durations. Refrigerated storage results in a “storage lesion” that is reflected by metabolic derangements, RBC shape change, rheologic changes, loss of membrane carbohydrates, oxidative injury to lipids and proteins, changes in oxygen affinity and delivery, increased adhesion of RBCs to endothelial cells, and reduced RBC lifespan as well as the secondary risks of accumulating concentrations of potassium and plasticizer, and shedding of active proteins, lipids, and microvesicles.9 These changes that take place with the aging of stored blood raised concern that stored older RBCs could increase patient risks.
At present, most adult and pediatric patients requiring transfusion receive blood of their specific type with the oldest compatible unit available given first.10 This first-in, first-out principle was designed to conserve a limited and perishable blood supply. If RBC's that have been stored for an extended period were found to pose clinically significant risks, the current strategies for blood storage and transfusion used worldwide would come into question. Reducing the maximum shelf life for RBCs would involve new approaches to inventory management and result in major operational and financial consequences.11
Published retrospective and prospective studies have raised concern that patients receiving older blood have an increased mortality risk compared to patients receiving newer stored units. Meta-analyses have struggled to summarize these studies because of variability in methods of reporting data in individual studies. In an attempt to summarize and give a full description of the previous published transfusion experience, we have used a methodology that allows the study endpoints to be comparable. Our primary objective is to determine the effect of storage on mortality and our secondary objective is to determine if effects are restricted to any subgroups.
MATERIALS AND METHODS
Data Sources, Searches and Study Selection
Three databases (PubMed, Scopus and Embase) were searched (all 3 were last searched May 6, 2011) to find all relevant articles published comparing patients receiving transfusions with new and old stored blood. The search was limited to “Humans” and publications in “English” but not to “Date” with additional search terms detailed for each database (see Appendix Table 1, Online Supplement), resulting in a total of 2640 articles. After removing duplicate articles, 1348 unique article citations remained. Each of these articles was reviewed. Trials were included if they compared survival rates after being transfused with blood that was stored over different durations in days, one having longer storage times (“old blood”) and the other having a shorter storage time (“new blood”). To perform a comprehensive analysis, studies were only excluded if they did not have mortality data, or did not refer to the age of the stored blood transfused, or the study was subsequently retracted. A total of 21 trials met these inclusion criteria (Fig 1).
Figure 1.
Study selection
Data Extraction
Two of us independently (DW and JS) reviewed the included studies. A third author (CN) resolved any discrepancies. Mortality was selected as the primary endpoint of interest. We also abstracted other descriptive data from included trials (Table 1) such as year published, enrollment dates, study design, population studied, volume of blood transfused, age of blood stored and solution used, if blood was leukoreduced, mortality endpoints used, and if there were severe adverse events.
Table 1.
Characteristics of studies included in meta-analysis
| Author (Reference) | Year Published | Years of Enrollment | Study Type | Study Population | No. of Patients in study | No. of Patients used in Metaanalysis* | Average Transfusion Volume per Patient (units of PRBC) | Age of Stored Blood Compared† | Blood Storage Solution‡ | Leuko-reduced | Mortality | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New Blood (days) | Old Blood (days) | |||||||||||
| Van Straten14 | 2011 | 1998−2007 | OBR | Cardiac surgery | 3597 | 3141 | new=2.6, old=2.4 | < 14 | ≥ 14 | SAGM | Yes | 30-d mortality |
| Pettila15 | 2011 | 2008 | OBP | ICU patient | 757 | 379 | 2 | ≤ 11 | ≥ 28 | NR | Partially | Hospital mortality |
| Edgren22 | 2010 | 1995−2002 | OBR | Transfused at least one RBC unit | 387,130 | 387,130 | 2 | < 9 | > 30 | SAGM | NR | Short term mortality (1 week) |
| Eikelboom21 | 2010 | 2002−2006 | OBP | Cardiac disease | 4933 | 4933 | 3 | < 10 | 31−42 | AS−3 | Yes | Hospital mortality |
| Robinson20 | 2010 | 1999−2005 | OBR | Cardiac surgery | 909 | 712 | NR | ≤ 21 | > 21 | NR | Yes | 30-d mortality |
| Weinberg13 | 2010 | 2000−2009 | OBR | Trauma | 1647 | 1647 | new=2.9, old=3.4 | < 14 | ≥ 14 | NR | Yes | Hospital mortality |
| Karam10 | 2010 | 2004−2005 | OBP | PICU patient | 296 | 296 | new=2.6, old=5.5 | < 14 | ≥ 14 | NR | Partially | 28-d mortality |
| Gauvin24 | 2010 | 2001−2005 | OBP | PICU patient | 455 | 224 | new=1.2, old=2.3 | ≤ 7 | > 21 | NR | Yes | 28-d mortality |
| Van Buskirk32 | 2009 | NR | OBR | ICU patient | 298 | 298 | NR | < 8 | > 14 | NR | NR | Hospital mortality |
| Spinella17 | 2009 | 2004−2007 | OBR | Trauma | 202 | 176 | 9 | <21 | ≥21 | NR | NR | Hospital mortality |
