Abstract
Objective
Screening for methicillin-resistant Staphylococcus aureus (MRSA) in high-risk patients is a legislative mandate in nine U.S. states and has been adopted by many hospitals. Definitions of “high-risk” differ among hospitals and state laws. A systematic evaluation of factors associated with colonization is lacking. We performed a systematic review of the literature to assess factors associated with MRSA colonization at hospital admission.
Design
We searched MEDLINE from 1966–2012 for articles comparing MRSA colonized and non-colonized patients on hospital or ICU admission. Data were extracted using a standardized instrument. Meta-analyses were performed to identify factors associated with MRSA colonization.
Results
We reviewed 4,381 abstracts; twenty-nine manuscripts met inclusion criteria (n=76,913 patients). MRSA colonization at hospital admission was associated with recent prior hospitalization (OR=2.4 95%-CI=1.3–4.7;p<0.01), nursing home exposure (OR=3.8 95%-CI=2.3–6.3;p<0.01) and history of exposure to healthcare-associated pathogens (MRSA carriage OR=8.0 95%-CI =4.2–15.1, C. difficile infection OR=3.4 95%-CI=2.2–5.3, vancomycin-resistant Enterococci carriage OR=3.1 95%-CI=2.5–4.0;p<0.01 for all). Select comorbidities were associated with MRSA colonization (congestive heart failure, diabetes, pulmonary disease, immunosuppression and renal failure; p<0.01 for all), while others were not (HIV, cirrhosis, and malignancy). ICU admission was not associated with an increased risk of MRSA colonization (OR=1.1 95%-CI =0.6–1.8;p=0.87).
Conclusions
MRSA colonization on hospital admission was associated with healthcare contact, previous healthcare-associated pathogens, and select comorbid conditions. ICU admission was not associated with MRSA colonization although this is commonly used in state mandates for MRSA screening. Infection prevention programs utilizing targeted MRSA screening may consider our results to define patients likely to have MRSA colonization.
Methicillin-resistant Staphylococcus aureus (MRSA) is a common cause of healthcare-associated infections across the globe.1–4 Many hospitals screen for MRSA colonization on admission as a key infection prevention strategy.5–11 Active MRSA surveillance combined with implementation of barrier precautions with or without decolonization protocols have been associated with reduced MRSA transmission in investigations conducted in high prevalence settings.11–15
Universal screening of all admitted patients for MRSA has been suggested as a means to prevent MRSA transmission by identifying and isolating MRSA carriers.6,16,17 However, such an approach can be resource intense and may pose practical challenges.18,19 An alternative to universal screening is to test for MRSA among populations at highest risk for colonization. In the United States, nine states have passed legislation mandating MRSA screening for high risk patients being admitted to the hospital, particularly those admitted to intensive care units (ICUs).20 Unfortunately, current laws have disparate definitions of “high-risk” patients. For example, California has defined specific patient groups for active surveillance, while Illinois mandated testing for all ICU admissions and “other at risk patients.” 21,22
Published medical literature can help determine which patients are most likely to be MRSA colonized. However, data from individual investigations are derived from specific populations that may not be generalizable to other geographic locales and populations. To provide more generalizable estimates, we performed a systematic review of the literature and meta-analysis of factors associated with MRSA colonization in patients admitted to hospitals and ICUs. The population of interest for the review included adults admitted to the hospital or intensive care unit. The intervention studied was testing for MRSA within 48 hours of admission. The comparator pairs included patient-level and clinical characteristics. The outcome was MRSA colonization and studies included retrospective and prospective reports of hospital- or unit wide surveillance, excluding case-control studies.
Methods
Search Strategy
To find published manuscripts evaluating factors associated with MRSA colonization upon hospital and/or ICU admission, we performed a literature search of Medline from 1966 to January 2012 and of EMBASE from January 1980 to January 2012 using the following terms: [((((screening) OR swab) OR surveillance) AND (((Methicillin) OR Meticillin) OR Oxacillin)) AND ((((((hospital) OR intensive care) OR ICU) OR inpatient) OR ward) OR Unit)]. We limited results to English language and human subjects. In addition, we examined the bibliography of all identified articles to look for additional relevant references. Attempts were made to contact primary authors when primary data were not available.
Study Selection
Each abstract identified by the search criteria was examined (J.M., S.E., E.C.) using a quality tool designed to assess the validity of the individual studies, including selection and measurement bias.23 To avoid potential selection bias, retrospective and prospective reports of hospital or unit-wide surveillance that contained data on factors associated with MRSA colonization in adults at hospital or ICU admission were included. Investigations were not excluded if they did not specifically state their MRSA screening methodologies or anatomic sites of screening, but would have been excluded if they only reported non-standardized methods of microbiologic testing. To avoid selection bias, investigations conducted during outbreaks were excluded. In addition, studies that collected data from pediatric patients or screened patients >48 hours after hospital admission (or >48 hours after ICU admission for ICU admission studies) were excluded. Reports describing clinical infections, non-hospitalized patients, laboratory-based surveys, or review articles were excluded. The full-text article was reviewed if two reviewers determined the manuscript potentially contained relevant data. Discrepant recommendations underwent arbitration by a third reviewer. Reviewers were not permitted to evaluate any manuscript that they authored.
Data Extraction
Each manuscript underwent independent, blinded, double-data extraction by two reviewers (J.M., S.E., or E.C.) using a standardized instrument. Discrepancies in data extraction underwent arbitration by a third reviewer and consensus was obtained by verbal discussion. Descriptive data collected for each study included time period of investigation, country of investigation, and hospital type (tertiary care, community, teaching, or other). Reviewers categorized the study population sampled (e.g. ICU population, total hospital population, orthopedics, etc.). Compliance with MRSA screening protocols, MRSA diagnostic testing method, and method of body swabbing were also captured when available.
Data Analysis
Data on factors potentially associated with MRSA colonization were extracted from all manuscripts. Mantel-Haenszel methods were used to calculate pooled odds ratios, 95% confidence intervals, and p-value associated with each factor and MRSA colonization. Random effects (DerSimonian and Laird) were utilized to adjust standard errors.24 To ensure that the pooled results of all studies were not biased by the process of combining results from multiple investigations (i.e. Simpson’s paradox), we performed graphical analysis and comparative analysis of data from each individual study.25,26 The I2 was calculated for each factor to determine the level of heterogeneity among the investigations analyzed.
