Abstract
Non-communicable diseases (NCDs), such as diabetes mellitus and coronary heart disease, are chronic, non-infectious diseases of long duration. NCDs are increasingly widespread worldwide and are becoming a serious health and economic burden. NCDs arise from complex interactions between the genetic make-up of an individual and environmental factors. Several epidemiological studies have revealed that the perinatal environment influences health later in life, and have proposed the concept of developmental programming or developmental origin of health and disease (DOHaD). These studies suggest the importance of life course health care from fetal life, early childhood, adulthood, and through to old age. Recent progress in genomics, proteomics and diagnostic modalities holds promise for identifying high risk groups, predicting latent diseases, and allowing early intervention. Preemptive medicine is the ultimate goal of medicine, but to achieve it, the full participation of the public and all sectors of society is imperative.
Keywords: non-communicable disease, developmental programming, life course health care, preemptive medicine, cohort study
Introduction
Non-communicable diseases (NCDs) are medical conditions or diseases which are non-infectious. NCDs are also known as chronic diseases and include cardiovascular diseases, diabetes mellitus, chronic respiratory diseases, cancers, and autoimmune diseases. The United Nations and the World Health Organization (WHO) have started initiatives to combat NCDs, which are becoming important health and economic burdens worldwide, including developing countries. WHO1) reported that NCDs kill more than 36 million people each year, and nearly 80% of these deaths occur in low- and middle-income countries. For example, type 2 diabetes, once thought of as a disease of developed countries, is increasing at an alarming rate in many countries, especially in Asia. Type 2 diabetes currently affects more than 300 million globally. The term “diabetes tsunami” has been used, even in medical literature,2) because the rapid increase of prevalence of the disease is like the force of an approaching tsunami. The reasons behind the diabetes tsunami in developing countries are not completely understood, but changes in lifestyle are important contributing factors. The term “lifestyle diseases” has been used extensively in Japan and is included in the broader category of NCDs.
This article discusses the pathogenesis of some common NCDs, but excludes cancers. The discussion draws from the recent literature and proposes the importance of life course health care and preemptive approaches to reduce the burden of NCDs.
Pathogenesis of NCDs —Missing heritability—
NCDs are defined as diseases of long duration and of generally slow progression; consequently, it is often difficult to determine when the disease started. A few NCDs are single gene disorders resulting from the mutation of a single gene with high penetrance. Most NCDs, however, are multifactorial in nature, meaning that the effects of multiple genes in combination with environmental factors are involved in developing the disease.
Since the completion of the international Human Genome Project in 2003, there has been much effort to elucidate associations of personal genome variations with NCDs or human phenotypes such as skin color, color of the iris, and height. Due to the study of haplotypes in different populations (HapMap project) and technological advances in microarrays, numerous genome-wide association studies (GWAS) have been performed and have identified many single nucleotide polymorphisms (SNPs) associated with diseases. However, for most diseases, individual SNPs have a weak effect and only modestly raise the risk which is insufficient for predicting whether someone will develop the disease. This “missing heritability” can be explained by common variants (more than 5% of the population) used in the present GWAS. It is possible that there are rare variants with substantial effect sizes which are not detected by GWAS. In order to find such rare variants, exome sequencing consortiums are now analyzing genomes of a large number of patients using next generation sequencers.
An alternative explanation for the genetic propensity to develop a given NCD is gene-gene interactions, in which a major gene may act in concert with other gene variants to cause a disease to develop. Such gene-gene interactions are known in Mendelian disorders. To elucidate gene-gene interactions or gene networks in common diseases, again a large number of patients must be studied.
GWAS and other genomic studies have discovered many variants associated with diseases, but there are still limitations in using these data for understanding disease processes and for realizing personalized medicine. Since the 1000 Genomes Project and other genomic studies are continuing, we may expect further progress in our understanding of the role of genomics in NCDs.
Another important research area for understanding pathogenesis of NCDs is epigenetics. Since NCDs are generally of late onset and are influenced by a variety of environmental factors, epigenetic modifications of genomes may contribute to the development of the disease. It has been reported that 88% of SNPs discovered by GWAS are located in introns or intergenic regions, and are possibly involved in gene expression.3) This observation is consistent with the contention that epigenetic changes are of great importance in the pathogenesis of NCDs.
Importance of prenatal environment —Lessons from the Dutch Hunger winter and other epidemiological studies—
During the winter of 1944–1945, near the end of World War II, the Allies were able to liberate the southern part of the Netherlands, but their effort suddenly halted because of operational failures. The exiled Dutch government appealed for Dutch laborers to strike and the German administration retaliated by stopping food transport to the western Netherlands. Some 4.5 million people were affected by the resulting famine and about 20,000 died during that time.4) Dutch medical scientists followed up people born right before, during and after the famine (1944–1946), comparing their health with those not exposed to the famine. They first reported that schizophrenia5) and schizophrenia-spectrum personality disorder6) were significantly associated with exposure to the famine during the early prenatal period. Further studies on the famine birth cohort showed that coronary heart disease, glucose intolerance, high blood pressure, decreased renal function as demonstrated by microalbuminuria and serum creatinine levels, obstructive airway diseases, and dyslipidemia were more prevalent in those who were exposed to the famine in their prenatal life4) (Table 1). More recently, as the birth cohort approached 56 to 59 years of age, they were noticed to have deteriorating cognitive function, although their cognitive ability at age 19 was the same as the control group.7) These observations suggest that maternal undernutrition during gestation has important effects on the physical and mental health of offspring later in life.
Table 1.
