Abstract
Of the protein synthesized and accumulated during differentiation of embryonic chick lens fibers, 70-80% is the tissue specific protein δ-crystallin. We have isolated and partially characterized the total cytoplasmic mRNA from purified lens fibers of 15-day-old embryos as an initial step toward understanding the regulated expression of δ-crystallin during development. Each lens fiber mass contained an average of 10 μg of cytoplasmic RNA; approximately 0.1 μg per fiber mass was recovered in the mRNA fraction by oligo(dT)-cellulose chromatography. The mRNA electrophoresed primarily as a single peak on a polyacrylamide-agarose gel with an apparent molecular weight of about 9 × 105 estimated by comparison with 28S and 18S rRNA markers. Of the protein synthesized in response to the mRNA in cell-free systems derived from Krebs II ascites tumor cells or rabbit reticulocytes, 70-80% comigrated with δ-crystallin on sodium dodecyl sulfatepolyacrylamide-agarose gels. Comparison of the tryptic peptides of δ-crystallin with those of the in vitro products from both heterologous systems established that the lens fiber mRNA contained δ-crystallin mRNA, and that no other functional mRNAs were present in detectable quantities. Thus, the specialization of protein synthesis in embryonic chick lens fibers apparently results from an accumulation of δ-crystallin mRNA in the cytoplasm.
Keywords: cell-free protein synthesis, oligo(dT)-cellulose, lens crystallins, differentiation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns A. J.M., De Abreu R. A., Van Kraaikamp M., Benedetti E. L., Bloemendal H. Synthesis of lens protein in vitro V. Isolation of messenger-like RNA from lens by high resolution zonal centrifugation. FEBS Lett. 1971 Oct 15;18(1):159–163. doi: 10.1016/0014-5793(71)80434-9. [DOI] [PubMed] [Google Scholar]
- Berns A. J., Strous G. J., Bloemendal H. Heterologous in vitro synthesis of lens -crystallin polypeptide. Nat New Biol. 1972 Mar 1;236(61):7–9. doi: 10.1038/newbio236007a0. [DOI] [PubMed] [Google Scholar]
- Berns A. J., van Kraaikamp M., Bloemendal H., Lane C. D. Calf crystallin synthesis in frog cells: the translation of lens-cell 14S RNA in oocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1606–1609. doi: 10.1073/pnas.69.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Chrambach A., Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971 Apr 30;172(3982):440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- Clayton R. M. Problems of differentiation in the vertebrate lens. Curr Top Dev Biol. 1970;5:115–180. doi: 10.1016/s0070-2153(08)60055-1. [DOI] [PubMed] [Google Scholar]
- Clayton R. M. Properties of the crystallins of the chick in terms of their subunit composition. Exp Eye Res. 1969 Jul;8(3):326–339. doi: 10.1016/s0014-4835(69)80046-1. [DOI] [PubMed] [Google Scholar]
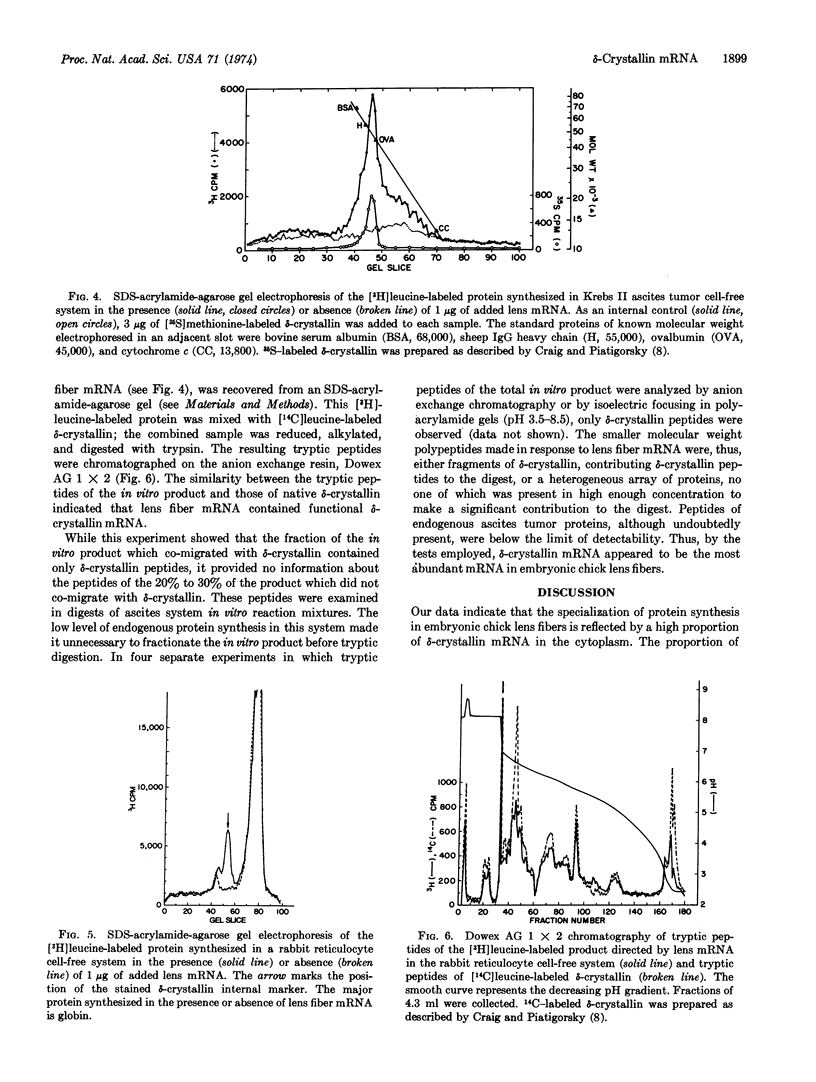
- Craig J. P., Piatigorsky J. Cell elongation and -crystallin synthesis without RNA synthesis in cultured early embryonic chick lens epithelia. Biochim Biophys Acta. 1973 Apr 11;299(4):642–653. doi: 10.1016/0005-2787(73)90237-2. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill P., Kabat D. Unexpectedly large size of globin messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1971 Jan;68(1):72–75. doi: 10.1073/pnas.68.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis-Galvez J. M., Castro J. M., Battaner E. Lens soluble proteins: correlation with the cytological differentiation in the young adult organ of the chick. Nature. 