| Koch16 | 2008 | 1998−2006 | OBR | Cardiac surgery | 6002 | 6002 | 2 | ≤ 14 | > 14 | NR | NR | Hospital mortality |
| Weinberg26 | 2008 | 2000−2007 | OBR | Trauma | 430 | 430 | 5.2 | < 14 | ≥ 14 | NR | Yes | Hospital mortality |
| Yap25 | 2008 | 2001−2007 | OBR | Cardiac surgery | 670 | 670 | 3 | 8 | 19 | NR | NR | Hospital mortality |
| Weinberg19 | 2008 | 2000−2007 | OBR | Trauma | 1813 | 1169 | 4.95 | <14 | ≥14 | NR | Yes | Hospital mortality |
| Leal-Noval23 | 2008 | 2004−2006 | OBP | Anemia | 66 | 34 | NR | < 10 | > 19 | SAGM | Yes | ICU mortality |
| Van-De-Watering18 | 2006 | 1993−1999 | OBR | Cardiac surgery | 2732 | 1895 | 4·77 | < 18 | ≥ 18 | SAGM | Yes | 30-d mortality |
| Murrell27 | 2005 | 2001−2002 | OBR | Trauma | 275 | 275 | 3 | NR | NR | NR | Yes | Hospital mortality |
| Fernandes-Da-Cunha33 | 2005 | 2002−2003 | RCT | Premature infant | 52 | 52 | NR | 1. 6 | 9 | CPDA−1 | Yes | Hospital mortality |
| Hebert34 | 2005 | 1999−2001 | RCT | Cardiac surgery | 57 | 57 | new=3, old=2 | 4 | 19 | CPD−2 , AS−3 | Yes | Hospital mortality |
| Schulman35 | 2002 | 2000−2001 | RCT | Trauma | 17 | 17 | new=9.3, old=10.6 | <11 | ≥20 | NR | Yes | Hospital mortality |
| Mynster12 | 2001 | 1991−1993 | OBP | Colorectal surgery | 740 | 429 | 3 | < 21 | ≥ 21 | SAGM | Yes | Long term mortality |
Abbreviations: NR, not reported; OBR, observational retrospective study; OBP, observational prospective study; RCT, randomized controlled trial; ICU, intensive care unit; PICU, pediatric intensive care unit; PRBC, packed red blood cells
When multiple ages of stored blood were presented only the extremes were analyzed (see statistical method)
See statistical method for how stored blood groups were determined for comparison
The numbers and letters indicate the type of storage solution: C=citrate, A=adenine, P=phosphate, D=dextrose, S=sodium chloride, M=mannitol, G=glucose
Data Synthesis and Analysis
In order to compare the effects of old versus new stored blood transfusion on the odds ratio (OR) of death across studies, each individual study was summarized as follows:
When both count data (survivor or non-survivor) and adjusted results [hazard ratio (HR), odds ratio (OR), or relative risk (RR)] were provided, the count data were used to calculate OR (old vs. new stored blood) for the analysis.12-20 Adjusted HR12, 18, 20-22 and RR13 were converted to OR (proportional hazard was assumed for HR). Using the adjusted ORs instead of counts in sensitivity analyses, we obtained similar results.
If more than one way of dividing the subgroups were reported, the division that provided the greatest degree of age separation between old and new stored blood was used.14,15,18,19,21-24 When count data were used and there were more than 2 blood age groups, only the patients from the newest and oldest groups were used in this meta-analysis. 17-19,23, 24 When the estimated effect (e.g., OR) used was derived from multivariate analysis, all patients from that study were used in this meta-analysis.
When more than one cut-off value was used to divide 2 groups of stored blood, the cut-off closest to 21 days was used.17,20
If more than one way of calculating blood age was used, the result, based on average blood age was used.25
When each transfused group (newest or oldest) was compared to a third group (an intermediate blood age group or “no transfusion” group), the comparison of oldest versus newest was calculated (based on the estimates and standard errors from the newest (or oldest) versus no transfusion) using an approximate t test.22,26
When the estimated effect was based on a continuous blood age (e.g., OR of death when blood age increases by 1 day), the estimated effect size of third-quartile blood age versus first-quartile blood age was used in the analysis.25 For one study, 27 the continuous blood age used was the sum of “proportionate age” (i.e., age divided by 42 days) of each blood unit. In this case, the first and third quartiles of this calculated blood age were used to calculate the estimated effect size. Adverse events were defined a priori and in studies meeting inclusion criteria were reported if significant and combined if appropriate when reported in at least two studies.
Heterogeneity among studies was assessed using the Q statistic and I2 value.28 A random effects model was used and not the fixed-effects model to estimate the overall effect of old stored blood versus new stored blood transfusion because we assumed it was unlikely effects would be identical across studies and wanted to make inferences for all potential studies.29 Summary ORs are reported when heterogeneity was low (≤ 30% I2 value). Subgroup analysis with different conditions was done using random-effects meta-analysis. Publication bias was assessed by funnel plot and Egger's regression30,31 (Fig 2). Conventional forest plots were prepared, with the sizes of point estimates proportional to the inverse variance of each estimate. The overall estimates for the increase in mortality with older transfused blood based on counts data versus adjusted results (among studies that provided both) were compared using exact Wilcoxon matched pairs signed rank test (StatXact-9, Cytel Software Corp., Cambridge, MA). All analyses were performed using Comprehensive Meta-analysis version 2 (http://www.meta-analysis.com/) except noted otherwise.