Associations of NCDs later in life with low birth weight
| Dutch Famine5–7) | British Studies8,9,27) | Helsinki Birth Cohort10–17) |
|---|---|---|
| CHD | CHD | CHD |
| T2D | T2D | T2D |
| Hypertension | Hypertension | Hypertension |
| Metabolic syndrome | Metabolic syndrome | Stroke |
| Decreased renal function | Dyslipidemia | Cognitive decline |
| Dyslipidemia | Decreased renal function | Dyslipidemia |
| Cognitive decline | Hypothyroidism | |
| Obstructive airway disease | Depression | |
| Schizophrenia, SSPD |
CHD: Coronary heart disease.
T2D: Type 2 diabetes.
SSPD: Schizophrenia spectrum personality disorder.
The Dutch famine seems different from under-nutrition in developing countries, because the Dutch famine occurred suddenly in a previously well-nourished society and was relieved quickly after liberation by the Allies. It lasted about 6 months, during which the daily caloric intake was 400 to 600 KCal. Slower and milder changes in nutrition may be different from such a drastic famine. In this respect, epidemiological studies performed in the United Kingdom are of great interest. After the Second World War, coronary heart disease increased in the United Kingdom, but the increase was more remarkable in the poorest areas and in lower income groups. This paradoxical observation prompted Barker and his associates8) to conduct epidemiological surveys. Using records of weights at birth and at one year of age in the county of Hertfordshire, they noticed that the lower birth weight or lower one year weight group was associated with a higher incidence of coronary heart disease in adulthood. Later studies by the same group showed that insulin resistance, metabolic syndrome, type 2 diabetes, hypertension, and dyslipidemia were associated with low birth weight (Table 1). Barker and coworkers proposed a “thrifty phenotype hypothesis”, implying that increased susceptibility to such diseases results from adaptations made by the fetus to survive an adverse environment.9)
Other studies performed later support the putative fetal origin of NCDs, as shown in Table 1. Based on the birth records of Helsinki University Central Hospital during 1934–1944, Eriksson et al.10) reported that low birth weight and low ponderal index (birth weight/length3) were associated with an increased risk of coronary heart disease. In the same cohort studies, several other diseases, such as type 2 diabetes, dyslipidemia, hypertension, stroke, cognitive decline, hypothyroidism, and depression, were more frequently observed in the low birth weight group.11–17) In Asian Population, similar results have been reported. For example, Song et al.18) observed that the prevalence of schizophrenia in early adulthood was significantly higher in the cohort born during the period of the Chinese Great Leap Famine than in control population. Fukushima et al.19) observed in a Japanese cohort that those with low birth weight tended to have a higher body mass index and higher fasting blood sugar levels later in life. These observations are consistent with Barker’s hypothesis, now more commonly called the Developmental Origins of Health and Disease (DOHaD) hypothesis, because both fetal life and more broadly plastic phases of human development such as early childhood are important, and because this concept is also applicable for promoting health.20)
Most of the studies mentioned above used birth weight or ponderal index as a rough indicator of intrauterine growth. Clearly, more precise indicators such as the size and shape of the placenta, the nutritional state of the mother, the adiposity of newborns, and other indices are needed. However, birth weight records are easily available and quantitative, and therefore most extensively used in such retrospective studies.
Possible mechanisms underlying DOHaD —Nature or nurture—
In the past decade, the DOHaD hypothesis indicating the importance of early environmental factors in developing NCDs later in life has been accepted by many scientists because of accumulating data from epidemiological studies. Although not discussed in this review, numerous experiments in rodents and other mammals also support the hypothesis.20) Furthermore, genomics has progressed remarkably during the same period and a great deal of data on genetic variants associated with a variety of NCDs have been reported, as mentioned above. In addition, family or twin studies have shown the importance of heritability in many NCDs. For example, type 2 diabetes is highly concordant in monozygotic twins compared with dizygotic twins,21) suggesting high heritability of the disease. However, the genetics of type 2 diabetes remains poorly understood: GWAS have revealed more than 60 SNPs that have small effect sizes and explain less than 10% of heritability,22) and the interplay between genetic and environmental factors remains unknown.
About two thirds of monozygotic twins share the same placenta and possibly experience a more adverse intrauterine environment than dizygotic twins. This may explain at least partly the high concordance of diabetes in monozygotic twins. Comparing elderly and young twins, Poulsen et al.23) reported that the effects of an adverse intrauterine environment appeared more frequently in the elderly monozygotic twin group than in the younger group, suggesting that time is important in unmasking prenatal environmental factors. This is consistent with the deterioration of cognitive function with aging in the Dutch Famine cohort.7) Poulsen et al.23) also observed that low birth weight was associated with insulin resistance and low insulin secretion after both intravenous and oral glucose administration when compared with monozygotic twins of normal birth weight.
Based on these observations, one can speculate that poor nutritional states in utero may program fetuses to survive similar adverse conditions after birth (Fig. 1). In most cases, this programming is beneficial for health and survival, because the offspring are likely to live in a nutritionally poor environment. On the other hand, if they live in an affluent society later in life, there will likely be a mismatch with this programming,24) which could lead to metabolic abnormalities such as diabetes mellitus, obesity, and vascular diseases. From an evolutionary viewpoint, this programming can be considered as developmental plasticity (change of phenotype or organ function) for better adapting to adverse living conditions. Such phenotypic plasticity is called predictive adaptive responses and seen not only in man but in animals.25,26) This developmental (or DOHaD) hypothesis may explain why the diabetes tsunami is occurring in rapidly developing countries. Needless to say, genetic make-up also plays important roles in developing metabolic abnormalities, and is likely to be related to varied risks of diabetes in different ethnic groups when lifestyle is suddenly westernized. The interplay of both nature and nurture shapes the human body.
