Abstract
Purpose
To determine if response to endocrine therapy of breast cancer can be predicted by either a metabolic “flare reaction” detected by positron emission tomography (PET) with 2-[18F]-fluoro-2-deoxyglucose (FDG), induced by an estradiol challenge, or by estrogen-receptor (ER) status, determined by PET with the estrogen analog 16α-[18F]fluoroestradiol-17β (FES).
Methods
Fifty-one post-menopausal women with advanced estrogen-receptor positive breast cancer were studied. Patients underwent FES-PET and FDG-PET at baseline and repeat FDG-PET after 30 mg estradiol. Tracer uptakes was measured as the standardized uptake value (SUV). Patients were subsequently treated with either an aromatase inhibitor or fulvestrant. A prospectively defined cut-off SUV ≥2 for FES was considered positive for ER expression. A cutoff of ≥12% increase in SUV for FDG, determined by ROC analysis, represented metabolic flare. PET results were correlated with responsiveness to endocrine therapy.
Results
Seventeen patients responded and 34 patients did not respond to endocrine therapy. Four responders and 1 non-responder had a clinical flare reaction, while only the responders demonstrated metabolic flare. After estradiol challenge, a significantly higher mean (±SD) percent change in SUV for FDG was noted in responders (20.9±24.2) compared with non-responders (−4.3±11.0, p<0.0001). On FES-PET, a higher tumor SUV was noted in responders (3.5±2.5) compared with non-responders (2.1±1.8, p=0.0049). There was significantly longer overall survival in patients with metabolic flare than in those without flare regardless of type of endocrine therapy (p=0.0062).
Conclusion
Baseline tumor FES uptake and metabolic flare after an estradiol challenge are both predictive of responsiveness to endocrine therapy in ER+ breast cancer.
Keywords: breast cancer, FDG, FES, flare reaction, hormone therapy, metabolic flare, PET, positron emission tomography
Introduction
Systemic treatment of metastatic breast cancer has been shown to provide palliation of symptoms and may also prolong survival. The majority of breast cancers express estrogen-receptors (ERs) and/or progesterone-receptor (PRs), and patients with such cancers are candidates for endocrine therapy. However, the presence of these hormone receptors predicts for clinical benefit in only 30–50% of women with advanced disease receiving first-line endocrine therapy and 15–30% receiving second-line therapy (1–3). Although breast tumor gene expression signatures with increasing predictive power are being developed, few data are available for their use in predicting endocrine sensitivity in advanced disease (4). Clearly, pathologic assessment of hormone-receptor status alone does not predict for hormone-dependent tumor growth.
We have utilized positron emission tomography (PET) for in vivo assessment of tumor hormone dependence in women with advanced breast cancer. Using the radiolabeled estrogen analog 16α-[18F]fluoroestradiol-17β (FES), we have assessed the functional status of tumor ERs in women with advanced breast cancer. In our experience, the tumor uptake on FES-PET correlates with quantitative ER levels measured in vitro, and may be more predictive of response to endocrine therapy than knowledge of the tumor ER status from analysis of tumor tissue by immunoassays or immunohistochemical assays (5–11).
The development of a “clinical flare reaction” after initiation of endocrine therapy may predict for response to that same endocrine therapy more accurately than does knowledge of the tumor ER status (11–15). Following initiation of endocrine treatment, the clinical picture in patients experiencing a flare suggests early disease progression and may be manifested by increased pain in areas of osseous metastasis or enlargement of soft tissue tumor foci. Often this is accompanied by increases in serum calcium, alkaline phosphatase and tumor markers, as well as apparent disease progression on bone scintigraphy (14, 15). Clinical flare reactions generally occur within two week after initiation of endocrine therapy and have been reported with both surgical ablative procedures and additive endocrine therapies (12, 16). The majority of women (65–90%), who experience a clinical flare reaction will subsequently experience clinical benefit when the endocrine therapy is continued (12, 13, 16). However, the clinical flare reaction is relatively infrequent, thus limiting its utility as a predictor of response. Moreover, it can be difficult to distinguish a “flare reaction” from disease progression clinically, such that potentially effective endocrine therapies may be discontinued because of concern about disease progression. Generally these patients are initiated on chemotherapy and considered “hormone resistant”. An accurate method for distinguishing ‘clinical flare reaction” from disease progression is therefore needed
We have previously shown that the metabolic correlates of a subclinical flare reaction caused by the initial agonist effects of tamoxifen can be documented by serial PET with FES or with 2-[18F]fluoro-2-deoxyglucose (FDG). Increased tumor FDG uptake 7–10 days after initiation of tamoxifen therapy predicts for clinical benefit more accurately than do either tumor FES uptake or ER status (9, 10). Because tamoxifen is a selective estrogen receptor modulator with a slow onset of action, estrogen agonist effects that peak 1–2 weeks after beginning therapy may contribute to the development of the “flare reaction”. Currently, aromatase inhibitors have largely replaced tamoxifen as first-line therapy for advanced disease because they produce a higher response rate and a longer duration of response than tamoxifen (17). We therefore sought to determine if we also could utilize the results of baseline FES-PET and serial FDG-PET as predictive biomarkers of response to agents other than tamoxifen. We hypothesized that the metabolic “flare reaction” induced by a brief challenge of estradiol, which is more potent, more agonistic, and more rapidly acting than tamoxifen,, would be similar to or greater than that we previously observed in our tamoxifen studies.
