Abstract
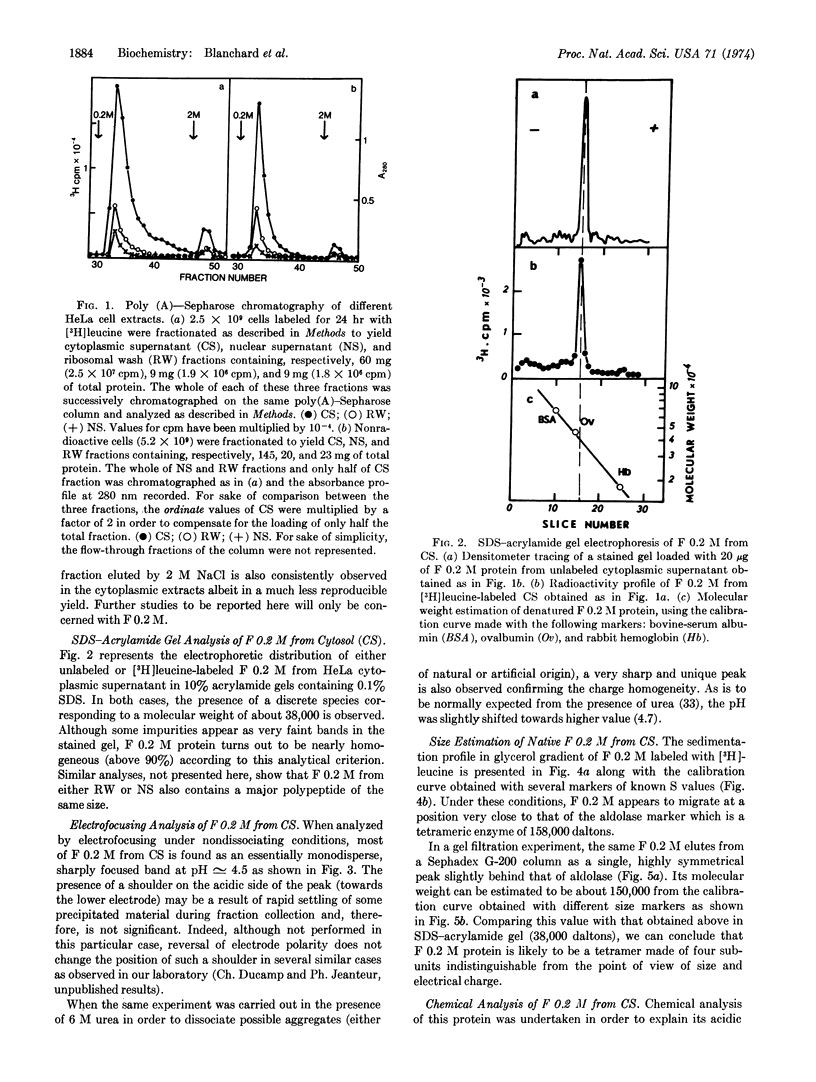
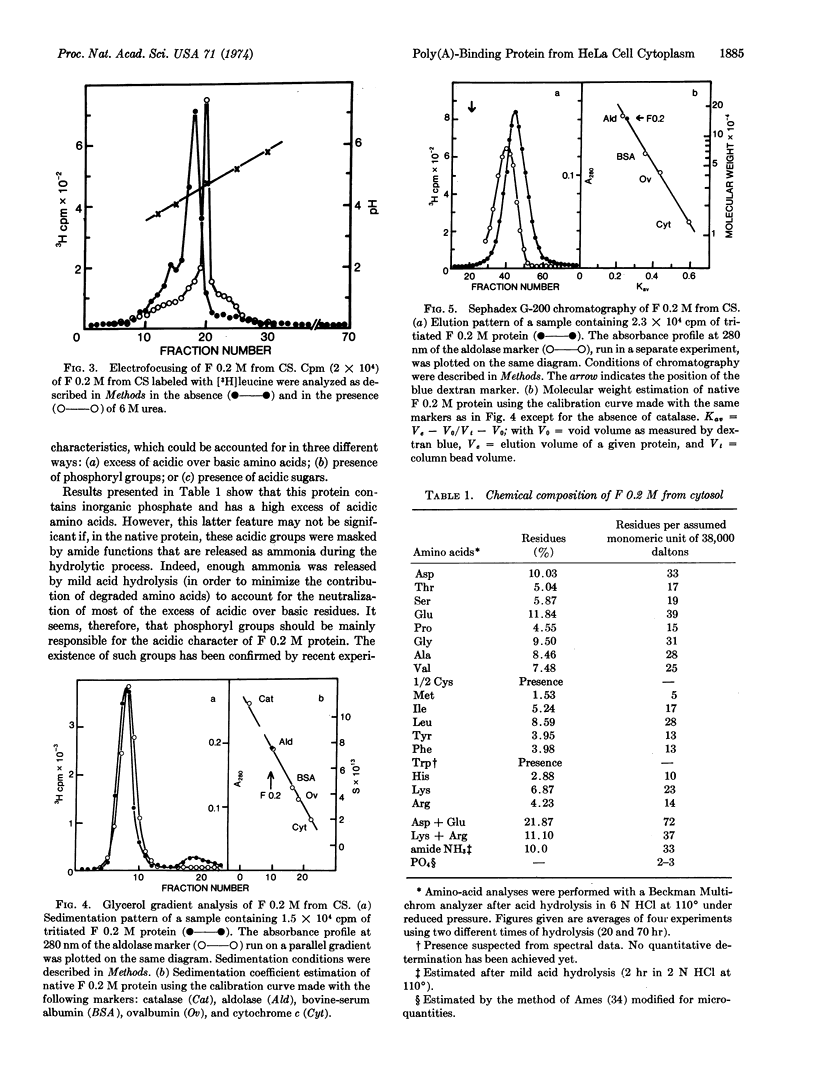
Chromatography of different soluble extracts from HeLa cells on poly(A)-Sepharose columns has allowed the isolation of a protein fraction eluted by 0.2 M NaCl and localized predominantly in the cytoplasmic supernatant and in the 0.5 M KCl ribosomal wash. This fraction is present in large amounts (around 3% of total cytosolic proteins) and appears to contain a major protein species that is acidic on electrofocusing (pI around 4.5) and phosphorylated. It runs on glycerol gradients and Sephadex G-200 chromatography close to the aldolase marker (158,000 daltons) and dissociates into apparently identical subunits of 38,000 ± 2,000 daltons on sodium dodecyl sulfate-acrylamide gels, suggesting a tetrameric structure.
Keywords: tetrameric structure, phosphorylated protein
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Edmonds M., Nakazato H., Phillips B. A., Vaughn M. H. Polyadenylic acid sequences in the virion RNA of poliovirus and Eastern Equine Encephalitis virus. Science. 1972 May 5;176(4034):526–528. doi: 10.1126/science.176.4034.526. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr H., Lingrel J. B. Poly A sequences at the 3' termini of rabbit globin mRNAs. Nat New Biol. 1971 Sep 8;233(36):41–43. doi: 10.1038/newbio233041a0. [DOI] [PubMed] [Google Scholar]
- Cartouzou G., Attali J. C., Lissitzky S. Acides ribonucléiques messagers de la glande thyroïde. I. RNA à marquage rapide des noyaux et des polysomes. Eur J Biochem. 1968 Mar;4(1):41–54. doi: 10.1111/j.1432-1033.1968.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducamp C., Jeanteur P. Characterization of nuclear RNP particles from Hela cells. Analysis of protein and RNA constituents. Presence of poly (A). Biochimie. 1973;55(10):1235–1243. doi: 10.1016/s0300-9084(74)80328-7. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Summers D. F. Adenylate-rich sequences in vesicular stomatitis virus messenger ribonucleic acid. J Virol. 