Results
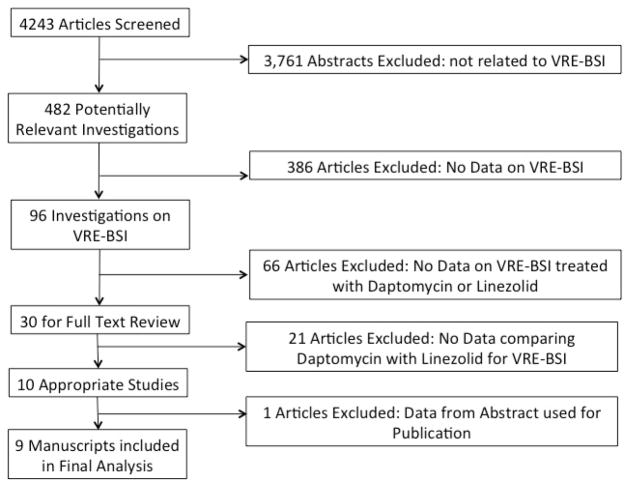
Our search criteria yielded 4,381 abstracts, of which we found 735 of potential interest and selected their manuscripts for full-text review. Abstracts were excluded from full text review because: limited to only clinical infections (n= 1,347), articles not pertinent to the subject matter (miscellaneous reasons) (n=718), laboratory based surveys (n= 658), pediatrics (n= 353), review articles (n= 205), outpatient investigations (n=192), or not about patients (n= 146) (Figure 1).
Figure 1.
Study Selection Process and Reasons for Exclusion
To ensure appropriate study quality the MOOSE criteria were applied to the 9 manuscripts included in the final analysis.
Review of the full-text manuscripts identified 24 investigations that had adequate data on factors associated with MRSA colonization. Manuscripts were excluded because screening did not occur at admission (n=344), the manuscript did not contain primary data on MRSA or was a review/opinion piece (n=328), the study was conducted in a long term care facility (n=13), screening occurred during an outbreak (n=10), the study was conducted in pediatric patients (n=9), and the study involved screening of healthcare workers only (n=7). (Figure 1) Bibliographic review of selected publications and expert opinion identified an additional 5 references for a total of 29 investigations included in the analysis.
Among the 29 investigations included in our analysis, 13 were conducted in Europe and 11 were conducted in North America. Other studies were conducted in Asia (n=4), and Australia (n=1).19,27–54 All studies were conducted between 1991 and 2009 and included a total of 76,913 patients. We identified 8 studies that focused solely on patients admitted to the ICU (Table 1).
Table 1.
Published manuscripts evaluating factors associated with MRSA colonization within 48 hours of hospital or ICU admission.
| Reference | Cohort | Location | Date of Study | Sample Size | Number of Patients MRSA+ (%) | Number of Patients MRSA− (%) |
|---|---|---|---|---|---|---|
| Mest 1994 | Patients admitted to a surgical intensive care unit | Long Beach, CA, USA | 1991–1992 | 484 | 19 (4%) | 465 (96%) |
| Troillet 1998 | Patients admitted to general medicine and select surgical services (vascular, podiatry & general surgery) | Boston, MA, USA | September 25– December 23, 1996 | 387 | 10 (3%) | 377 (97%) |
| Campillo 2001 | Patients with cirrhosis admitted to a chronic liver disease unit for more than 2 weeks | Paris, France | 1996–2000 | 748 | 125 (17%) | 623 (83%) |
| Eveillard 2002 | Patients admitted to a Geriatric Ward | Amiens, France | 2000 | 239 | 35 (15%) | 204 (85%) |
| Samad 2002 | Patients admitted to general surgery or orthopedic surgical service | Wales, UK | 2000–2001 | 430 | 23 (5%) | 407 (95%) |
| Lucet 2003 | Patients admitted to an intensive care unit | Paris, France | 1997–1997 | 746 | 53 (7%) | 693 (93%) |
| Ho 2003 | Patients admitted to an intensive care unit | Hong Kong, China | 1999 | 1,697 | 206 (12%) | 1,491 (88%) |
| Marshall 2003 | Patients admitted to an intensive care unit | Victoria, Australia | 2000–2001 | 1,185 | 80 (7%) | 1,105 (83%) |
| Corea 2003 | Patients admitted for routine surgery | Colombo, Sri Lanka | 1998–1999 | 269 | 20 (7%) | 249 (83%) |
| Merrer 2004 | Patients admitted with femoral neck fracture | Paris, France | 2000 | 179 | 15 (8%) | 164 (82%) |
| Fukuda 2004 | All inpatients | Hirado, Japan | 2000 | 136 | 12 (9%) | 124 (91%) |
| Lucet 2005 | Patients older than 75 years old admitted to the hospital | Paris, France | 2002 | 797 | 63 (8%) | 734 (92%) |
| Hidron 2005 | All admission on Tuesday, Thursday, and Sunday | Atlanta, GA, USA | 2003 | 726 | 53 (7%) | 673 (93%) |
| Sax 2005 | All inpatients, excluding former known MRSA carriers | Geneva, Switzerland | 2001, 2003 | 672 | 31 (5%) | 641 (95%) |
| Dupeyron 2006 | Patients admitted to the gastroenterology unit | Paris, France | 2000–2004 | 2,242 | 206 (9%) | 2,036 (91%) |
| Warren 2006 | Patients admitted to a surgical intensive care unit | St. Louis, MO, USA | 2002–2004 | 775 | 82 (11%) | 693 (89%) |
| Casas 2007 | All inpatients, excluding previously known MRSA colonized or infected patients, and obstetrics or pediatrics patients | Barcelona, Spain | 2001–2003 | 1128 | 17 (2%) | 1109 (98%) |
| Russell 2008 | All patients admitted to a liver transplant unit | Los Angeles, CA, USA | 2000–2005 | 706 | 47 (7%) | 659 (93%) |
| Riedel 2008 | All inpatients | Iowa City, IA, USA | 2006 | 421 | 43 (10%) | 378 (90%) |
| Chabernay 2008 | All inpatients | Hanover, Germany | 2005 | 509 | 27 (5%) | 482 (95%) |
| Baykam 2009 | All inpatients | Ankara, Turkey | 2005 | 900 | 11 (1%) | 889 (99%) |