1968 Feb 17;217(5129):652–654. doi: 10.1038/217652a0. [DOI] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M., Berns A. J., Bloemendal H. Translation of two messenger RNAs from lens in a cell free system from Krebs II ascites cells. Nat New Biol. 1972 Mar 1;236(61):5–7. doi: 10.1038/newbio236005a0. [DOI] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Means A. R., Comstock J. P., Rosenfeld G. C., O'Malley B. W. Ovalbumin messenger RNA of chick oviduct: partial characterization, estrogen dependence, and translation in vitro. Proc Natl Acad Sci U S A. 1972 May;69(5):1146–1150. doi: 10.1073/pnas.69.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy G. R., Sporn M. B., Kelley D. E., Perry R. P. Localization of polyadenylic acid sequences in messenger ribonucleic acid of mammalian cells. Biochemistry. 1972 Aug 15;11(17):3256–3260. doi: 10.1021/bi00767a020. [DOI] [PubMed] [Google Scholar]
- PHILPOTT G. W., COULOMBRE A. J. LENS DEVELOPMENT. II. THE DIFFERENTIATION OF EMBRYONIC CHICK LENS EPITHELIAL CELLS IN VITRO AND IN VIVO. Exp Cell Res. 1965 Jun;38:635–644. doi: 10.1016/0014-4827(65)90387-3. [DOI] [PubMed] [Google Scholar]
- Partington G. A., Kemp D. J., Rogers G. E. Isolation of feather keratin mRNA and its translation in a rabbit reticulocyte cell-free system. Nat New Biol. 1973 Nov 14;246(150):33–36. doi: 10.1038/newbio246033a0. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Rothschild S. S., Milstone L. M. Differentiation of lens fibers in explanted embryonic chick lens epithelia. Dev Biol. 1973 Oct;34(2):334–345. doi: 10.1016/0012-1606(73)90362-x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. D., Craig S. P. Protein synthesis and ultrastructure during the formation of embryonic chick lens fibers in vivo and in vitro. Dev Biol. 1972 Feb;27(2):176–189. doi: 10.1016/0012-1606(72)90096-6. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. de F., Wollberg M. Cell elongation in the cultured embryonic chick lens epithelium with and without protein synthesis. Involvement of microtubules. J Cell Biol. 1972 Oct;55(1):82–92. doi: 10.1083/jcb.55.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler H. S., Housman D., Scher W., Friend C. Effects of 5-bromo-2' -deoxyuridine on production of globin messenger RNA in dimethyl sulfoxide-stimulated Friend leukemia cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2956–2959. doi: 10.1073/pnas.70.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Ikawa Y., Leder P. Globin messenger-RNA induction during erythroid differentiation of cultured leukemia cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3620–3623. doi: 10.1073/pnas.69.12.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Isolation of messenger RNA coding for mouse heavy-chain immunoglobulin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1127–1131. doi: 10.1073/pnas.70.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Cantor L., Metafora S., Rifkind R. A., Bank A., Marks P. A. Globin messenger RNA activity in erythroid precursor cells and the effect of erythropoietin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3575–3579. doi: 10.1073/pnas.69.12.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman D. E., Brown A. G., Rao K. V. Estimation of the molecular weights of chick -and -crystallins and their subunits by gel filtration. Exp Eye Res. 1971 Nov;12(3):304–310. doi: 10.1016/0014-4835(71)90154-0. [DOI] [PubMed] [Google Scholar]
- Williamson R., Clayton R., Truman D. E. Isolation and identification of chick lens crystallin messenger RNA. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1936–1943. doi: 10.1016/0006-291x(72)90073-3. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Katoh A. Crystallin synthesis by chick lens. II. Changes in synthetic activities of epithelial and fiber cells during embryonic development. Exp Eye Res. 1971 Mar;11(2):184–194. doi: 10.1016/s0014-4835(71)80022-2. [DOI] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]