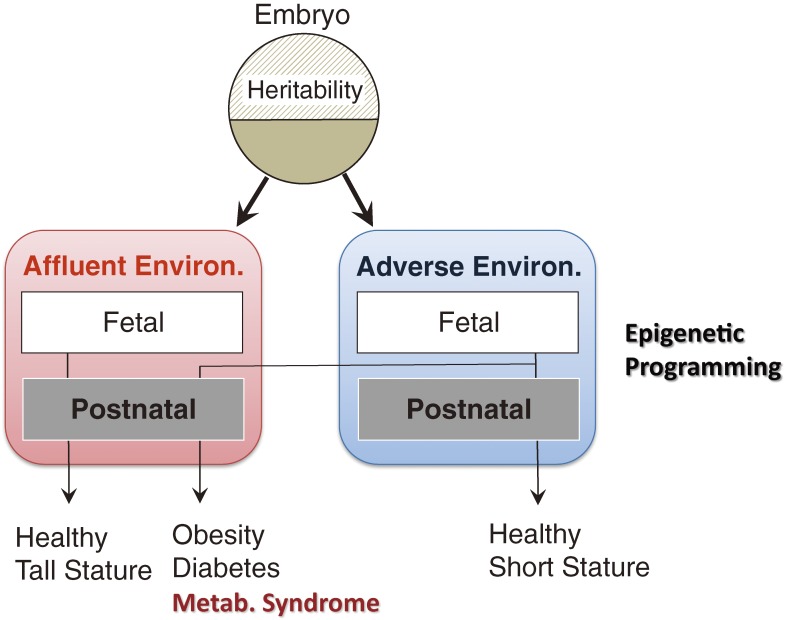
Figure 1.
Schematic representation of developmental programming. When insults such as undernutrition affect fetuses in utero, they are programmed to live in similar adverse environment after birth and body sizes are usually small. If intrauterine life is rich and problem-less, vice versa. If those who are programmed to adapt adverse environment actually live in affluent society in later life, they develop NCDs due to mismatch with programs.
During fetal development, there are critical periods during which a system or organ matures. If malnutrition, stress, or other insults afflict the fetus during these periods, we can assume that long-lasting effects would occur in the developing system or organ, culminating in diseases later in life. For example, in utero undernutrition affects renal organogenesis, resulting in reduced nephron number. This can provide the basis for developing hypertension and chronic kidney disease later in life.20) In a British birth cohort, low birth weight was associated with decreased renal function as measured by estimated glomerular filtration rate (eGFR), and was independent of hypertension or diabetes.27) The association was stronger in members who were overweight in adulthood, which might be a contributing factor for deteriorating renal function. The authors recommended that individuals with low birth weight should avoid becoming overweight in order to prevent renal diseases.
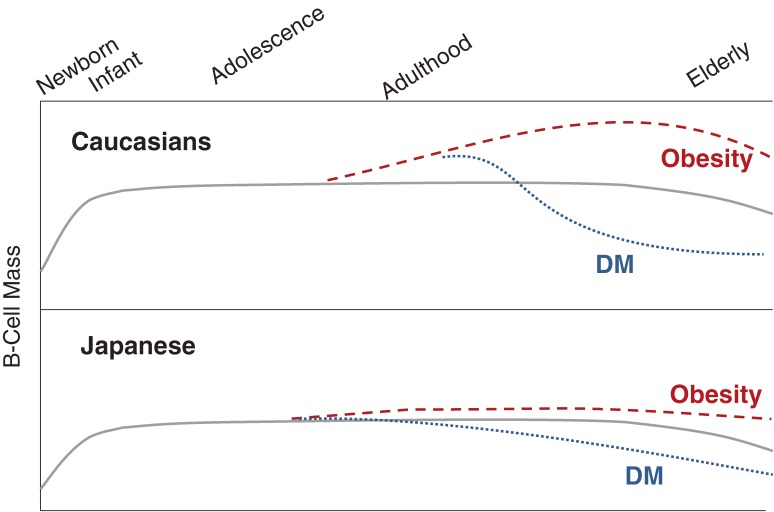
The pancreas is another organ that may be programmed by the fetal environment. Factors controlling the birth, growth, and death of pancreatic beta-cells have not been fully elucidated, but beta-cells are known to increase during fetal life and to replicate during early childhood before the age of 5. The mass of beta-cells is maintained in adulthood.28–30) If the requirement for insulin is increased, for example due to pregnancy or obesity, the beta-cell mass must increase in order to maintain glucose homeostasis. If beta-cells cannot proliferate to meet the increased insulin requirement, then diabetes develops. Indeed, a postmortem study in Caucasians found that obese non-diabetic people exhibited increased beta-cell mass, whereas beta-cell mass was much smaller in obese diabetic subjects.31) A similar postmortem study performed in Japan showed no significant increase in beta-cell mass in obese Japanese people.32) Although further studies are needed to provide conclusive evidence, we can hypothesize that genetic factors and the intrauterine environment may determine beta-cell mass at a relatively early period in life. This hypothesis may explain why Japanese people develop diabetes without a significant increase in body weight, whereas diabetes tends to be accompanied by a significant increase in body weight in Caucasians. Figure 2 shows a model of beta-cell mass throughout life and possible differences between Caucasians and Japanese.
Figure 2.
Models of growth and maintenance of islet beta cells throughout life and their response to increased insulin requirement in Caucasians and in Japanese. The Caucasian’s model is reproduced from the paper of Rhodes.25)
Glucose intolerance is a system disorder of energy homeostasis caused not only by impaired insulin secretion, but also by insulin resistance.33) Muscle, adipose tissue, the liver, and the hypothalamus are involved in impaired insulin action. It remains unknown to what extent insulin resistance is programmed in utero or whether it is secondary to obesity in postnatal life. Insulin resistance is often associated with diabetes of possible prenatal origin. Chronic inflammatory processes are now postulated to be involved in causing abnormal energy homeostasis, especially in excessive obesity.34) It is still not known whether and how chronic inflammation is directly related to an adverse prenatal environment. Another system that is important in metabolic programming is the hypothalamo-pituitary system, in which leptin, corticotropin-releasing hormone, adrenocorticotropic hormone, and other peptides are key players.