1972 Oct;10(4):683–688. doi: 10.1128/jvi.10.4.683-688.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinaro-Matringe H., Jacob M. Nuclear particles from rat brain: complexity of the major proteins and their phosphorylation in vivo. FEBS Lett. 1973 Oct 1;36(1):105–108. doi: 10.1016/0014-5793(73)80348-5. [DOI] [PubMed] [Google Scholar]
- Girard M., Baltimore D. The effect of HeLa cell cytoplasm on the rate of sedimentation of RNA. Proc Natl Acad Sci U S A. 1966 Sep;56(3):999–1002. doi: 10.1073/pnas.56.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Cartas M. The genome of RNA tumor viruses contains polyadenylic acid sequences. Proc Natl Acad Sci U S A. 1972 Apr;69(4):791–794. doi: 10.1073/pnas.69.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. Correlation of messenger RNA function with adenylate-rich segments in the genomes of single-stranded RNA viruses. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1514–1516. doi: 10.1073/pnas.69.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S. W., Brawerman G. A particle associated with the polyadenylate segment in mammalian messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3247–3250. doi: 10.1073/pnas.69.11.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler K., Arends S. Particles of nuclear origin carrying rapidly labelled RNA. Eur J Biochem. 1968 Sep 24;5(4):500–506. doi: 10.1111/j.1432-1033.1968.tb00398.x. [DOI] [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. I. PURIFICATION AND PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1287–1293. [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukanidin E. M., Zalmanzon E. S., Komaromi L., Samarina O. P., Georgiev G. P. Structure and function of informofers. Nat New Biol. 1972 Aug 16;238(85):193–197. doi: 10.1038/newbio238193a0. [DOI] [PubMed] [Google Scholar]