| Nishikawa 2009 | All inpatients aged over 65 | Aichi, Japan | 2003 | 138 | 11 (8%) | 127 (82%) |
| Kock 2009 | All inpatients | Germany/Netherlands | 2006 | 21,190 | 354 (2%) | 20,836 (98%) |
| Niven 2009 | Patients admitted to an intensive care unit | Calgary, Canada | 2005–2006 | 1,308 | 50 (4%) | 1,258 |
| Keene 2010 | Patients with a risk factor for MRSA nasal colonization, excluding those with a S. aureus clinical isolate within 3 months | New York, NY, USA | 2007–2008 | 200 | 29 (15%) | 171 (85%) |
| Creamer 2010 | All inpatients | Dublin, Ireland | 2008–2009 | 489 | 115 (24%) | 374 (76%) |
| Honda 2010 | Patients admitted to an intensive care unit | St. Louis, MO, USA | 2002–2007 | 9,523 | 674 (7%) | 4,487 (93%) |
| Parvez 2010 | All inpatients | Temple, TX, USA | 2008 | 5,375 | 581 (11%) | 4,794 (89%) |
| Robiscek 2011 | All inpatients, excluding those with prior MRSA cultures | Chicago, IL, USA | 2007–2008 | 23,314 | 520 (2%) | 22,794 |
Factors Associated with MRSA Carriage at Hospital Admission
Among the 21 studies evaluating MRSA colonization at hospital admission, we found that MRSA colonization was associated with prior healthcare exposure such as history of hospitalization in the last 12 months (OR = 2.4, 95% CI 1.3–4.7, p<0.01, n=15 studies, 44,902 patients) and having been transferred from a nursing home (OR = 3.8, 95% CI 2.3–6.3, p,0.01, n=18 studies, 57,666 patients). (Table 2) Being transferred from an outside hospital was not associated MRSA colonization at screening (OR = 1.3, 95% CI 0.7–2.3, p=0.36, n=10 studies, 31,881 patients).
Table 2.
Meta-analysis of Risk Factors Associated with MRSA Colonization at Admission to the Hospital
| Variable | Manuscripts | Sample Size | Odds Ratio | Lower Limit | Upper Limit | P-value | I2 |
|---|---|---|---|---|---|---|---|
| Prior Healthcare Contact | |||||||
| Nursing home resident | 18 | 57,666 | 3.84 | 2.34 | 6.30 | <0.01 | 27.23 |
| Hospitalization in the past 12 Months | 15 | 44,902 | 2.43 | 1.26 | 4.70 | <0.01 | 0.00 |
| Transfer from outside hospital | 10 | 31,881 | 1.31 | 0.74 | 2.33 | 0.36 | 55.17 |
| Contact with Nosocomial Pathogens | |||||||
| History of MRSA carriage | 7 | 29,145 | 8.01 | 4.24 | 15.14 | <0.01 | 15.67 |
| -Carriage in past 6 Months | 2 | 5,936 | 14.42 | 10.98 | 18.93 | <0.01 | 0.00 |
| History of Clostridium difficile infection | 3 | 29,250 | 3.43 | 2.21 | 5.32 | <0.01 | 2.84 |
| Any infection in the prior 3 Months | 3 | 12,299 | 3.61 | 2.61 | 4.98 | <0.01 | 0.00 |
| Vancomycin-resistant enterococci carriage | 4 | 29,671 | 3.12 | 2.46 | 3.95 | <0.01 | 0.00 |
| Recent antibiotic use* | 14 | 31,429 | 3.33 | 2.42 | 4.56 | <0.01 | 50.15 |
| Type of Admission | |||||||
| Medical admission | 5 | 27,022 | 2.29 | 1.09 | 4.79 | 0.03 | 21.90 |
| Surgical admission | 9 | 36,863 | 1.22 | 0.77 | 1.93 | 0.41 | 27.37 |
| ICU admission | 4 | 29,377 | 1.05 | 0.60 | 1.82 | 0.87 | 34.85 |
| Comorbid Conditions | |||||||
| Skin lesion present | 5 | 25,707 | 2.63 | 1.02 | 6.77 | 0.05 | 0.00 |
| Wounds/bedsores present | 10 | 31,875 | 3.02 | 1.57 | 5.78 | <0.01 | 0.00 |
| Congestive heart failure | 3 | 29,250 | 2.31 | 1.94 | 2.74 | <0.01 | 0.00 |
| Diabetes | 9 | 38,669 | 2.30 | 1.56 | 3.40 | <0.01 | 0.00 |
| Chronic obstructive pulmonary disease | 4 | 30,150 | 2.37 | 1.77 | 3.16 | <0.01 | 0.00 |
| Chronic renal failure | 2 | 10,992 | 1.77 | 1.42 | 2.20 | <0.01 | 0.00 |
| Renal failure requiring dialysis | 7 | 52,494 | 1.50 | 1.20 | 1.88 | <0.01 | 0.00 |
| Immunosuppression | 5 | 30,664 | 1.45 | 1.15 | 1.84 | <0.01 | 16.26 |
| HIV | 3 | 29,201 | 2.19 | 0.96 | 4.96 | 0.06 | 0.00 |
| Transplant candidate | 3 | 6,642 | 0.95 | 0.66 | 1.36 | 0.76 | 0.00 |
| Malignancy | 2 | 5,936 | 0.85 | 0.65 | 1.13 | 0.27 | 0.00 |
| Cirrhosis | 4 | 6,274 | 0.99 | 0.73 | 1.33 | 0.92 | 7.11 |
| History of IV drug use | 3 | 8,478 | 1.16 | 0.73 | 1.85 | 0.53 | 8.64 |
| Presence of a Medical Device | |||||||
| Central venous catheter present | 6 | 51,586 | 1.72 | 0.70 | 4.23 | 0.23 | 0.00 |
| Urinary catheter present | 6 | 5,205 | 2.32 | 0.99 | 5.45 | 0.05 | 0.00 |
The I2 value for this investigation was 55.7, suggesting heterogeneity amongst studies on the association between recent antibiotic use and MRSA colonization.
Results of our meta-analysis of risk factors associated with MRSA colonization at admission to the hospital demonstrates that exposure to nosocomial pathogens and history of healthcare exposure were strongly associated with MRSA carriage, whereas comorbid conditions had a lesser association. Type of admission, ICU versus routine ward admission or medical versus surgical, had no clear association with MRSA carriage.