Recent studies have focused on molecular mechanisms underlying metabolic programming, which is a key missing link in this research field. Epigenetic changes that alter gene expression through DNA methylation, histone modifications, and posttranscriptional regulation of gene expression via non-coding microRNAs are among the most plausible mechanisms.35) A variety of animal experiments have provided evidence of epigenetic events evoked by nutritional alteration during the prenatal period. However, it is difficult to unequivocally extrapolate findings obtained by animal experiments to humans. Human studies have been performed mostly using DNA from easily accessible umbilical blood cells, and usually with selected candidate loci such as the IGF2/H19 locus, which is crucial for prenatal growth.36) Epigenetic events occurring in pancreatic beta-cells, muscle, adipose tissue, and hypothalamic nuclei would be more pertinent for understanding metabolic programming under normal and undernourished conditions.
Another potential mechanism is mitochondrial function, which plays central roles in cellular energy homeostasis and regulation of reactive oxygen species (ROS) production and oxidative stress. It is therefore likely that oxidative stress is involved in metabolic programming under adverse conditions in prenatal life.34) The main challenge in future will be to elucidate how the intrauterine environment interacts with programmed phenotypes to trigger diseases later in life.
Postnatal environment —Does childhood affect health later in life?—
As mentioned in the previous sections, the thrifty phenotype or DOHaD hypothesis was proposed on the basis of observations that low birth weight is associated with NCDs later in life, suggesting the importance of the prenatal environment. Later studies expanded the hypothesis to include early postnatal life and used the names perinatal programming or early programming. For example, Eriksson37) reported that those who were small at birth and in early infancy, followed by an increase in body size in late childhood, had a higher risk of coronary heart disease and diabetes later in life. Timing of catch-up growth, and especially compensatory weight gain in children who are born small, appears to be important because they have a tendency to become obese in late childhood. Obesity is a critical factor in developing diabetes and coronary heart disease later in life. In Japan, the number of low birth weight babies has increased in the past two decades, probably due to dieting by young women, causing concerns among specialists of the increasing number of patients with metabolic abnormalities in future.
Another point that should be mentioned is an increasing number of large babies born to diabetic or prediabetic mothers. These babies become obese in childhood and show decreased physical functioning with increased prevalence of metabolic abnormalities after middle age.37) Childhood obesity and type 2 diabetes are increasing worldwide, and have reached an alarming level. Therefore, all children, especially those who were born small or large, must take care not to become obese.
The British Post War Birth Cohort, a part of which was mentioned above, was initiated by the British Medical Council in 1946 and comprised 2547 females and 2815 males, born the same week in March 1946.38) Some studies were performed in childhood, but for most of the cohort, follow-up started at age 26 and has continued to the present. Subsequent cohorts were started in 1956 and 1970. When participants reached the age of retirement (60–64 years), most of them had on average two common diseases, such as hypertension, diabetes, obesity, or dyslipidemia, and only one out of six was disease-free.39) Premature death between 26–54 years was significantly related to smoking habits, and to socioeconomic conditions in childhood and in adulthood. Using childhood cognitive ability data, Henderson et al.40) also reported that better childhood cognitive ability at age 10 to 11 was significantly associated with reduced odds of long-term sickness in adults, and that education correlated with improved health of people with lower cognitive ability. They suggested that education should form part of the policy response to long-term sickness so that young people are better equipped with skills needed in a flexible labor market.
Another point is mental health problems in childhood and adolescence, which is fairly common and casts a shadow over their lives. In British cohort studies,41,42) mild and severe adolescent behavioral problems were associated with never marrying in women, divorce, and teenage parenthood. People with emotional problems in childhood are much more likely to have emotional problems in adult life. Since mental diseases are increasing worldwide, more study is required on the trajectory from childhood mental states to adulthood mental disorders. The relationship between mental disorders and perinatal environment also requires investigation, as highlighted by the increased prevalence of schizophrenia in the Dutch Hunger cohort.
In contrast to other primates, humans have a long childhood and adolescence, which may be important for maturing cognitive function, social skills, and behaviors. This period is also important for both physical and mental health later in life.
Life course health care —A new public health paradigm—
As mentioned above, ample evidence now suggests the importance of the prenatal and postnatal environment for developing NCDs later in life through interactions with the genetic make-up. Further genetic, epigenetic, and biomedical studies should clarify the mechanisms by which such long-lasting effects are programmed. Public health social security policies should aim to improve the adverse environments that pregnant mothers and infants face. Previously, public health policies focused on apparently healthy middle-aged subjects in order to detect early signs of NCDs and treat them to stop or slow down the disease process. This is the secondary prevention of diseases; to achieve primary prevention, a larger scope is required.
Although it is not in the scope of this review, periconceptual events are also important for the physical and mental health of offspring. It is well established that the risk of chromosome anomalies such as Down’s syndrome increases with the mother’s age, especially after age 35. More recently, whole genome sequencing of trios (father, mother, and child) performed on Icelanders showed 40 to 100 de novo mutations in one generation. The number of mutations in the child increases when the father is over 40 years of age, especially when the child had either schizophrenia or autism spectrum disorders.43) Since couples are having children later in life in developed countries, public education about the potential hazards of having children later in life is needed.
Although the importance of health care for pregnant women was discussed already, it should be added that one must pay attention to not only macronutrients such as total caloric intake and protein, but also micronutrients such as folate and other vitamins and minerals. Emetic symptoms that occur in the first trimester of pregnancy should be carefully managed. Both physical and mental stresses must be avoided as much as possible, and the expectant mother should be urged to refrain from smoking, drinking, and drug use. If necessary, vitamin and mineral supplements should be encouraged. It is imperative that health care providers make pregnant women understand the importance of a healthy intrauterine environment for the health of the child later in life.