- Matringe H., Jacob M. Brain nuclear and cytoplasmic ribonucleoproteins carrying DNA-like RNA: comparison of their proteins with those from soluble nuclear and cytoplasmic supernatants by polyacrylamide gel electrophoresis. Biochimie. 1972;54(9):1169–1178. doi: 10.1016/s0300-9084(72)80021-x. [DOI] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Sporn M. B., Kelley D. E., Perry R. P. Localization of polyadenylic acid sequences in messenger ribonucleic acid of mammalian cells. Biochemistry. 1972 Aug 15;11(17):3256–3260. doi: 10.1021/bi00767a020. [DOI] [PubMed] [Google Scholar]
- Moulé Y., Chauveau J. Particules ribonucléoprotéiques 40 s des noyaux de foie de rat. J Mol Biol. 1968 Apr 28;33(2):465–481. doi: 10.1016/0022-2836(68)90203-9. [DOI] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas J., Green H. Proteins binding to DNA and their relation to growth in cultured mammalian cells. Nat New Biol. 1971 Feb 10;229(6):165–169. doi: 10.1038/newbio229165a0. [DOI] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. The second Sir Hans Krebs Lecture. Informosomes. Eur J Biochem. 1969 Aug;10(1):20–35. doi: 10.1111/j.1432-1033.1969.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Mandel P., Jacob M. Forme particulaire du dRNA géant dans les noyaux de cerveau de rat. Bull Soc Chim Biol (Paris) 1970 Sep 15;52(7):703–720. [PubMed] [Google Scholar]
- Ui N. Isoelectric points and conformation of proteins. I. Effect of urea on the behavior of some proteins in isoelectric focusing. Biochim Biophys Acta. 1971 Mar 23;229(3):567–581. [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Wagner A. F., Bugianesi R. L., Shen T. Y. Preparation of sepharose-bound poly (rI:rC). Biochem Biophys Res Commun. 1971 Oct 1;45(1):184–189. doi: 10.1016/0006-291x(71)90067-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Poly A associated with SV40 messenger RNA. Nat New Biol. 1972 Jul 26;238(82):111–113. doi: 10.1038/newbio238111a0. [DOI] [PubMed] [Google Scholar]