Materials and Methods
Patients
Women with locally advanced or metastatic breast cancer were eligible for participation in this study if they were postmenopausal, had measurable or evaluable estrogen-receptor-positive (ER+) tumors, had an ECOG performance status 0–2, and were to be treated either with an aromatase inhibitor or with the ER blocker/down regulator, fulvestrant. All patients had biopsy-proven ER+ cancer confirmed by immunohistochemical staining on the primary breast cancer or a recurrent or metastatic lesion. HER2 amplification was assessed by in situ hybridization (FISH) and was considered positive if the gene amplification was ≥ 2.0. The study was approved by the Institutional Review Board and the Radioactive Drug Research Committee of Washington University School of Medicine, as well as by the Protocol Review and Monitoring Committee of the Alvin J. Siteman Cancer Center. All patients gave written informed consent for study participation.
The pretreatment evaluation included a complete history and physical examination, complete blood count, liver function studies, and necessary radiological examinations such as computed tomography of the chest, abdomen and pelvis, and skeletal scintigraphy, as was medically indicated. After the initial evaluation and completion of pretherapy PET studies (see below), treatment with an aromatase-inhibitor (AI) or fulvestrant was instituted. The AI dosing was as follows: anastrozole, 1 mg/day; exemestane, 25 mg/day; and letrozole, 2.5 mg/day. The fulvestrant dose was 250 mg/month by intramuscular injection. Patients were re-examined approximately once a month (or as frequently as medically required) by the treating medical oncologist, who was blinded to the results of PET imaging. Selected radiologic imaging and blood tests were repeated as clinically indicated; typically repeated every 2 months (or as frequently as medically required) to assess response at sites of known disease.
PET Imaging and Estradiol Challenge
FES was prepared by a robotic adaptation of previously described methods (18). FDG was prepared based on a published procedure (19) with the PETrace FDG MicroLab synthesis module (General Electric Medical Systems, Uppsala, Sweden).
The overall imaging protocol required imaging on three separate days. Patients underwent FES-PET and FDG-PET at baseline, typically on two subsequent days. Whenever possible, baseline FDG imaging was performed prior to FES imaging so that the additional information gained from FDG imaging could be used to optimize patient positioning for the FES study. A repeat FDG-PET study was performed on another day after the estradiol challenge (see below). The majority of the PET examinations were performed with a Siemens ECAT HR+; 7 FDG and 6 FES studies were performed with an ECAT EXACT (Siemens/CTI PET Systems, Knoxville, TN). The performance specifications of these scanners have been reported (20–23) Emission images were reconstructed by filtered back projection with use of a Hanning filter (cutoff frequency=0.6 × Nyquist frequency) and were corrected for attenuation. Reconstructed spatial resolution under clinical imaging conditions was approximately 10 mm.
For FES-PET imaging at baseline, 6 mCi (222 MBq) of FES was injected intravenously. There was no significant difference in blood glucose levels measured at the time of the pre- and post-estradiol FDG studies, or in the duration of fasting, the dose of FDG, or the duration of the FDG uptake phase between the pre- and post-estradiol studies. FDG Beginning 90 min after the injection, PET imaging was performed (2-min segmented transmission and 15- to 30-min emission) at the level of the known tumor. In patients with multiple lesions, imaging was performed at up to 2 or 4 bed positions.