Both physical and mental health in childhood is important, as discussed above, although more studies are needed to clarify many aspects. One important finding is that childhood obesity is highly associated with adult metabolic syndrome, type 2 diabetes, and cardiovascular diseases. The British birth cohort demonstrated that cognitive ability in late childhood was significantly associated with health in late adulthood.42) It is still not clear to what extent education can improve the situation. Since the British birth cohort showed that smoking is an important single cause of premature death or long sickness, health education during late childhood and adolescence is extremely important.
Preventive medicine, started as the science and art of preventing infectious diseases, is now moving toward prevention of NCDs. Since NCDs develop through the interplay of genetic and environmental factors, and proceed during a very long course, health care for preventing such diseases requires a different viewpoint which begins at the time of conception and continues throughout the person’s entire life. This is what I call life course health care. Figure 3 schematically shows how NCDs develop during a long course and what public health policies should do. The question remains how to organize the efforts of society to achieve life course health care. This will be discussed later.
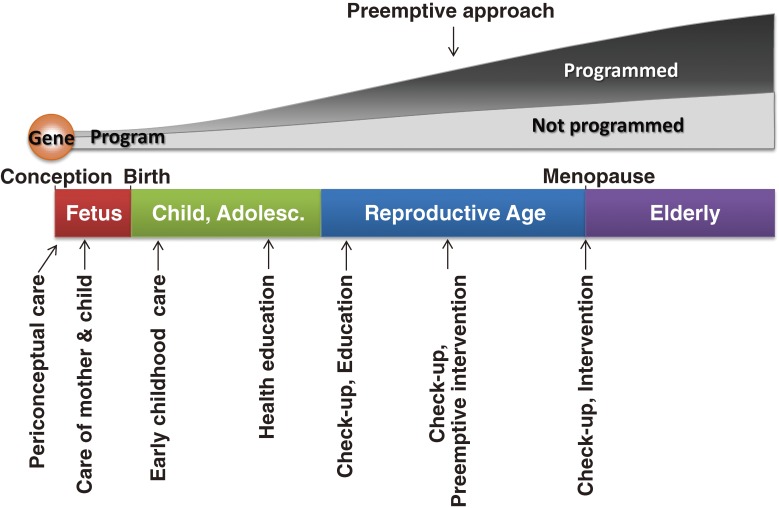
Figure 3.
Human life history and life course health care. Life history in human is unique in some aspects. It has a long childhood and adolescence with delays of the first breeding. This might be evolved for maturing the brain, its cognitive ability and social behaviors. Another characteristic feature is menopause in females and long period as grandmothers that have helped probably the growth of grandchildren. “Programmed” means that a person is programmed to live in adverse environment after birth. Life course health care begins at periconceptual period, followed by care of pregnant mothers and fetuses. Early childhood care is as important as discussed in the text. Since human brain matures over a long period, physical and mental care of childhood and adolescence as well as health education are of great importance. In adulthood, regular check-ups are of importance and, if necessary, early intervention (preemptive approach) is effective for obviating serious diseases.
Preemptive medicine —A new approach toward NCDs—
Since NCDs develop over a long period, it is impossible to pinpoint when the disease in question started. It certainly has a long latent period without symptoms or notable abnormalities in routine laboratory examinations. Historically, health care providers attempted to diagnose patients as early as possible after onset of the disease in order to minimize tissue damage and slow the progress of the disease. Although our understanding of the genetics of NCDs is still limited, whole genome sequencing using next generation sequencers is ongoing and will provide the information required for stratifying high risk groups.
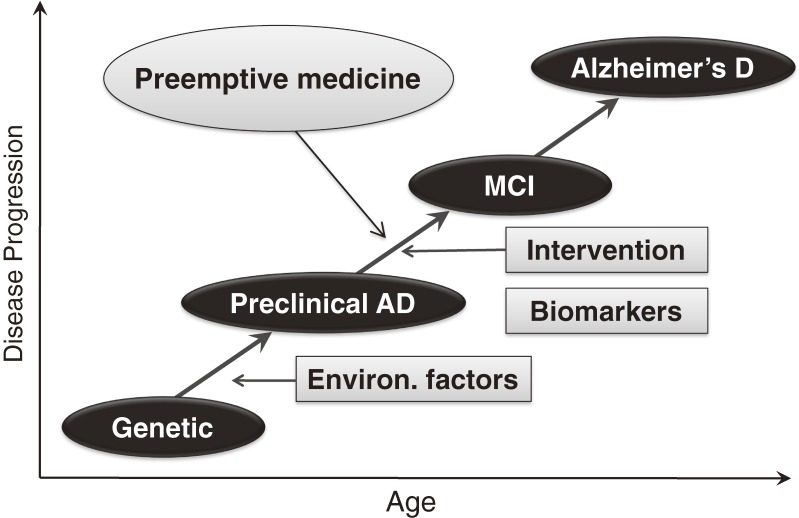
In addition, progress in epigenetics, proteomics, metabolomics and imaging modalities is revolutionizing procedures for diagnosing NCDs even in the latent period. One example is Alzheimer’s disease, the prevalence of which is increasing worldwide in parallel with an enormous increase in the aged population. Although the pathogenesis of the disease is not fully understood, it is characterized by deposition of amyloid β (Aβ) protein in the brain. Postmortem studies demonstrated that deposition of Aβ in the brain begins long before the onset of mild cognitive impairment (MCI), a prodromal stage of Alzheimer’s disease. Recent developments in positron emission tomography (PET) have allowed the demonstration of Aβ deposits in the brain. In addition, the concentrations of Aβ and tau protein in the cerebrospinal fluid also change before the onset of MCI. Using these and other biomarkers, it is possible to diagnose preclinical Alzheimer’s disease with high probability.44) At present there are no unequivocally effective drugs, but clinical trials of drug candidates are currently ongoing to treat patients at the preclinical stage. If an effective drug is found, it will be possible to preemptively treat Alzheimer’s disease and prevent the neuronal loss that causes irreversible damage in the brain (Fig. 4). Since genetic studies have shown several genes associated with Alzheimer’s disease,45) such as the ε 4 allele of apolipoprotein E, high risk groups can be identified. The preemptive approach will likely become the major path for preventing or treating Alzheimer’s disease in the near future.