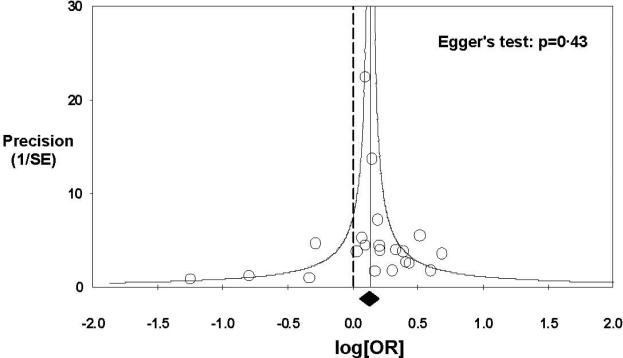
Figure 2. Funnel plot of studies included in meta-analysis.
Each open circle represents one of 21 studies in our meta-analysis. The precision for each study is plotted versus log (OR). The circles are distributed equally around the solid vertical line with a solid diamond at the bottom, representing the overall treatment effect in this study. There is no skewed distribution of studies giving evidence for a publication bias. Eggers test for publication bias (or small study effect) is non-significant (P=0.43) and confirm what is seen on visual expectedness.
RESULTS
Study Characteristics
Twenty-one studies10,12-27,32-35 that were published from 2001 to 2011 met inclusion criteria (Fig. 1, Table 1). Three of these studies were randomized controlled trials33-35 and eighteen observational studies, six prospective studies,10,12,15,21,23,24 and twelve retrospective.13,14,16-20,22,25-27,32 The patients were enrolled in these studies from as early as 1991 to 1993 to as late as 2000-2009. The three randomized controlled trials33-35 were much smaller in size, ranging from 17 to 57 patients and the observational studies10,12-27,32 much larger, ranging in size from 66 to 387,130 patients. Most studies were in cardiac surgery14,16,18,20,25,34 (n = 6) or trauma patients13,17,19,26,27,35 (n = 6). The age of new blood in these 21 studies ranged from 1.6 days to ≤ 21 days and the old blood, 9 days to 31 to 42 days. The blood was reported to be leukoreduced in 1610,12-15,18-21,23,24,26,27,33-35 out of 21 studies. The average volume of blood transfused per patient in studies ranged from 1 unit to almost 10 units.
Effect of Old versus New Stored Blood on Mortality
There was a total of 555 deaths among 7611 patients transfused with new blood (7.3%) and a total of 774 deaths among 9114 patients transfused with old blood (8.5%) from sixteen studies that provided such data.10,12-20,23,24,32-35 There was no significant heterogeneity among these 21 studies for the mortality endpoint (I2 = 3.7%; p = 0.41) (Fig 3). The overall estimate of mortality with transfusion of old blood compared to new stored blood was a significantly increased odds ratio (OR) of death, 95% confidence interval (CI) of 1.16 (1.07, 1.24). Using the available mortality data from the current studies (7.3% for new blood group), based on our meta-analysis, the number needed to treat (NNT) (95% CI) is 97 (63, 199) patients with only new blood to save one life.
Figure 3. Mortality.
The size of the data markers is proportional to the inverse variance of each point estimate.
Effect of Old versus New Stored Blood on Severe Adverse Effects
Seven10,16,17,24-26,33 of 21 studies included in our meta-analysis described one or more severe adverse effects: intubated ventilation > 72 hours, pneumonia, renal failure, sepsis, acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome (MODS), and deep vein thrombosis. Averaging over the three studies reporting the incidence of MODS10,16,24 and the three studies reporting the incidence of pneumonia,16,25,26 there was no significant heterogeneity among these studies (I2 = 0%; p = 0.44 and I2 = 0%; p = 0.50, respectively) and the overall estimate showed a significant increase in MODS and pneumonia with transfusion of old stored blood OR 2.26, 95% CI (1.56, 3.25) and 1.17 (1.08,1.27), respectively. Two studies found a non-significant overall increase in the incidence of ARDS26,33 with transfusion of older stored blood (Fig. 4). Three studies reported the incidence of renal dysfunction; two16,26 found significant increases with transfusion of older blood, and the third, 25 no significant effect of stored blood age transfused. Two studies reported the incidence of sepsis; one found a significant increase with transfusion of older stored blood,16 the other no significant effect of the age of stored blood transfused.33 The above studies of either renal dysfunction or sepsis could not be combined because of significant heterogeneity among studies for each condition (I2 = 62.6% and 77.6% respectively). The single studies reporting intubated ventilation for > 72 hours16 and deep vein thrombosis17 both found a significant increase with transfusion of older stored blood.