For FDG-PET, patients were required to fast for at least 4 hours before the study. A blood sample for glucose determination was obtained immediately prior to the injection of FDG; all except one patient with poorly-controlled diabetes mellitus had blood glucose ≤ 150 mg/dL at the time of both FDG-PET studies. There was no significant difference in blood glucose levels measured at the time of the pre- and post-estradiol FDG studies, or in the duration of fasting, the dose of FDG, or the duration of the FDG uptake phase between the pre- and post-estradiol FDG studies. Beginning 40–60 minutes after intravenous injection of 15 mCi (555 MBq) of FDG, PET imaging was performed (2-min segmented transmission and 5- to 10-min emission imaging) at the level of the known tumor. In each patient, the post estradiol FDG-PET study was performed similar to the pretherapy scan in regard to the FDG dose, uptake phase, duration of the fasting and medications. In patients with multiple lesions, imaging was performed at up to 5 or 6 bed positions.
The estradiol challenge was performed beginning on the day before the repeat FDG-PET study and consisted of 30 mg of estradiol, taken as three oral 10 mg doses. The first and second doses of estradiol were taken approximately 8–12 hours apart. The third dose was taken typically 1–3 hours before the expected time of injection of FDG. A 30-mg dose of estradiol was selected because this is the currently recommended dose for treatment of metastatic breast cancer. The rationale for performing the repeat FDG-PET study at the end of a one-day challenge with estradiol was based on in vitro and in vivo studies of breast cancer cell lines and rat uterus indicating that the effect of estrogen on glucose metabolism in ER+ tissues occurs rapidly (within 1 hr) (24, 25).
Response Criteria
Assessment of clinical response to endocrine therapy was performed by the treating oncologists who were blinded to the PET findings and was based on all clinical and radiological examinations in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) (26). Briefly, response was characterized by a 30% or greater decline in the average diameter of measurable tumor site(s), progressive disease by a 20% or greater increase in longest measurable tumor diameters, and stable disease for all others not meeting criteria for response or progression. For patients with osseous metastasis only, a complete response was defined as disappearance of all objective and clinical disease, including complete normalization of radiological studies and tumor markers. A partial response was defined as a decrease in pain with evidence of recalcification of known osseous lesions on radiography. Disease progression was defined as worsening of disease on bone scintigraphy or radiographs or worsening of pain and decline in performance status. Any response that did not meet the criteria for complete response, partial response, or progression was defined as stable disease. A clinical flare reaction was considered to have occurred if the patient developed worsening pain or an increase in cutaneous and soft tissue disease shortly after the estradiol challenge. The treating medical oncologists were ultimately responsible for determining if these signs and symptoms were more likely attributable to disease progression or to flare reaction. For analysis of the prediction of response based on PET findings, response was dichotomized as follows: patients with response or stable disease for at least 6 months were considered responders and those with progressive disease as non-responders.
Data Analysis
All PET images were evaluated semiquantitatively by determination of the standardized uptake values (SUVs) of tumor foci. The SUV is the ratio of the decay-corrected activity per unit volume of tissue (nCi/mL) to the administered activity per unit of body weight (nCi/g) (27). Maximum SUV was used for calculation of both FDG and FES tumor uptake. The absolute SUVs for FDG and FES and percent changes in SUVs for FDG were recorded. Baseline FDG-PET studies were reviewed prior to post estradiol FDG-PET to select metastatic lesion(s) for analysis. Baseline FDG-PET studies were reviewed prior to post estradiol FDG-PET to select metastatic lesion(s) for analysis. In patients with multiple lesions, the SUVs of up to seven lesions (typically, the most intense lesions) seen on PET images were determined and the overall average values for all selected lesions in a given patient were recorded; these overall average values were used in the comparisons of PET findings with clinical response. Based on our previous experience (9, 10), a cut-off SUV ≥ 2 for baseline FES was considered positive for functional ERs. In our previous experience with tamoxifen, we arbitrarily selected a ≥ 10% increase in FDG uptake as the criterion of metabolic flare (9, 10). Because of the expected greater agonistic effect of estradiol on tumor ERs, we performed a receiver-operating-characteristic (ROC) analysis on %SUV changes for FDG after estradiol therapy, to determine the appropriate threshold for metabolic flare.
Statistics
The absolute pretreatment SUVs for FES and FDG and the percent changes in SUVs for FDG after estradiol challenge were compared in responders and nonresponders with use of the Wilcoxon-Mann-Whitney test. The frequencies of metabolic flare and of baseline SUV ≥2.0 in responders and non-responders were compared by Fisher’s exact test. To determine the strongest predictor(s) of response, logistic regression analyses were performed with odds ratios computed for each unit increase in baseline SUVs, for each unit increase in absolute changes in SUVs, and for each percent change in SUVs. The overall patient survival was compared by baseline FES, percent change in FDG (metabolic flare) and clinical response using the Kaplan-Meier method.