Figure 4.
Schematic representation of natural course of Alzheimer’s disease and importance of preemptive approach. Alzheimer’s disease develops through interactions of genetic and environmental factors. The role of perinatal environment is still not understood, but some cohort studies mentioned in the text showed that those who were exposed to adverse perinatal environment develop early decline of cognitive function, suggesting the possible involvement of fetal life. As environmental factors in adulthood, metabolic abnormalities such as type 2 diabetes become risk factors. It is possible to diagnose preclinical Alzheimer’s disease by some biomarkers or PET scanning, intervention before the onset of MCI will become the most important approach. In other words, preemptive medicine will become a standard approach for preventing Alzheimer’s disease.
Many NCDs will become targets of preemptive medicine. The development of type 2 diabetes is based on genetic factors, the prenatal environment, and postnatal nutritional deviations such as obesity. If we can measure pancreatic beta-cell mass or beta cell reserves in future, we will be able to predict diabetes and allow much earlier intervention that will prevent the progress of the disease, for example by changing lifestyle or with drugs. Since arteriosclerosis progresses at the stage of impaired glucose tolerance, a prodromal stage of diabetes, earlier intervention is preferable for preventing vascular complications of diabetes.
There are two levels in preemptive medicine for some NCDs.46) Level 1 is to predict the underlying disease and try early intervention in order to interrupt the progress of the disease (Table 2). In coronary sclerosis, a basic pathological change, atherosclerosis, begins in adolescence as fatty streaks and progresses slowly. Preventing the basic processes of atherosclerotic changes is a level 1 preemptive approach. However, it is currently difficult to detect such subtle changes in the arterial walls, so the level 2 preemptive approach is more practical and important. This involves the detection of high risk plaque with threat of rupture or thrombosis by blood biomarker screening and then imaging procedures such as CT, MRI, and ultrasonography. Early detection by these approaches enables us to intervene early and prevent serious events. Another example is osteoporosis, which is characterized by reduction of bone mass. Both genetic and environmental factors, and possibly the perinatal environment, together provide the basis for osteoporosis. The level 1 approach is to minimize bone mass loss by lifestyle changes or medication, but this is not easy, especially in aged subjects. More practically, therefore, a level 2 approach is needed to predict the threat of bone fracture, which is one of the most common causes of disability in an aged population. Measurement of bone mineral content significantly contributes to delineating high risk groups, although biomarkers that reflect deteriorating bone microstructure are still lacking. Therefore, the World Health Organization proposed FRAX (fracture risk assessment tool) to delineate high risk groups and to start drug therapy.47)
Table 2.
Two levels in preemptive medicine
| Level 1 | Inhibiting progression of basic disease process |
| Level 2 | Diagnosing high risk group to prevent serious events |
Preemptive medicine differs from preventive medicine in several ways. Preventive medicine used to take a population-based approach. One famous study in this field is the Framingham Heart Study, a cohort study, which aimed to elucidate risk factors of coronary heart disease. The risk factors discovered by these researchers have contributed greatly to preventive medicine. There is, however, currently no individualized approach; consequently, those who have no or only one risk factor may develop the disease, and those who have multiple risks may not have the disease. Differences in the hazard ratio can be calculated by statistical methods, although the high risk group certainly has an increased incidence of heart attack. Preemptive medicine aims for individualized medicine, although this has not yet been established, in order to stratify high risk groups based on genetics. Then, using biomarkers, preemptive medicine tries to detect latent disease with high probability for earlier intervention before the onset of clinical disease by changing lifestyle or by medication. Preemptive medicine is personalized, predictive and preventive medicine.
Conclusion
Life course health care as proposed above is the best way to achieve a healthy aging society in which most aged persons are free of serious health problems and enjoy high quality of life. To this end, we need to re-organize our social systems to collect health data from infancy and throughout life, in addition to individual genome, epigenome, proteome, and metabolome data. ICT and other new laboratory technologies are rapidly developing and will certainly help in analyzing enormous amounts of data, but we need to develop a new discipline of systems medicine in order to manage these enormous amounts of data and use them in daily medical practice.
Life course health care and preemptive medicine will be the best way to reduce ever-growing health care costs. They will certainly revolutionize the healthcare system, changing it from reactive to preventive: in other words, from waiting until the patient is sick to going out into the community to assess and help his or her healthcare activities before disease onset. This is a paradigm change in medicine from reactive to proactive attitudes. All sectors of society, not only healthcare providers but also local government, industrial companies, NPOs, and individuals, are required to participate in life course health care activities. P4 medicine, coined by Hood,48) is personalized, predictive, preventive, and participatory medicine, and is the medicine of the future.