In addition to history of exposure to healthcare settings, MRSA colonization at hospital admission was associated with a history of infection or colonization with MRSA. Specifically, MRSA colonization was associated with both a history of a MRSA carriage in the last 6 months (OR = 14.4, 95% CI 11.0–18.9, p<0.01, n=2 studies, 5,936 patients) and a history of MRSA carriage at any time (OR = 8.0, 95% CI 4.2–15.1, p<0.01, n=7 studies, 29,145 patients).
Notably, MRSA colonization was associated with history of non-MRSA healthcare-associated infections. History of other exposure to healthcare-associated pathogens, including history of Clostridium difficile infection (OR = 3.4, 95% CI 2.2–5.3, p<0.01, n=3 studies, 29,250 patients) and vancomycin-resistant Enterococci spp. carriage (VRE) (OR = 3.1, 95% CI 2.4–4.0, p<0.01, n=4 studies, 29,671 patients) were also associated with MRSA colonization. Any infection, including community-onset infections, in the prior 3 months (OR = 3.6, 95% CI 2.6–5.0, p<0.01, n=3 studies, 12,299 patients) and recent antibiotic use (OR = 3.3, 95% CI 2.4–4.5, p<0.01, n= 14 studies, 31,429 patients) were also associated with MRSA colonization on admission. (Table 2)
Comorbidities associated with an increased likelihood of MRSA carriage at hospital admission included congestive heart failure, diabetes, chronic obstructive pulmonary disease (COPD), renal failure and immunosuppression (p<0.01 for all). MRSA colonization at admission screening was not associated with HIV infection, use of intravenous drugs, malignancy, or cirrhosis. (Table 2)
Four manuscripts examined the association between MRSA colonization at the time of hospital admission when admitted to an ICU as compared to a lower level of care. These investigations included data on 29,377 patients, including 2,469 admissions to the ICU. None of the individual papers found admission to an ICU to be associated with increased probability of MRSA colonization compared to routine ward level admissions. In the meta-analysis, ICU admission was not significantly associated with MRSA colonization (OR = 1.05, 95% CI 0.6–1.82, p=0.87).
Analysis of Risk Factors for MRSA carriage at ICU Admission
Data from papers limited to those assessing MRSA colonization upon ICU admission are summarized in Table 3. MRSA colonization at ICU admission was similarly associated with recent hospitalization (prior 12 months) (OR = 2.4, 95% CI 1.7–3.4, p<0.01, n=5 studies, 7,587 patients) and exposure to MRSA in the last 6 months (OR = 14.4, 95% CI 11.0–18.9, p<0.01, n=2 studies, 5,936 patients). We again noted an association of MRSA colonization with non-MRSA HAI; VRE carriage (OR = 3.3, 95% CI 2.4–4.5, p<0.01, n= 3 studies, 6,357 patients) and C. difficile infection (OR = 4.0, 95% CI 1.9–8.4, p<0.01, n=2 studies, 5,936 patients). In addition, similar comorbid conditions present on ICU admission were associated with MRSA colonization including congestive heart failure, COPD, diabetes, immunosuppression, and chronic renal failure (p<0.01 for all associations, see Table 3).
Table 3.
Meta-analysis of Risk Factors Associated with MRSA Colonization at Admission to the ICU
| Variable | Manuscripts | Sample Size | Odds Ratio | Lower Limit | Upper Limit | P-value | I2 |
|---|---|---|---|---|---|---|---|
| Prior Healthcare Contact | |||||||
| Hospitalization in the past 12 months | 5 | 7,587 | 2.38 | 1.69 | 3.36 | <0.01 | 15.76 |
| Nursing home | 6 | 8,333 | 2.62 | 0.74 | 9.25 | 0.14 | 0.00 |
| Transfer from Outside hospital | 4 | 8,430 | 1.08 | 0.73 | 1.59 | 0.70 | 31.24 |
| Contact with Nosocomial Pathogens | |||||||
| History of MRSA carriage | 3 | 6,357 | 12.83 | 8.51 | 19.33 | <0.01 | 10.75 |
| -Carriage in past 6 Months | 2 | 5,936 | 14.42 | 10.98 | 18.93 | <0.01 | 0.00 |
| History of Clostridium difficile infection | 2 | 5,936 | 3.98 | 1.89 | 8.37 | <0.01 | 0.00 |
| Any infection in the prior 3 Months | 3 | 12,299 | 3.61 | 2.61 | 4.98 | <0.01 | 0.00 |
| Vancomycin-resistant Enterococci infection | 3 | 6,357 | 3.27 | 2.37 | 4.52 | <0.01 | 0.00 |
| Recent antibiotic use | 6 | 5,568 | 2.84 | 2.10 | 3.84 | <0.01 | 0.00 |
| Type of Admission | |||||||
| Medical ICU admission | 3 | 3,219 | 3.21 | 2.29 | 4.49 | <0.01 | 0.00 |
| Surgical ICU admission | 5 | 10,618 | 1.38 | 0.69 | 2.73 | 0.36 | 0.00 |
| Comorbid Conditions | |||||||
| Congestive heart failure | 2 | 5,936 | 2.09 | 1.73 | 2.53 | <0.01 | 0.00 |
| Chronic obstructive pulmonary disease | 2 | 5,936 | 1.98 | 1.67 | 2.36 | <0.01 | 9.18 |
| Diabetes | 2 | 10,992 | 3.78 | 3.24 | 4.41 | <0.01 | 0.00 |
| Immunosuppression | 3 | 8,125 | 1.46 | 1.22 | 1.75 | <0.01 | 0.00 |
| Chronic renal failure | 2 | 10,992 | 1.77 | 1.42 | 2.2 | <0.01 | 0.00 |
| Renal failure requiring dialysis | 2 | 5,936 | 1.34 | 0.98 | 1.82 | 0.07 | 0.00 |
| Wounds/bedsores | 3 | 8,125 | 1.65 | 0.96 | 2.85 | 0.07 | 0.00 |
| HIV | 1 | 5,161 | 1.41 | 0.65 | 3.03 | 0.38 | - |
| Skin lesion | 3 | 3,653 | 2.02 | 0.70 | 1.30 | 0.20 | 0.94 |
| Cirrhosis | 2 | 5,936 | 0.99 | 0.74 | 1.34 | 0.97 | 0.00 |
| Transplant candidate | 3 | 6,642 | 0.95 | 0.66 | 1.36 | 0.76 | |
| IV drug | 1 | 5,161 | 0.95 | 0.78 | 1.16 | 0.60 | - |
| Malignancy | 2 | 5,936 | 0.85 | 0.65 | 1.13 | 0.27 | 0.00 |
| Presence of a Medical Device | |||||||
| Central Venous Line | 1 | 2,189 | 2.01 | 1.31 | 3.10 | <0.01 | - |
| Urinary catheter | 2 | 2,428 | 2.38 | 0.93 | 6.07 | 0.07 | 0.00 |
Results of our meta-analysis of risk factors associated with MRSA colonization at admission to the ICU demonstrates again that exposure to nosocomial pathogens and history of healthcare exposure were associated with MRSA carriage. Interestingly, type of ICU admission, medical versus surgical, did have an association with MRSA carriage whereas type of non-ICU admission did not. As with hospital admissions, comorbid conditions had a lesser association with MRSA carriage.