Figure 4.
Severe adverse effects.
Subgroup Analysis
Figure 5 shows the odds ratio of death according to different patient categories. In trauma patients, cardiac surgery patients and the non-cardiac, non-trauma patients (including those with anemia, cardiac disease, undergoing colorectal surgery, ICU patients, premature infants, and for one study, all patients regardless of type of illness that were transfused at least one unit), the OR of death significantly increased in all three subgroups with old vs. new blood. There was no significant heterogeneity among studies in any of the three subgroups or significant difference comparing these subgroup’ increases in the OR of death (p = 0.60). In cardiac surgery patients14,16,18,20,25,34 the OR (95% CI) of death with old verse new blood was 1.26 (1.04, 1.53), somewhat larger than the overall estimate for the 21 studies. In trauma patients13,17,19,26,27,35 the OR (95% CI) of 1.18 (1.02, 1.35) was very similar to the overall estimate of the increase in mortality with old vs. new blood. The smallest increase in OR of death with old vs. new blood was in patients with non-trauma, non-cardiac surgery10,12,15,21-24,32,33 related transfusions with OR (95% CI) of 1.12 (1.00, 1.26).
Figure 5.
Subgroup analysis (Patient types).
Figure 6 shows odds ratio of death for pediatric and adult patients. There was no significant heterogeneity among the studies in either of these two subgroups (I2 = 0%; p = 0.86 and I2 = 14.9%; p = 0.28, respectively). In the pediatric group,10,24,33 the overall estimate of death was increased but not significantly, OR (95% CI) 1.43 (0.76, 2.69) and in the adult group,12-23,25-27,32,34,35 the overall estimate of mortality was significantly increased with transfusion of older blood 1.16 (1.07, 1.25). There was no significant difference in overall estimates comparing adults and children (p= 0.51); however, the estimate was nominally larger in children and less significant potentially because of a lack of power, there being far fewer pediatric studies.
Figure 6.
Subgroup analysis (Pediatric and Adult).
Figure 7 shows the odds ratio of death according to studies that provided counts data10,12-18,20,23,24,26,32-35 (survivor/non-survivor) or adjusted mortality results.19,21,22,25,27 There was no significant heterogeneity (I2 = 12.6%, p = 0.31 and I2 = 0%, p = 0.57, respectively) within either of these two subgroups and no significant difference in the OR of death (comparing the two subgroups) (p = 0.55). In the counts group the OR (95% CI) was 1.20 (1.05, 1.36); in the adjusted group, 1.14 (1.04, 1.26). There were eight studies12-15,17-20 which provided both counts data and adjusted mortality analysis comparing new and old stored blood transfusion. The overall estimates for the increase in mortality with older transfused blood were similar (P=0.30) comparing counts, 1.14 (0.96, 1.36), p = 0.13 and adjusted mortality analysis 1.42 (1.00, 2.01), p = 0.05. If there is any difference at all, adjustment resulted in a larger treatment effect that was more significant than counts. However there was more marked heterogeneity using adjusted mortality compared to counts (adjusted I2 = 66.6%, p = 0.004 versus counts I2=23.4%, p = 0.24), potentially reflecting the different methods across studies used to adjust mortality. Thus counts give a similar but somewhat more conservative estimate of the overall effect of older transfused stored blood on mortality with less variability.
Figure 7.
Subgroup analysis (Adjusted mortality versus counts).
From 2001 to 2008, there were 11 studies published comparing the effect of age of stored blood on mortality12,16,18,19,23,25-27,33-35 approximately one per year. From 2009 to 2011, there were 10 such studies published 10,13-15,17,20-22,24,32 approximately two per year. An observational study of 6002 cardiac surgery patients from a single institution published in 2008 showed a highly significant increase in mortality with older stored transfused blood, received wide publicity in the medical and lay press, and may have influenced this doubling.16 However, the increase in mortality with older stored transfused blood OR (95% CI) was similar (p = 0.81) in the studies published before 2009 [1.18 (1.06, 1.31); p = 0.03 (I2 = 23.8%; p = 0.22)] and in those published 2009 to 2011 [1.15 (1.01, 6.31); p = 0.03 (I2 = 0%; p = 0.50)].