Profile
Hiroo Imura was born in Shiga Prefecture in 1931 and graduated from Kyoto University School of Medicine in 1954. After internship, he majored in internal medicine, especially endocrinology and diabetes. He received PhD degree in 1962 and worked as a postdoctoral fellow at the University of California San Francisco between 1963 and 1965. He became Professor of Medicine at Kobe University School of Medicine in 1971 and Professor of Medicine at Kyoto University in 1977. He was elected to be President of Kyoto University in 1991 and served 6 year’s term. He was appointed to be an executive member of Science and Technology Policy of the Cabinet Office from 1998 to 2004. Since then, he has been President, Foundation for Biomedical Research and Innovation. He was also a Principal Fellow, Center for Research and Development Strategy, Japan Science and Technology Agency between 2005 and 2011. He has done pioneering works on biosynthesis of adrenocorticotropic hormone, regulation of growth hormone and prolactin and pathophysiology of natriuretic peptides. For his Accomplishment, he received Dale Medal, Asia and Oceania Medal (from UK), Robert H Williams Distinguished Leadership Award (from U.S.A.), Takeda Medical Award, Japan Medical Association Award and some other awards (from Japan). He is currently a member of the Japan Academy and a Foreign Honorary Member of the American Academy of Arts and Sciences.

References
- 1).World Health Organization (2010) Global status report on noncommunicable diseases 2010. Available from <http://www.who.int/nmh/publications/ncd_report_full_en.pdf>.
- 2).Sherwin R., Jastreboff A.M. (2012) Year in diabetes 2012: The diabetes tsunami. J. Clin. Endocrinol. Metab. 97, 4293–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).ENCODE Project Consortium. Bernstein B.E., Birney E., Dunham I., Green E.D, Gunter C., Snyder M. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Roseboom T., de Rooij S., Painter R. (2006) The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 82, 485–491 [DOI] [PubMed] [Google Scholar]
- 5).Susser E., Neugebauer R., Hoek H.W., Brown A.S., Lin S., Labovitz D., Gorman J.M. (1996) Schizophrenia after prenatal exposure to famine: further evidence. Arch. Gen. Psychiatry 53, 25–31 [DOI] [PubMed] [Google Scholar]
- 6).Hoek H.W., Susser E., Buck K.A., Lumey L.H., Lin S.P., Gorman J.M. (1996) Schizoid personality disorder after prenatal exposure to famine. Am. J. Psychiatry 153, 1637–1639 [DOI] [PubMed] [Google Scholar]
- 7).de Rooij S.R., Wouters H., Yonker J.E., Painter R.C., Roseboom T.J. (2010) Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl. Acad. Sci. U.S.A. 107, 16881–16886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Barker D.J.P., Winter P.D., Osmond C., Margetts B., Simmonds S.J. (1989) Weight in infancy and death from ischemic heart diseases. Lancet ii, 577–580 [DOI] [PubMed] [Google Scholar]
- 9).Hales C.N., Barker D.J. (1992) Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35, 595–601 [DOI] [PubMed] [Google Scholar]
- 10).Eriksson J.G., Forsén T., Tuomilehto J., Osmond C., Barker D.J. (2001) Early growth and coronary heart disease in later life: longitudinal study. BMJ 322, 949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Forsén T., Eriksson J., Tuomilehto J., Reunanen A., Osmond C., Barker D. (2000) The fetal and childhood growth of persons who develop type 2 diabetes. Ann. Intern. Med. 133, 176–182 [DOI] [PubMed] [Google Scholar]
- 12).Raikkonen K., Kajantie E., Pesonen A.K., Heinonen K., Alastalo H., Leskinen J.T., Nyman K., Henriksson M., Lahti J., Lahti M., Pyhälä R., Tuovinen S., Osmond C., Barker D.J., Eriksson J.G. (2013) Early life origins cognitive decline: findings in elderly men in the Helsinki Birth Cohort Study. PLoS ONE 8, e54707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Eriksson J., Forsén T., Tuomilehto J., Osmond C., Barker D. (2000) Fetal and childhood growth and hypertension in adult life. Hypertension 36, 790–794 [DOI] [PubMed] [Google Scholar]
- 14).Osmond C., Kajantie E., Forsén T.J., Eriksson J.G., Barker D.J. (2007) Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke 38, 264–270 [DOI] [PubMed] [Google Scholar]
- 15).Räikkönen K., Pesonen A.K., Heinonen K., Kajantie E., Hovi P., Järvenpää A.L., Eriksson J.G., Andersson S. (2008) Depression in young adults with very low birth weight: the Helsinki study of very low-birth-weight adults. Arch. Gen. Psychiatry 65, 290–296 [DOI] [PubMed] [Google Scholar]
- 16).Kajantie E., Phillips D.I., Osmond C., Barker D.J., Forsén T., Eriksson J.G. (2006) Spontaneous hypothyroidism in adult women is predicted by small body size at birth and during childhood. J. Clin. Endocrinol. Metab. 91, 4953–4956 [DOI] [PubMed] [Google Scholar]
- 17).Wahlbeck K., Forsén T., Osmond C., Barker D.J., Eriksson J.G. (2001) Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch. Gen. Psychiatry 58, 48–52 [DOI] [PubMed] [Google Scholar]
- 18).Song S., Wang W., Hu P. (2009) Famine, death, madness: Schizophrenia in early adulthood after prenatal exposure to the Chinese Great Leap Famine. Soc. Sci. Med. 68, 1315–1321 [DOI] [PubMed] [Google Scholar]
- 19).Fukushima, M. unpublished observation.