Results
Patient Characteristics
Fifty-nine women were enrolled in this study between October 2001 and March 2005. Eight patients were excluded: 4 were noncompliant with the PET protocol, 1 declined endocrine therapy, and 3 had tumor deposits that were judged after baseline FDG imaging to be too small to be assessed reliably by PET (the remainder of the protocol was not performed in these three subjects).
The demographic characteristics of the 51 evaluable patients are summarized in Table 1. The median age was 59 years (range 35–83 years); 41 patients (82%) had metastatic disease and 10 (18%) had either locally advanced primary cancers (n = 5) or locally recurrent disease (n = 5) (Table 1). The receptor status was determined for the original locally advanced primary tumor or the histologically proven recurrent or metastatic lesion in 18 patients; in the remaining 33 patients, the receptor status of metastatic or recurrent disease was assumed to be the same as that of the original primary tumor. All tumors were ER+ and 38 were also progesterone-receptor positive (PgR+). HER2 was assessed in 45 patients and was amplified in 18 (Table 1). Although 11 patients (21%) were treatment-naïve, the majority had received various forms of prior therapy. Two had received prior chemotherapy alone. Fifteen had received prior endocrine therapy alone: 12 of these were treated initially with tamoxifen, and 5 of these were treated with other hormonal agents once they progressed on tamoxifen (3 with AIs, 1 with megestrol acetate, and 1 with an AI followed by fulvestrant) (Table 2). The remaining 3 who initially received endocrine therapy with agents other than tamoxifen included one each treated with an AI, megestrol acetate, and estradiol, respectively). In 23, there had been both prior chemotherapy and endocrine therapy: 21 of these had initially been treated with tamoxifen, and 9 of these 21 had been treated with other endocrine agents once they progressed on tamoxifen (6 with AIs, 1 with megestrol acetate, and 2 with fulvestrant), and the remaining 2 initially treated by other endocrine therapeutic agents had received an AI and fulvestrant, respectively (Table 2).
Table 1.
Patient Profile
| Number of patients | 51 |
| Median age | 59 yrs (range 35–83 yrs) |
| Caucasian | 42 |
| African American | 8 |
| Hispanic | 1 |
| Disease Status | |
| Metastatic disease | 41 |
| Locally advanced/chest wall recurrence | 10 |
| Receptor status | |
| ER+ | 51 |
| PgR+ | 38 |
| HER2+ | 18 |
Table 2.
Treatment Summary
| Prior Treatment | |
| None | 11 |
| Chemotherapy alone | 2 |
| Endocrine therapy alone | 15 |
| Tamoxifen | 7 |
| Tamoxifen, AI | 3 |
| Tamoxifen, megestrol acetate | 1 |
| Tamoxifen, AI, fulvestrant | 1 |
| AI | 1 |
| Megestrol acetate | 1 |
| Estradial | 1 |
| Chemotherapy and endocrine therapy | 23 |
| Chemotherapy, tamoxifen | 12 |
| Chemotherapy, tamoxifen, AI | 6 |
| Chemotherapy, tamoxifen, megestrol acetate | 1 |
| Chemotherapy, tamoxifen, fulvestrant | 2 |
| Chemotherapy, AI | 1 |
| Chemotherapy, fulvestrant | 1 |
| Endocrine Therapy during Study | |
| Anastrozole | 15 |
| Exemestane | 12 |
| Letrozole | 13 |
| Fulvestrant | 11 |
Different types of endocrine therapies were used during the period of observation for this study: estrogen ablation by the aromatase inhibitors anastrozole in 15, exemestane in 12, and letrozole in 13, and estrogen receptor blockade/downregulation by the pure antiestrogen, fulvestrant in 11.
Clinical Response
Seventeen patients (33%) experienced clinical benefit (responders), including 14 patients (27%) with an objective response to endocrine therapy (median duration of response 15.0 months) and 3 (6%) with stable disease. The remaining 34 patients (67%) were non-responders, experiencing disease progression during endocrine therapy (Table 3). The tumors were PgR+ in 15 (88%) of the responding patients and 23 (68%) of the non-responding patients (p = 0.18 by Fisher’s exact test). The lack of amplification of HER2 also did not predict response to endocrine therapy. The tumors in 7/15 (47%) of responding patients and 11/30 (37%) of non-responding patients were HER2+ (p = 0.55).
Table 3.