We found no association between MRSA colonization at ICU admission and nursing home residency (OR 2.6, 95% CI 0.7–9.2, p=0.14, n=6 studies, 8,333 patients), nor transfer from another hospital (OR = 1.1, 95% CI 0.7–1.6, p=0.70, n=4 studies, 8,430 patients).
Discussion
Our systematic review of the literature provides a robust analysis of the factors associated with MRSA colonization at the time of hospital and ICU admission. We reviewed over 4,000 abstracts to identify 29 manuscripts with data of sufficient quality to warrant analysis. The investigations included in this review incorporate more than 75,000 patient admissions from diverse medical centers worldwide.
Our data are important to help improve and refine the growing practice of screening for MRSA colonization at hospital admission. Screening for MRSA is increasingly performed as a matter of routine clinical care.7–9 Current data indicates that active surveillance combined with infection prevention and control measures may reduce MRSA transmission.11–15 Unfortunately, despite the promise of screening programs, MRSA testing consumes a large amount of personnel time and hospital financial resources. Balancing the potential benefit of screening against the cost of program administration has hindered the widespread adoption of MRSA screening.18,19
Some programs have adopted targeted MRSA screening protocols to optimize potential benefit, while limiting cost. Nine U.S. states have legislated mandates that hospitals must screen for MRSA at hospital admission. Many states target “high risk” hospital admissions, particularly patients admitted to intensive care units (ICUs).20 Unfortunately, definitions of “high risk” are not consistent. The state of California requires screening for patients from a skilled nursing facility, dialysis patients, pre-operative patients, ICU/Burn unit admission, and those discharged from an acute care hospital in the last 30 days. In contrast, the state of Illinois requires surveillance of all ICU admissions and “other at risk patients.”21,22 Our systematic review and meta-analysis provide data on specific populations and specific factors that are associated with MRSA colonization. Our data can provide guidance as to which populations could be selected for targeted MRSA screening and may suggest an opportunity to optimize patient selection through hospital based, clinical databases.55
Despite the rising community carriage of MRSA, our analysis found that factors indicative of prior healthcare contact were strongly and consistently associated with MRSA colonization. Patients with recent hospitalization and nursing home residence were more likely to be MRSA carriers, perhaps suggestive of exposure to high risk settings for MRSA acquisition. As hospital systems become increasingly electronic and able to readily signal readmission and prior discharge disposition to a healthcare facility, these data can and have been purposed for targeted MRSA screening protocols.56
Further supporting evidence that exposure to high risk healthcare settings is a strong predictor of MRSA colonization is its association with other healthcare pathogens, such as a history of Clostridium difficile infection or VRE carriage. Beyond high risk healthcare exposure, such pathogens may also be a proxy measure for underlying factors that increase acquisition risk, i.e.. antibiotic exposure which is thought to increase the risk of MRSA colonization through selective pressure.57,58 Data from our analysis supports the observation that recent antibiotic exposure was associated with MRSA colonization. While a history of VRE may obviate the need for screening since contact precautions are usually already applied, a history of VRE or C. difficile infection may be suggestive of the need for decolonization or other strategies that target a range of multi-drug resistant pathogens. With an increasing number of hospitals tracking a history of multi-drug resistant pathogens, an opportunity may exist to focus efforts on a high risk population at hospital admission.
The strong association of MRSA colonization with history of MRSA is well documented and supported by this analysis.59–61 In contrast to the above risk factors which may hone a target population for screening, this information may be used to prevent re-screening of patients who are unlikely to have lost carriage. This may also provide cost-savings.
Our review identified select comorbid conditions, such as diabetes, COPD, and congestive heart failure that were associated with MRSA colonization at hospital and ICU admission. Reasons for these associations may be repeated hospital exposure or other host related factors that increases the chance of acquiring MRSA. We report a trend toward an association between HIV infection and MRSA colonization, but this does not quite reach statistical significance. Other investigations have associated HIV infection with MRSA colonization.62–64 Prior publications have also suggested that patients with intravenous drug use or cirrhosis were at higher risk for MRSA carriage, but we did not find such an association in our review.65,66 These data may provide further opportunities to develop targeted screening protocols by linking screening to clinical pathways for the management of congestive heart failure or insulin dosing protocols for diabetic patients.
Interestingly, we did not find an increased likelihood of MRSA colonization among hospitalized patients being admitted to ICUs (compared to non-ICUs). Moreover, the four studies included in our meta-analysis comparing ICU admissions to routine ward admissions contained robust data from a range of geographic and clinical practice, including more than 2,000 ICU admissions and more than 25,000 hospital admissions. The investigations were conducted both in the United States (n=3) and Europe (n=1) and includes both tertiary and community hospitals (tertiary=4, community hospital 2, one investigation was a multi-site study). We note that only one of the four investigations, Robiscek et al, attempted to adjust for comorbid conditions or other factors associated with MRSA colonization, but this may have been expected to have increased rather than diminished an association with ICU admission.55
As evidence indicates a rising prevalence of MRSA colonization in the general U.S. community, it is plausible that MRSA prevalence in the non-ICU setting may be becoming similar to ICU populations.67,68 Regardless, while ICU patients may not be more likely to have MRSA colonization, the potential consequences of colonization or MRSA infection, may be more grave in ICU patients. Thus screening may be reasonable in this population for clinical, rather than epidemiologic reasons.