- 20).Gluckman, P. and Hanson, M. (eds.) (2006) Developmental Origins of Health and Diseases. Cambridge University Press, Cambridge UK. [Google Scholar]
- 21).Poulsen P., Kyvik K.O., Vaag A., Beck-Nielsen H. (1999) Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—a population-based twin study. Diabetologia 42, 139–145 [DOI] [PubMed] [Google Scholar]
- 22).Mühlenbruch K., Jeppesen C., Joost H.G., Boeing H., Schulze M.B. (2013) The value of genetic information for diabetes risk prediction—differences according to sex, age, family history and obesity. PLoS ONE 8, e64307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Poulsen P., Levin K., Beck-Nielsen H., Vaag A. (2002) Age-dependent impact of zygosity and birth weight on insulin secretion and insulin action in twins. Diabetologia 45, 1649–1657 [DOI] [PubMed] [Google Scholar]
- 24).Gluckman, P. and Hanson, M. (2006) Mismatch: The Lifestye Diseases Timebomb. Oxford University Press, Oxford UK. [Google Scholar]
- 25).Gluckman P.D., Hanson M.A., Spencer H.G. (2005) Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533 [DOI] [PubMed] [Google Scholar]
- 26).Nettle D., Frankenhuis W.E., Rickard I.J. (2013) The evolution of predictive adaptive responses in human life history. Proc. Biol. Sci. 280, 20131343, doi:10.1098/rspb.2013.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Silverwood R.J., Pierce M., Hardy R., Sattar N., Whincup P., Ferro C., Savage C., Kuh D., Nitsch D. (2013) Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. doi:10.1038/ki.2013.223 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Weir G.C., Bonner-Weir S. (2013) Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann. N. Y. Acad. Sci. 1281, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Rhodes C.J. (2005) Type 2 diabetes–a matter of beta-cell life and death? Science 307, 380–384 [DOI] [PubMed] [Google Scholar]
- 30).Gregg B.E., Moore P.C., Demozay D., Hall B.A., Li M., Husain A., Wright A.J., Atkinson M.A., Rhodes C.J. (2012) Formation of a human β-cell population within pancreatic islets is set early in life. J. Clin. Endocrinol. Metab. 97, 3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. (2003) β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 32).Kou K., Saisho Y., Satoh S., Yamada T., Itoh H. (2013) Change in β cell mass in Japanese non-diabetic obese individuals. J. Clin. Endocrinol. Metab. 98, 3724–3730 [DOI] [PubMed] [Google Scholar]
- 33).Jones R.H., Ozanne S.E. (2009) Fetal programming of glucose-insulin metabolism. Mol. Cell. Endocrinol. 297, 4–9 [DOI] [PubMed] [Google Scholar]
- 34).Martínez J.A., Cordero P., Campión J., Milagro F.I. (2012) Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc. Nutr. Soc. 71, 276–283 [DOI] [PubMed] [Google Scholar]
- 35).Sebert S., Sharkey D., Budge H., Symonds M.E. (2011) The early programming of metabolic health: is epigenetic setting the missing link? Am. J. Clin. Nutr. 94, 1953S–1958S [DOI] [PubMed] [Google Scholar]
- 36).Herzog E., Galvez J., Roks A., Stolk L., Verbiest M., Eilers P., Cornelissen J., Steegers E., Steegers-Theunissen R. (2013) Tissue-specific DNA methylation profiles in newborns. Clin. Epigenetics 5, 8, doi:10.1186/1868-7083-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Eriksson J.G. (2011) Early growth and coronary heart disease and type 2 diabetes: findings from the Helsinki Birth Cohort Study (HBCS). Am. J. Clin. Nutr. 94 (Suppl. 6), 1799S–1802S [DOI] [PubMed] [Google Scholar]
- 38).Wadsworth M., Kuh D., Richards M., Hardy R. (2006) Cohort Profile: The 1946 National Birth Cohort (MRC National Survey of Health and Development). Int. J. Epidemiol. 35, 49–54 [DOI] [PubMed] [Google Scholar]
- 39).Pierce M.B., Silverwood R.J., Nitsch D., Adams J.E., Stephen A.M., Nip W., Macfarlane P., Wong A., Richards M., Hardy R., Kuh D., NSHD Scientific and Data Collection Teams (2012) Clinical disorders in a post war British cohort reaching retirement: evidence from the First National Birth Cohort study. PLoS ONE 7, e44857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Henderson M., Richards M., Stansfeld S., Hotopf M. (2012) The association between childhood cognitive ability and adult long-term sickness absence in three British birth cohorts: a cohort study. BMJ Open 2, e000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Sainsbury Center for Mental Health (2009) Childhood mental health and life chances in post-war Britain. Insights from three national cohort studies. Available from <http://www.centreformentalhealth.org.uk/pdfs/life_chances_report.pdf>.
- 42).Goodman A., Joyce R., Smith J.P. (2011) The long shadow cast by childhood physical and mental problems on adult life. Proc. Natl. Acad. Sci. U.S.A. 108, 6032–6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Kong A., Frigge M.L., Masson G., Besenbacher S., Sulem P., Magnusson G., Gudjonsson S.A., Sigurdsson A., Jonasdottir A., Jonasdottir A., Wong W.S., Sigurdsson G., Walters G.B., Steinberg S., Helgasson H., Thorleifsson G., Gudbjartsson D.F., Helgason A., Magnusson O.T., Thorsteinsdottir U., Stefansson K. (2012) Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488, 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Jr., Kaye J., Montine T.J., Park D.C., Reiman E.M., Rowe C.C., Siemers E., Stern Y., Yaffe K., Carrillo M.C., Thies B., Morrison-Bogorad M., Wagster M.V., Phelps C.H. (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer Association workshops on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Mayeux R., Stern Y. (2012) Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect Med. 2, a006239, doi:10.1101/cshperspect.a006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Imura, H. (2012) For Future of Japanese Medicine: From Traditional Medical Treatment to Preemptive Medicine. Shindan-to-Chiryo Publishing Co., Tokyo (in Japanese). [Google Scholar]
- 47).World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. WHO, Geneva. [PubMed]
- 48).Hood L. (2013) Systems biology and p4 medicine: past, present, and future. Rambam Maimonides Med J. 4, e0012. [DOI] [PMC free article] [PubMed] [Google Scholar]