We further divided these 21 studies into nine large studies (n > 500)13,14,16,18-22,25 and twelve small studies (n ≤ 500).10,12,15,17,23,24,26,27,32-35 There was no significant heterogeneity in each subgroup (I2 = 0%, p = 0.49 and I2 = 17.4%, p = 0.27, respectively). The OR (95% CI) were similar for large and small studies (p = 0.92), being 1.17 (1.06, 1.30) and 1.16 (1.01, 1.34), respectively. We also divided these 21 studies according to the number of red blood cell units that were transfused to patients on average in each study: in ten trials ≤3 units group,12,14-16,21,22,24,25,27,34 in seven trials >3 units group,10,13,17-19,26,35 and in four trials20,23,32,33 this information was not provided. There was significant heterogeneity among the trials receiving ≤3 units (I2 = 49.0%, p = 0.04), but not among the studies receiving >3 units, and unknown number of units (I2 = 0%, p = 0.65 and I2 = 0%, p = 0.85, respectively). The OR (95% CI) were very similar (p = 0.96), regardless of number of units transfused, 1.19 (1.05, 1.35), 1.16 (1.00, 1.35) and 1.20 (0.87, 1.66), respectively. In five studies 15,18,19,23,24 our a priori analytic plan reduced the sample size; if we include all these patients in our analysis, the OR (95% CI) for mortality is 1.20 (1.10, 1.31), very similar to our overall estimate 1.16 (1.07, 1.24) (Fig. 3). Finally, with the removal of the three small randomized controlled trials,33-35 the OR (95% CI) for the remaining 18 observational studies of 1.16 (1.08, 1.24) compared to the overall estimate (Fig. 3) is essentially unchanged.
Publication Bias
In Figure 2 is shown a funnel plot. The 21 studies in our meta-analysis are equally distributed in Figure 2 on both sides of the line, representing the significant increase in OR of death with old vs. new blood. Moreover, the Eggers test for publication bias is non-significant. This indicates absence of statistical evidence for a publication bias (i.e. that unpublished studies exist that are influencing the finding of an increase in mortality with old vs. new blood).
DISCUSSION
Our literature search and inclusion/exclusion criteria resulted in a total of 21 unique studies10,12-27,32-35 that compared survival rates with blood that was stored over different durations in days, one being longer “old” and one being shorter “new” blood. These studies were published from 2001 to 2010, included 18 observational studies,10,12-27,32 6 prospective10,12,15,21,23,24 and 12 retrospective,13,14,16-20,22,25-27,32 and 3 randomized controlled trials33-35. The studies enrolled patients from 1991 to 2009 and ranged in size from 17 to 387,130 patients. Sixty percent of the studies were in cardiac surgery and trauma patients, but in the remaining forty percent of studies the patients varied from neonates to patients with anemia or colorectal cancer (Table 1). Nonetheless, the findings across these 21 studies were statistically very similar (I2 = 3.7%, p = 0.41) (Fig. 3) and showed a significant increase in mortality for those receiving old blood rather than new blood [odds ratio 95% CI 1.16 (1.07, 1.24)]. Taking into account both the mortality data found in these studies and the amount of blood transfusions per patient (range from 1-10 units on average), our data suggest one would need to treat 97 patients [95% CI, (63, 199)] exclusively with newly stored blood to save one life.
We performed multiple analyses to determine whether our results were consistent across potentially important subgroups. In our meta-analysis, approximately thirty percent of the studies were in trauma patients,13,17,19,26,27,35 thirty percent in cardiac surgery patients,14,16,18,20,25,34 and forty percent were a mix of varied populations.10,12,15,21-24,32,33 The results were similar comparing these three subgroups (Fig. 5) and very consistent with the overall increase in mortality found with old blood vs. new blood (Fig. 3). In our meta-analysis, the results of three pediatric patient studies,10,24,33 compared to the 18 adult studies,12-23,25-27,32,34,35 were similar (Fig. 6) and consistent with the overall increase in mortality found with transfusion of older stored blood (Fig. 3). In studies having ≤ 500 or > 500 patients, as well as in studies of patients receiving on average ≤ 3 units versus > 3 units per patient, the results in all these subgroups were likewise similar to the overall findings of our study showing old blood versus new blood increases mortality. In studies published before 2009 (n = 11)12,16,18,19,23,25-27,33-35 and afterward 2009 to 2011 (n = 10),10,13-15,17,20-22,24,32 the increases in mortality with older stored blood were statistically significant and similar to the overall findings in this meta-analysis. Many of these studies were observational; however, we found that if we examined only the five studies19,21,22,25,27 using adjusted mortality rates, the results were similar (p = 0.55) to the 16 studies10,12-18,20,23,24,26,32-35 that used count data (survivor/non-survivor) [1.14 (1.04, 1.26) vs. 1.20 (1.05, 1.36)] (Fig. 7), and were consistent with the overall findings of our study that older blood increases mortality (Fig. 3). Moreover, if we compare the studies that reported both counts and adjusted mortality (Fig. 7 top), the results are similar and comparable to the overall finding showing old blood versus new increases mortality (Fig. 3). Finally, if we remove the three small-randomized controlled trials, the overall results are unchanged. Thus, our results are statistically very similar across individual trials, similar with different types of analysis, and also very consistent across multiple different clinically important subgroups.
Only 710,16,17,24-26,33 of the 21 studies in our meta-analysis reported serious adverse events (Fig. 4). Three studies16,25,26 suggested an overall significant increase in pneumonia and three other studies10,16,24 showed an overall increase in MODS associated with transfusion of old blood. Two16,26 of three16,25,26 studies showed significant increase in renal failure and one16 of two16,33 studies, a significant increase in sepsis with transfusion of older blood. The available data on serious side effects suggest, similar to mortality, an increase in risk using old versus new blood.