There are limitations to our investigation. First, despite the number of investigations included in our analysis and the robust sample size of many of the comparisons, our findings may not be generalizable to all practice settings. Many studies included in our analysis were done in academic medical centers, which may not reflect patient populations at other types of medical centers, and studies often did not control for the same factors. However, the heterogeneity among the studies was generally low for each factor. The only significant factor with a moderate I2 was recent antibiotic use at admission to the hospital. This may be due to the variable ways in which “recent” antibiotic use were determined. Additionally, our data are focused on identifying MRSA colonization and do not consider the impact of MRSA infections on the patient populations who may be screened. The grave consequence of MRSA infection for critically ill or immunocompromised populations may justify screening, regardless of a low colonization probability, especially if a history of MRSA would broaden empiric antibiotic regimens to include MRSA. In addition, screening may be justified in patients with extensive or infected wounds because they may present a high risk for transmission to others. Finally, data from our review focused on nasal MRSA colonization. We found no systematic data on risk of extra-nasal MRSA colonization (e.g., pharyngeal, inguinal) on admission. Extra-nasal colonization may be an important reservoir of MRSA and does not always correlate with nasal colonization.69,70
In summary, our systematic literature review and meta-analysis identifies patient characteristics that may enhance detection of MRSA colonization upon admission to the hospital. These results continue to support healthcare-associated exposures as the major source of MRSA, despite the fact that MRSA carriage is now common in the community. These data may help inform hospital policies on MRSA screening and enable electronic targeting of screening using electronic medical records. While a few academic centers have developed screening algorithms tailored to their specific patient populations71, these results may assist hospitals select screening criteria when resources for tailored algorithms are not available.
Figure 2.
Meta-Analysis Comparing Mortality in Patients treated with Linezolid versus Daptomycin for VRE-BSI
Graphical Presentation of results for overall mortality in patients treated with linezolid versus daptomycin for VRE-BSI. No weighting criteria were applied to the calculations. The overall trend is for improved survival with linezolid versus daptomycin (OR 1.3), but this is not statistically significant (p=0.053). Dapto-Daptomycin, LZD-Linezolid.
Acknowledgments
Financial Support:
The current project was supported by the Agency for Healthcare Research and Quality (AHRQ) grant number RC4AI092327. J.M.’s effort was also supported NIH/NCRR/NCATS Grant Number KL2TR000122 to the UCLA Clinical and Translational Science Institute. This manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ or NIH.
Footnotes
Potential Conflicts of Interest:
None of the authors have any conflicts of interest to disclose in relation to this manuscript.
References
- 1.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 2.Sader HS, Streit JM, Fritsche TR, Jones RN. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002–2004) Clin Microbiol Infect. 2006;12(9):844–852. doi: 10.1111/j.1469-0691.2006.01550.x. [DOI] [PubMed] [Google Scholar]
- 3.Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl VT, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis. 1994;13(1):50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]
- 4.Fluit AC, Wielders CL, Verhoef J, Schmitz FJ. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. J Clin Microbiol. 2001;39(10):3727–3732. doi: 10.1128/JCM.39.10.3727-3732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 6.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008;148(6):409–418. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 7.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 8.Coia JE, Duckworth GJ, Edwards DI, et al. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect. 2006;63 (Suppl 1):S1–44. doi: 10.1016/j.jhin.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Siegel JD, Rhinehart E, Jackson M, Chiarello L. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35(10 Suppl 2):S165–193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 11.Huang SS, Yokoe DS, Hinrichsen VL, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2006;43(8):971–978. doi: 10.1086/507636. [DOI] [PubMed] [Google Scholar]
- 12.West TE, Guerry C, Hiott M, Morrow N, Ward K, Salgado CD. Effect of targeted surveillance for control of methicillin-resistant Staphylococcus aureus in a community hospital system. Infect Control Hosp Epidemiol. 2006;27(3):233–238. doi: 10.1086/500372. [DOI] [PubMed] [Google Scholar]
- 13.Safdar N, Marx J, Meyer NA, Maki DG. Effectiveness of preemptive barrier precautions in controlling nosocomial colonization and infection by methicillin-resistant Staphylococcus aureus in a burn unit. Am J Infect Control. 2006;34(8):476–483. doi: 10.1016/j.ajic.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Lucet JC, Paoletti X, Lolom I, et al. Successful long-term program for controlling methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med. 2005;31(8):1051–1057. doi: 10.1007/s00134-005-2679-0. [DOI] [PubMed] [Google Scholar]
- 15.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37(6):1858–1865. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 16.Lee BY, Bailey RR, Smith KJ, et al. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infect Control Hosp Epidemiol. 2010;31(6):598–606. doi: 10.1086/652524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacek DM, Paule SM, Thomson RB, Jr, Robicsek A, Peterson LR. Implementation of a universal admission surveillance and decolonization program for methicillin-resistant staphylococcus aureus (MRSA) reduces the number of MRSA and total number of S. aureus isolates reported by the clinical laboratory. J Clin Microbiol. 2009;47(11):3749–3752. doi: 10.1128/JCM.01223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dancer SJ. Considering the introduction of universal MRSA screening. J Hosp Infect. 2008;69(4):315–320. doi: 10.1016/j.jhin.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Parvez N, Jinadatha C, Fader R, et al. Universal MRSA nasal surveillance: characterization of outcomes at a tertiary care center and implications for infection control. South Med J. 2010;103(11):1084–1091. doi: 10.1097/SMJ.0b013e3181f69235. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed 3/2/2012];Association for Professionals in Infection Control and Hospital Epidemiology. 2011 http://www.apic.org/Resource_/TinyMceFileManager/Advocacy-PDFs/MRSA_map.gif.
- 21.California Health and Safety Code Section 1255.8. September 25, 2008.
- 22.Illinois Compiled Statutes 210 ILCS 83, MRSA Screening and Reporting Act, Section 5. Augst 20, 2007.
