Abstract
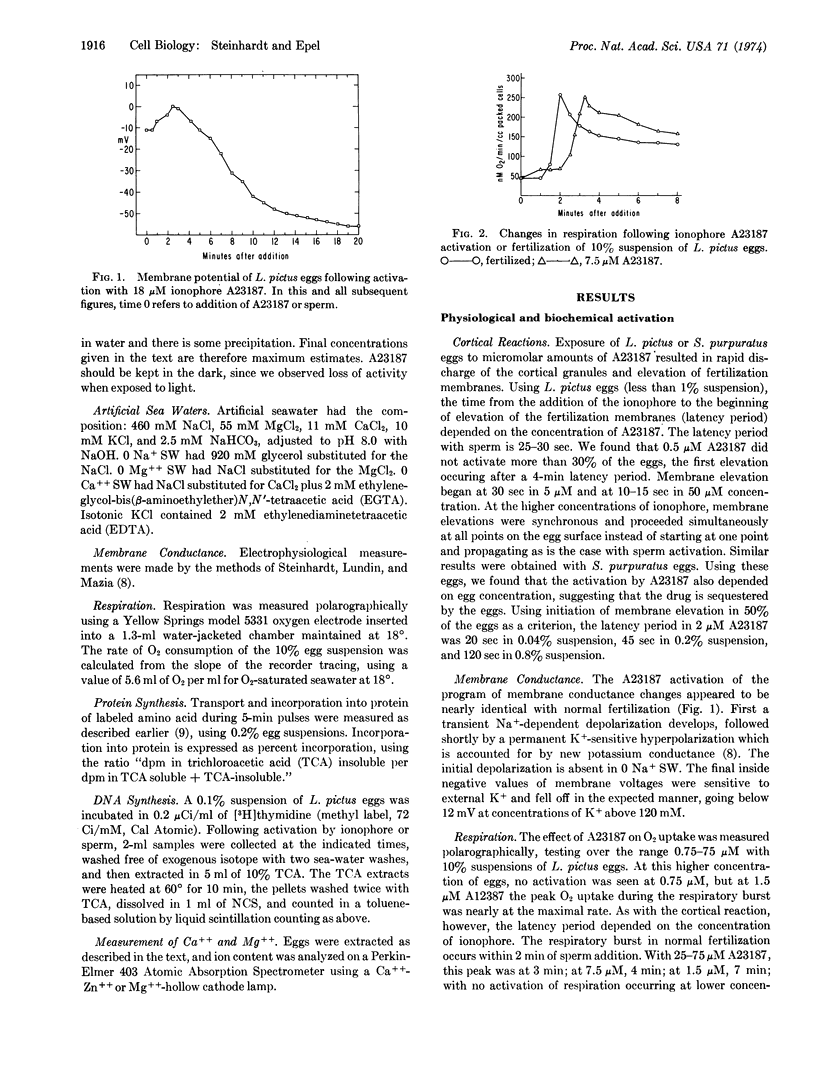
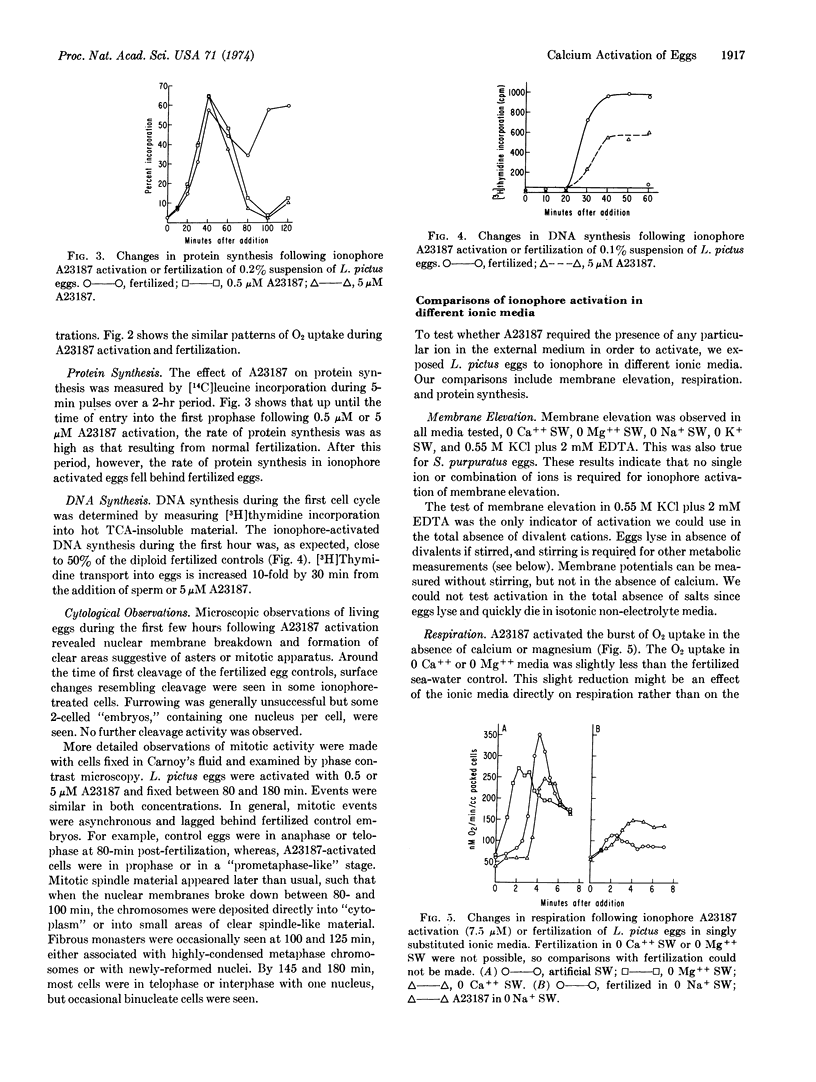
Micromolar amounts of the divalent ionophore A23187 can activate echinoderm eggs. The activations by ionophore A23187 were examined in terms of membrane elevation, the program of membrane conductance changes, the respiratory burst, and the increases in protein and DNA synthesis which normally accompany activation by sperm. In all these respects activation by the ionophore was fairly normal although subsequent cleavage and embryonic development was limited.