Four previously published reviews36-39 examined the available clinical studies comparing the effects on outcome of old versus new blood. These four papers were accepted for publication from September 2008 to August 2009. Since that time, nine studies,10,13-15,20-22,24,32 including 400,042 patients providing mortality data comparing old versus new blood have been published. These nine studies10,13-15,21,22,24,33 are included in our main analysis but not the four previous reviews.36-39 Three of these four previous reviews did not include a formal meta-analysis36-38. All three indicated that the currently available data set was insufficient to draw the conclusion that old blood is harmful. It is not possible to compare these studies and ours, since the data sets are markedly different and no formal analysis was done in these three other studies. One of these three reviews 38 stated the studies examined were too heterogeneous to allow an overall analysis. No formal analysis is provided to support this conclusion..
One review39 did a formal meta-analysis but only analyzed subgroups of studies that were homogenous. This study concluded that the available data are not adequate to confirm the suspicion that older blood increases morbidity and mortality.39 We believe that overall our results do not differ from this meta-analysis , but rather represent an increase in numbers and therefore in power. More studies are included in our meta-analysis and each of our subgroup analyses. For example, in this previous review,39 an OR (95%) CI for mortality was reported for trauma patients of 1.24 (1.06 – 1.44) based on three studies markedly similar to our result 1.18 (1.02 – 1.35) based on six studies which reached statistical significance (Fig.5).
Most of the available data in our meta-analysis derive from observational studies and conclusions must be interpreted cautiously because of the potential for unintentional bias. It is possible, for example, that some practitioners selected fresher units for children and older units for elderly patients with a poor prognosis, or there could be some other systematic unknown confounder across studies giving us this signal. However, the consistency of the data in this meta-analysis supports the hypothesis that older stored blood increases mortality. Nonetheless, until well designed, multicenter, adequately powered, randomized controlled trials confirm these findings, the evidence is inadequate to institute major changes in blood collection and transfusion practice. Three such trials, one in cardiac surgery patients, one in critically ill patients and another in neonates have been initiated.40-42
Extrapolation of these findings to the general population should be done with caution. Many of these studies involved critically ill trauma victims or patients with serious cardiac disease. Older blood may amplify the injury of certain diseases or interventions. It is possible that old blood acts as a “second hit” for patients with severely compromised organ function or immune competence.43 If the risk of death from a unit of blood as estimated by the US Government Accountability Office is of the order of magnitude of one in 10,000, and transfusion is the only cause of death for a general patient population, the mortality for that population would be 0.01%.44 Using this estimate of mortality and the OR estimate we found in our meta-analysis between old and new blood, one would have to treat 69,428 patients with exclusively new blood to save one life. If old blood increases risk, then these two estimates of 97 versus 69,428 likely encompass the number of patients needed to be treated with exclusively newer blood to save one life. The ongoing randomized controlled trials should help determine which one of the above estimates is closer to the actual risk for the “average” patient in need of a transfusion and for specific categories of patients whose clinical status might render them unusually vulnerable to changes that occur during the storage of RBCs.
Clinical studies comparing day-old blood with blood at the end of its storage life are operationally difficult and ethically challenging. RBCs must be processed, tested and shipped prior to issue for transfusion which places some limit on the extent of “freshness.” Blood stored for 42 days deteriorates by most measures of red blood cell function and older stored blood has been associated with increased mortality in several published studies. It is hard to imagine that a patient given this information would consent to receive only blood at the very end of its shelf life. The ongoing human clinical trials have been designed appropriately to compare fully tested, compatible newer blood with current transfusion practice or with older RBCs of mixed age available in the hospital inventory.40,41,42 Animal models might be better able to assess the clinical differences, if any, between the very youngest units and those at the end of shelf life and if older blood is found to increase risks, help define after how many days of storage and in which type of clinical circumstances.
Two endpoints other than morbidity and mortality have been investigated in relation to the age of stored blood transfused: tumor recurrence and oxygen delivery. Edna and Bjerkeset45 found that blood storage time had no effect on local recurrences and distant metastases in 336 patients who had colorectal cancer surgery, yet Mynster and Nielsen12 found that, compared with patients who did not undergo transfusion, those who received allogeneic blood stored for less than 21 days had increased cancer recurrence after colorectal surgery; those who underwent transfusion with blood with a storage age older than 21 days had a recurrence rate similar to that of non-transfused patients. The clinical importance of the RBC storage lesion on oxygen delivery in adults varied, some studies suggesting the age of blood has little effect on oxygen delivery, using measures as varied as neurocognitive function and gastric tonometry.46-48 Still other studies suggest impairment of oxygen delivery using measures such as cerebral oxygenation in patients with traumatic brain injury or peripheral tissue oxygenation in pediatric or elderly patients.23,49,50 Conclusions drawn using these two endpoints are much less consistent than what we found for mortality in association with older blood.