- 23.Zaccai JH. How to assess epidemiological studies. Postgraduate medical journal. 2004;80(941):140–147. doi: 10.1136/pgmj.2003.012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedenreich CM. Methods for Pooled Analysis of Epidemiologic Studies. Epidemiology. 1993;4(4):295–302. doi: 10.1097/00001648-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Bickel PJ, Hammel EA, O’Connell JW. Sex bias in graduate admissions: data from berkeley. Science. 1975;187(4175):398–404. doi: 10.1126/science.187.4175.398. [DOI] [PubMed] [Google Scholar]
- 26.Pearl J. Causality: models, reasoning, and inference. Cambridge, U.K.; New York: Cambridge University Press; 2000. [Google Scholar]
- 27.Mest DR, Wong DH, Shimoda KJ, Mulligan ME, Wilson SE. Nasal colonization with methicillin-resistant Staphylococcus aureus on admission to the surgical intensive care unit increases the risk of infection. Anesth Analg. 1994;78(4):644–650. doi: 10.1213/00000539-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Troillet N, Carmeli Y, Samore MH, et al. Carriage of methicillin-resistant Staphylococcus aureus at hospital admission. Infect Control Hosp Epidemiol. 1998;19(3):181–185. doi: 10.1086/647791. [DOI] [PubMed] [Google Scholar]
- 29.Campillo B, Dupeyron C, Richardet JP. Epidemiology of hospital-acquired infections in cirrhotic patients: effect of carriage of methicillin-resistant Staphylococcus aureus and influence of previous antibiotic therapy and norfloxacin prophylaxis. Epidemiol Infect. 2001;127(3):443–450. doi: 10.1017/s0950268801006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eveillard M, Ernst C, Cuviller S, et al. Prevalence of methicillin-resistant Staphylococcus aureus carriage at the time of admission in two acute geriatric wards. J Hosp Infect. 2002;50(2):122–126. doi: 10.1053/jhin.2001.1152. [DOI] [PubMed] [Google Scholar]
- 31.Samad A, Banerjee D, Carbarns N, Ghosh S. Prevalence of methicillin-resistant Staphylococcus aureus colonization in surgical patients, on admission to a Welsh hospital. J Hosp Infect. 2002;51(1):43–46. doi: 10.1053/jhin.2002.1182. [DOI] [PubMed] [Google Scholar]
- 32.Corea E, de Silva T, Perera J. Methicillin-resistant Staphylococcus aureus: prevalence, incidence and risk factors associated with colonization in Sri Lanka. J Hosp Infect. 2003;55(2):145–148. doi: 10.1016/s0195-6701(03)00256-1. [DOI] [PubMed] [Google Scholar]
- 33.Ho PL. Carriage of methicillin-resistant Staphylococcus aureus, ceftazidime-resistant Gram-negative bacilli, and vancomycin-resistant enterococci before and after intensive care unit admission. Crit Care Med. 2003;31(4):1175–1182. doi: 10.1097/01.CCM.0000059437.01924.97. [DOI] [PubMed] [Google Scholar]
- 34.Lucet JC, Chevret S, Durand-Zaleski I, Chastang C, Regnier B. Prevalence and risk factors for carriage of methicillin-resistant Staphylococcus aureus at admission to the intensive care unit: results of a multicenter study. Arch Intern Med. 2003;163(2):181–188. doi: 10.1001/archinte.163.2.181. [DOI] [PubMed] [Google Scholar]
- 35.Marshall C, Harrington G, Wolfe R, Fairley CK, Wesselingh S, Spelman D. Acquisition of methicillin-resistant Staphylococcus aureus in a large intensive care unit. Infect Control Hosp Epidemiol. 2003;24(5):322–326. doi: 10.1086/502215. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda M, Tanaka H, Kajiwara Y, et al. High-risk populations for nasal carriage of methicillin-resistant Staphylococcus aureus. J Infect Chemother. 2004;10(3):189–191. doi: 10.1007/s10156-004-0318-2. [DOI] [PubMed] [Google Scholar]
- 37.Merrer J, Pisica-Donose G, Leneveu M, Pauthier F. Prevalence of methicillin-resistant Staphylococcus aureus nasal carriage among patients with femoral neck fractures: implication for antibiotic prophylaxis. Infect Control Hosp Epidemiol. 2004;25(6):515–517. doi: 10.1086/502432. [DOI] [PubMed] [Google Scholar]
- 38.Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41(2):159–166. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 39.Lucet JC, Grenet K, Armand-Lefevre L, et al. High prevalence of carriage of methicillin-resistant Staphylococcus aureus at hospital admission in elderly patients: implications for infection control strategies. Infect Control Hosp Epidemiol. 2005;26(2):121–126. doi: 10.1086/502514. [DOI] [PubMed] [Google Scholar]
- 40.Sax H, Harbarth S, Gavazzi G, et al. Prevalence and prediction of previously unknown MRSA carriage on admission to a geriatric hospital. Age Ageing. 2005;34(5):456–462. doi: 10.1093/ageing/afi135. [DOI] [PubMed] [Google Scholar]
- 41.Dupeyron C, Campillo B, Richardet JP, Soussy CJ. Long-term efficacy of mupirocin in the prevention of infections with meticillin-resistant Staphylococcus aureus in a gastroenterology unit. J Hosp Infect. 2006;63(4):385–392. doi: 10.1016/j.jhin.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Warren DK, Guth RM, Coopersmith CM, Merz LR, Zack JE, Fraser VJ. Epidemiology of methicillin-resistant Staphylococcus aureus colonization in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2006;27(10):1032–1040. doi: 10.1086/507919. [DOI] [PubMed] [Google Scholar]
- 43.Casas I, Sopena N, Esteve M, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus carriage at hospital admission. Infect Control Hosp Epidemiol. 2007;28(11):1314–1317. doi: 10.1086/520738. [DOI] [PubMed] [Google Scholar]
- 44.Chaberny IF, Bindseil A, Sohr D, Gastmeier P. A point-prevalence study for MRSA in a German university hospital to identify patients at risk and to evaluate an established admission screening procedure. Infection. 2008;36(6):526–532. doi: 10.1007/s15010-008-7436-1. [DOI] [PubMed] [Google Scholar]
- 45.Riedel S, Von Stein D, Richardson K, et al. Development of a prediction rule for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus carriage in a Veterans Affairs Medical Center population. Infect Control Hosp Epidemiol. 2008;29(10):969–971. doi: 10.1086/590662. [DOI] [PubMed] [Google Scholar]
- 46.Russell DL, Flood A, Zaroda TE, et al. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant. 2008;8(8):1737–1743. doi: 10.1111/j.1600-6143.2008.02304.x. [DOI] [PubMed] [Google Scholar]