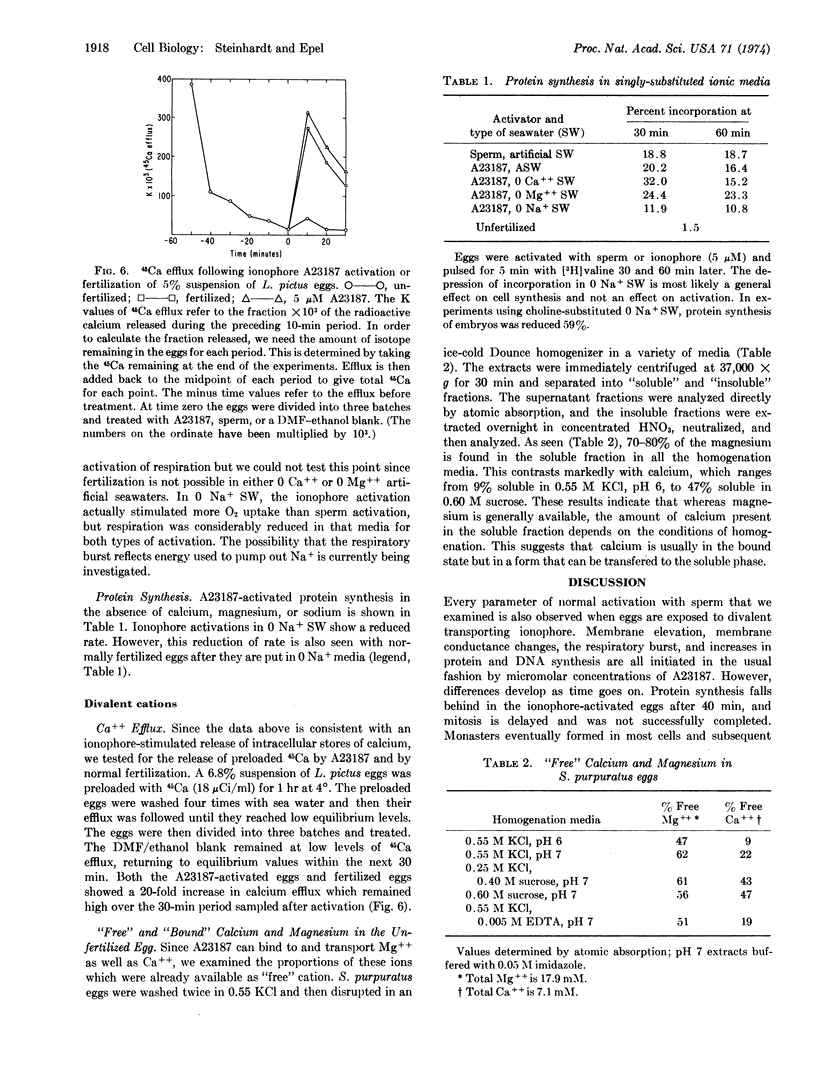
Ionophore A23187 activations of the cortex of Lytechinus pictus and Strongylocentrotus purpuratus eggs were compared in various ionic media and were found to be completely independent of the ionic composition of the external solution. Respiration and protein synthesis of L. pictus eggs in singly substituted ionic media also indicated that these activations were independent of external sodium, calcium, or magnesium. These results suggest that the ionophore acts by releasing intracellular Ca++. Consistent with this interpretation is the finding that eggs preloaded with 45Ca show a 20-fold increase in 45Ca-efflux when activated by ionophore A23187 or fertilization. Measurements of the “free” and “bound” calcium and magnesium in homogenates of the unfertilized eggs show that most of the Mg++ is already available in the soluble form, whereas Ca++ is sequestered but available for release.
We propose that both normal fertilization and ionophore activation affect the metabolism of the egg by releasing Ca++ sequestered in intracellular stores.
Keywords: fertilization, membrane conductance, respiration, protein synthesis, DNA synthesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clothier G., Timourian H. Calcium uptake and release by dividing sea urchin eggs. Exp Cell Res. 1972 Nov;75(1):105–110. doi: 10.1016/0014-4827(72)90525-3. [DOI] [PubMed] [Google Scholar]
- Epel D. Activation of an Na + -dependent amino acid transport system upon fertilization of sea urchin eggs. Exp Cell Res. 1972 May;72(1):74–89. doi: 10.1016/0014-4827(72)90569-1. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardy H. A., Graven S. N., Estrada S. Specific induction and inhibition of cation and anion transport in mitochondria. Fed Proc. 1967 Sep;26(5):1355–1360. [PubMed] [Google Scholar]
- Levy J. V., Cohen J. A., Inesi G. Contractile effects of a calcium ionophore. Nature. 1973 Apr 13;242(5398):461–463. doi: 10.1038/242461a0. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E., Strickland D. L. Ionophore A23187, calcium and contractility in frog eggs. Exp Cell Res. 1974 Jan;83(1):139–142. doi: 10.1016/0014-4827(74)90696-x. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Lundin L., Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ledebur-Villiger M. Cytology and nucleic acid synthesis of parthenogenetically activated sea urchin eggs. Exp Cell Res. 1972 May;72(1):285–308. doi: 10.1016/0014-4827(72)90591-5. [DOI] [PubMed] [Google Scholar]