We conclude that the published clinical experience to date suggests that newer blood if used exclusively might save lives. This effect is based largely on observational studies and could be confounded by some unrecognized bias that should be addressed in the three randomized controlled trials ongoing testing older versus newer blood.40-42 Nonetheless, the data available to date cause concern that our current blood banking storage practices may not adequately protect patients. If older RBCs are found to pose clinically significant risks, current systems of blood collection, storage and transfusion would need to be revised and would likely entail major operational and financial impacts. Blood collectors should be developing strategies to assure that blood availability can be maintained should our results be confirmed by the ongoing clinical trials.
ACKNOWLEDGMENTS
We thank J. Welsh, librarian, and J. Maltagliati, typist, for their support in the production of this report.
The content of this publication does not necessarily represent the views or policies of the National Institutes of Health, the Department of Health and Human Resources, or the U.S. Federal Government.
Appendix
Appendix Table 1.
Online supplement search strategies used for each database
| Data Base | Search Strategy | Terms | Records Found |
|---|---|---|---|
| PubMed | blood preservation[mesh] AND erythrocyte transfusion[mesh] AND time factors[mesh] Limits: Humans, English | MeSH terms (PubMed controlled vocabulary) | 116 |
| PubMed | (“red cell”[ti] OR “red cells”[ti] OR “red blood cell”[ti] OR “red blood cells”[ti] OR “transfused blood”[ti] OR “transfused cells”[ti]) AND (duration[ti] OR storage[ti] OR stored[ti] OR age[ti] OR time[ti] OR old[ti] OR older[ti]) Limits: Humans, English | 765 | |
| PubMed | (“red cell”[ti] OR “red cells”[ti] OR “red blood cell”[ti] OR “red blood cells”[ti] OR “transfused blood”[ti] OR “transfused cells”[ti]) AND (duration[ti] OR storage[ti] OR stored[ti] OR age[ti] OR time[ti] OR old[ti] OR older[ti]) NOT medline[subset] Limits: English | 42 | |
| Scopus | (TITLE(“red cell” OR “red cells” OR “red blood cell” OR “red blood cells” OR “transfused blood” OR “transfused cells”) AND TITLE(duration OR storage OR stored OR age OR time OR old OR older)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(LANGUAGE, “English”)) | 813 | |
| EMBASE | ‘erythrocyte preservation’/exp/mj OR ‘blood storage’/exp/mj AND (‘blood transfusion’/exp/mj OR ‘erythrocyte transfusion’/exp/mj) AND [humans]/lim AND [english]/lim AND ([embase]/lim OR [embase classic]/lim) | EMTREE terms (EMBASE controlled vocabulary) | 328 |
| EMBASE | ‘red cell’:ti OR ‘red cells’:ti OR ‘red blood cell’:ti OR ‘red blood cells’:ti OR ‘transfused blood’:ti OR ‘transfused cells’:ti AND (duration:ti OR storage:ti OR stored:ti OR age:ti OR time:ti OR old:ti OR older:ti) AND [humans]/lim AND [english]/lim AND ([embase]/lim OR [embase classic]/lim) | 576 |
Footnotes
Conflict-of-interest disclosure: The authors declare that they have no competing financial interests
Contributions: D.W. and S.B.S. managed the research process, including methods development, search for studies, extraction and analysis of data, and writing the first draft. C.N. conceived the research idea. C.N. and J.S. participated in methods development, data interpretation, and in the writing of the final manuscript. J.S. participated in data analysis. H.G.K. participated in data interpretation and in the writing of the final manuscript. All authors agreed on the final version of the manuscript.
REFERENCES
- 1.Blundell J. Some account of a case of obstinate vomiting, in which an attempt was made to prolong life by the injection of blood into the veins. Med Chir Trans. 1819;10(pt2):296–311. doi: 10.1177/09595287190100p204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Database on Blood Safety: Report 2004-2005. World Health Organization; Geneva, Swizerland: 2008. [Google Scholar]
- 3.Whitaker BI, Schlumpf K Schulman J, Green J. The 2009 Nationwide Blood Collection and Utilization Survey Report. Besthesda, MD: 2011. ISBN. [Google Scholar]
- 4.Hill HR, Oliver CK, Lippert LE, Greenwalt TJ, Hess JR. The effects of polyvinyl chloride and polyolefin blood bags on red blood cells stored in a new additive solution. Vox Sang. 2001;81(3):161–166. doi: 10.1046/j.1423-0410.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- 5.Heaton WA, Holme S, Smith K, et al. Effects of 3-5 log10 pre-storage leucocyte depletion on red cell storage and metabolism. Br J Haematol. 1994;87(2):363–368. doi: 10.1111/j.1365-2141.1994.tb04923.x. [DOI] [PubMed] [Google Scholar]
- 6.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 7.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91(1):13–19. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 8.FDA . Workshop on Red Cell Stored in Additive Solution Systems. Bethesda, MD: Apr 25, 1985. [Google Scholar]
- 9.Hess JR, Greenwalt TG. Storage of red blood cells: new approaches. Transfus Med Rev. 2002;16(4):283–295. doi: 10.1053/tmrv.2002.35212. [DOI] [PubMed] [Google Scholar]