- 47.Baykam N, Esener H, Ergonul O, et al. Methicillin-resistant Staphylococcus aureus on hospital admission in Turkey. Am J Infect Control. 2009;37(3):247–249. doi: 10.1016/j.ajic.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Kock R, Brakensiek L, Mellmann A, et al. Cross-border comparison of the admission prevalence and clonal structure of meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2009;71(4):320–326. doi: 10.1016/j.jhin.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Nishikawa M, Tanaka T, Nakashima K, et al. Screening for methicillin-resistant Staphylococcus aureus (MRSA) carriage on admission to a geriatric hospital. Arch Gerontol Geriatr. 2009;49(2):242–245. doi: 10.1016/j.archger.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Niven DJ, Laupland KB, Gregson DB, Church DL. Epidemiology of Staphylococcus aureus nasal colonization and influence on outcome in the critically ill. J Crit Care. 2009;24(4):583–589. doi: 10.1016/j.jcrc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Creamer E, Dolan A, Sherlock O, et al. The effect of rapid screening for methicillin-resistant Staphylococcus aureus (MRSA) on the identification and earlier isolation of MRSA-positive patients. Infect Control Hosp Epidemiol. 2010;31(4):374–381. doi: 10.1086/651093. [DOI] [PubMed] [Google Scholar]
- 52.Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol. 2010;31(6):584–591. doi: 10.1086/652530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keene A, Lemos-Filho L, Levi M, et al. The use of a critical care consult team to identify risk for methicillin-resistant Staphylococcus aureus infection and the potential for early intervention: a pilot study. Crit Care Med. 2010;38(1):109–113. doi: 10.1097/CCM.0b013e3181b42d03. [DOI] [PubMed] [Google Scholar]
- 54.Wright MO, Kharasch M, Beaumont JL, Peterson LR, Robicsek A. Reporting catheter-associated urinary tract infections: denominator matters. Infect Control Hosp Epidemiol. 2011;32(7):635–640. doi: 10.1086/660765. [DOI] [PubMed] [Google Scholar]
- 55.Robicsek A, Beaumont JL, Wright MO, Thomson RB, Jr, Kaul KL, Peterson LR. Electronic prediction rules for methicillin-resistant Staphylococcus aureus colonization. Infect Control Hosp Epidemiol. 2011;32(1):9–19. doi: 10.1086/657631. [DOI] [PubMed] [Google Scholar]
- 56.Control DoHEaI; Francisco UoCMCS, editor. Policy 4.6. 2011. Active Surveillance Testing (AST) for Methicillin-Resistant Staphylococcus aureus (MRSA) [Google Scholar]
- 57.Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. The Journal of antimicrobial chemotherapy. 2008;61(1):26–38. doi: 10.1093/jac/dkm416. [DOI] [PubMed] [Google Scholar]
- 58.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38 (Suppl 4):S341–345. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]
- 59.Robicsek A, Beaumont JL, Peterson LR. Duration of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2009;48(7):910–913. doi: 10.1086/597296. [DOI] [PubMed] [Google Scholar]
- 60.Scanvic A, Denic L, Gaillon S, Giry P, Andremont A, Lucet JC. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001;32(10):1393–1398. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 61.Larsson AK, Gustafsson E, Nilsson AC, Odenholt I, Ringberg H, Melander E. Duration of methicillin-resistant Staphylococcus aureus colonization after diagnosis: a four-year experience from southern Sweden. Scand J Infect Dis. 2011;43(6–7):456–462. doi: 10.3109/00365548.2011.562530. [DOI] [PubMed] [Google Scholar]
- 62.Popovich KJ, Hota B, Aroutcheva A, et al. Community-associated methicillin-resistant Staphylococcus aureus colonization burden in HIV-infected patients. Clin Infect Dis. 2013;56(8):1067–1074. doi: 10.1093/cid/cit010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shet A, Mathema B, Mediavilla JR, et al. Colonization and subsequent skin and soft tissue infection due to methicillin-resistant Staphylococcus aureus in a cohort of otherwise healthy adults infected with HIV type 1. J Infect Dis. 2009;200(1):88–93. doi: 10.1086/599315. [DOI] [PubMed] [Google Scholar]
- 64.Miller M, Cespedes C, Bhat M, Vavagiakis P, Klein RS, Lowy FD. Incidence and persistence of Staphylococcus aureus nasal colonization in a community sample of HIV-infected and -uninfected drug users. Clin Infect Dis. 2007;45(3):343–346. doi: 10.1086/519429. [DOI] [PubMed] [Google Scholar]
- 65.El-Sharif A, Ashour HM. Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) colonization and infection in intravenous and inhalational opiate drug abusers. Exp Biol Med (Maywood) 2008;233(7):874–880. doi: 10.3181/0711-RM-294. [DOI] [PubMed] [Google Scholar]
- 66.Chang FY, Singh N, Gayowski T, Wagener MM, Marino IR. Staphylococcus aureus nasal colonization in patients with cirrhosis: prospective assessment of association with infection. Infect Control Hosp Epidemiol. 1998;19(5):328–332. doi: 10.1086/647823. [DOI] [PubMed] [Google Scholar]
- 67.Kuehnert MJ, Kruszon-Moran D, Hill HA, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193(2):172–179. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 68.Gorwitz RJ, Kruszon-Moran D, McAllister SK, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197(9):1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 69.McKinnell JA, Huang SS, Eells SJ, Cui E, Miller LG. Quantifying the Impact of Extranasal Testing of Body Sites for Methicillin-Resistant Staphylococcus aureus Colonization at the Time of Hospital or Intensive Care Unit Admission. Infect Control Hosp Epidemiol. 2013;34(2) doi: 10.1086/669095. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matheson A, Christie P, Stari T, et al. Nasal swab screening for methicillin-resistant Staphylococcus aureus--how well does it perform? A cross-sectional study. Infect Control Hosp Epidemiol. 2012;33(8):803–808. doi: 10.1086/666639. [DOI] [PubMed] [Google Scholar]
- 71.Morgan DJ, Day HR, Furuno JP, et al. Improving efficiency in active surveillance for methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus at hospital admission. Infect Control Hosp Epidemiol. 2010;31(12):1230–1235. doi: 10.1086/657335. [DOI] [PMC free article] [PubMed] [Google Scholar]