- 10.Karam O, Tucci M, Bateman ST, et al. Association between length of storage of red blood cell units and outcome of critically ill children: a prospective observational study. Crit Care. 2010;14(2):R57. doi: 10.1186/cc8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontaine MJ, Chung YT, Erhun F, Goodnough LT. Age of blood as a limitation for transfusion: potential impact on blood inventory and availability. Transfusion. 2010;50(10):2233–2239. doi: 10.1111/j.1537-2995.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- 12.Mynster T, Nielsen HJ. Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44(7):955–964. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg JA, McGwin G, Jr., Vandromme MJ, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69(6):1427–1431. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Straten AH, Soliman Hamad MA, van Zundert AA, et al. Effect of duration of red blood cell storage on early and late mortality after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;141(1):231–237. doi: 10.1016/j.jtcvs.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 15.Pettila V, Westbrook AJ, Nichol AD, et al. Age of red blood cells and mortality in the critically ill. Crit Care. 2011;15(2):R116. doi: 10.1186/cc10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 17.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46(10):1712–1718. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg JA, McGwin G, Jr., Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65(2):279–282. doi: 10.1097/TA.0b013e31817c9687. discussion 282-284. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SD, Janssen C, Fretz EB, et al. Red blood cell storage duration and mortality in patients undergoing percutaneous coronary intervention. Am Heart J. 2010;159(5):876–881. doi: 10.1016/j.ahj.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Eikelboom JW, Cook RJ, Liu Y, Heddle NM. Duration of red cell storage before transfusion and in-hospital mortality. Am Heart J. 2010;159(5) doi: 10.1016/j.ahj.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Edgren G, Kamper-Jørgensen M, Eloranta S, et al. Duration of red blood cell storage and survival of transfused patients. Transfusion. 2010;50(6):1185–1195. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leal-Noval SR, Muñoz-Gómez M, Arellano-Orden V, et al. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36(4):1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- 24.Gauvin F, Spinella PC, Lacroix J, et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50(9):1902–1913. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 25.Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86(2):554–559. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg JA, McGwin Jr G, Marques MB, et al. Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65(4):794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 27.Murrell Z, Haukoos JS, Putnam B, Klein SR. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am Surg. 2005;71(9):781–785. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Brit Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 32.Van Buskirk CM, Kashyap R, Thakur SJ, et al. Red blood cell storage age has no impact on clinical outcome in critically ill patients. Transfusion. 2009;49:10A. [Google Scholar]
- 33.Fernandes da Cunha DH, Nunes Dos Santos AM, Kopelman BI, et al. Transfusions of CPDA-1 red blood cells stored for up to 28 days decrease donor exposures in very low-birth-weight premature infants. Transfus Med. 2005;15(6):467–473. doi: 10.1111/j.1365-3148.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 34.Hébert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100(5):1433–1438. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 35.Schulman CI, Nathe K, Brown M, Cohn SM. Impact of age of transfused blood in the trauma patient. J Trauma. 2002;52(6):1224–1225. doi: 10.1097/00005373-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 36.Van De Watering LMG, Brand A. Effects of storage of red cells. Transfus Med Hemoth. 2008;35(5):359–367. doi: 10.1159/000155221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96(2):93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 38.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49(7):1384–1394. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 39.Vamvakas EC. Meta-analysis of clinical studies of the purported deleterious effects of “old” (versus “fresh”) red blood cells: are we at equipoise? Transfusion. 2010;50(3):600–610. doi: 10.1111/j.1537-2995.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 40.Steiner ME, Assmann SF, Levy JH, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7). Transfus Apher Sci. 2010;43(1):107–116. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 42.Fergusson D, Hutton B, Hogan DL, et al. The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfus Med Rev. 2009 Jan;23(1):55–61. doi: 10.1016/j.tmrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre LA, Hebert PC. Can we safely restrict transfusion in trauma patients? Curr Opin Crit Care. 2006;12(6):575–583. doi: 10.1097/MCC.0b013e32801067f0. [DOI] [PubMed] [Google Scholar]
- 44.U.S. General Accounting Office Blood Supply: Transfusion-Associated Risks. GAO report. Feb;25:1997. PEMD-97-2. [Google Scholar]
- 45.Edna TH, Bjerkeset T. Perioperative blood transfusions reduce long-term survival following surgery for colorectal cancer. Dis Colon Rectum. 1998;41(4):451–459. doi: 10.1007/BF02235758. [DOI] [PubMed] [Google Scholar]
- 46.Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104(5):911–920. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32(2):364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 48.Keidan I, Amir G, Mandel M, Mishali D. The metabolic effects of fresh versus old stored blood in the priming of cardiopulmonary bypass solution for pediatric patients. J Thorac Cardiovasc Surg. 2004;127(4):949–952. doi: 10.1016/s0022-5223(03)01316-3. [DOI] [PubMed] [Google Scholar]
- 49.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67(1):29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 50.Schroeder TH, Hansen M. Effects of fresh versus old stored blood in the priming solution on whole blood lactate levels during paediatric cardiac surgery. Perfusion. 2005;20(1):17–19. doi: 10.1191/0267659105pf784oa. [DOI] [PubMed] [Google Scholar]