Clinical Response
| Number of patients | 51 |
| Responders | 17 (33%) |
| Objective response | 14 (27%) |
| Stable disease | 3 (6%) |
| Non-responders (disease progression) | 34 (67%) |
| PgR + status | |
| Responders | 15 (88%) |
| Non-responders | 23 (68%) |
| HER2+ | |
| Responders | 7/15 (47%) |
| Non-responders | 11/30 (37%) |
Imaging data were derived from 120 different sites of disease involving bone (n=58), pleura (n=1), chest wall and soft tissue (n=6), lymph node (n=28), lung (n=16) or breast (n=11).
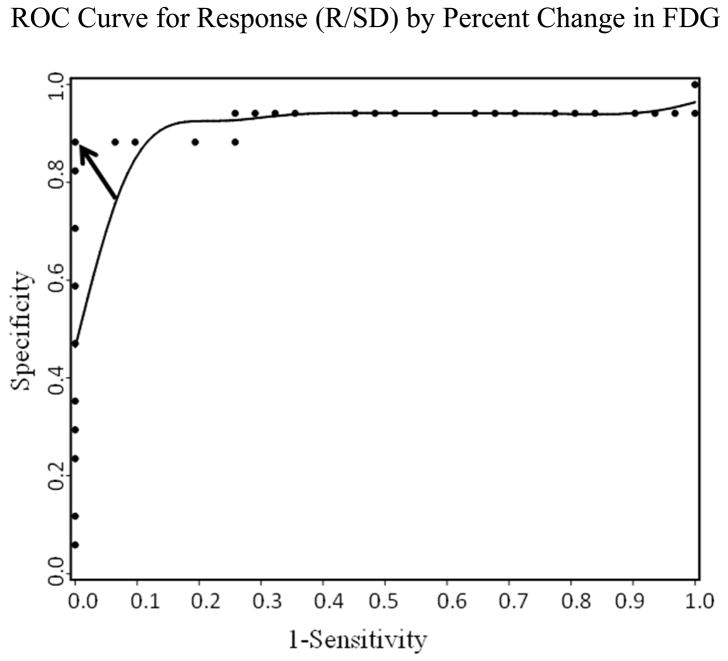
The ROC analysis for % SUV changes for FDG is shown in Figure 1. In this sample, a cutoff of 12% increase in FDG SUV represented the optimal tradeoff between sensitivity and specificity, providing sensitivity of 0.88 and specificity of 1.00. On FDG-PET, the tumors in responding patients had a significantly higher mean (± SD) percent change in SUV for FDG (20.9 ± 24.2) than did tumors in the non-responding patients −4.3 ± 11.0, p < 0.0001) (Table 4). Based on a ≥ 12% increase in FDG uptake after estradiol administration, metabolic flare was observed in 15 of the 17 responding patients (88%) and none of the 34 non-responding patients (p < 0.0001).
Figure 1.
The ROC analysis for % SUV changes for FDG demonstrated that a cutoff of 12% (arrow) increase in SUV for FDG shows the optimal tradeoff between sensitivity and specificity.
Table 4.
Summary of PET Findings
| Clinical Effect of Endocrine Therapy | P value | ||
|---|---|---|---|
| Response (N=17) | No Response (N=34) | ||
| Mean ± SD | Mean ± SD | ||
| FES Uptake | |||
| Pretreatment SUV | 3.5 ± 2.5 | 2.1 ± 1.8 | 0.0049 |
| FDG Uptake After Estradiol Challenge | |||
| Percent change in SUV | 20.9 ± 24.2 | −4.3± 11.0 | < 0.0001 |
SD = Standard deviation; SUV = standardized uptake value
On baseline FES imaging, tumors in the responding patients had a higher mean (± SD) SUV for FES (3.5 ± 2.5) than did tumors in the non-responding patients (2.1 ± 1.8, p= 0.0049) (Table 4). ROC analysis (not shown) confirmed that the FES cutoff of 2.0, defined by our previous study, was optimal for this sample, as well. Twelve of the 17 responding patients (71%) had tumor FES uptake with SUV ≥ 2, and 12 of the 34 non-responding patients (35%, specificity 0.65) had SUV ≥ 2 (p =0.036).
The PET results for individual responding and non-responding patients are summarized graphically in Figure 2. With an increase in tumor FDG uptake ≥ 12% as the criterion for defining estradiol-induced metabolic flare, the positive-predictive value (PPV) for response to endocrine therapy was 100% (all 15 of such patients responded) and the negative-predictive value (NPV) was 94% (2 of 36 such patients responded). The PPV and NPV for baseline FES uptake (using cutoff SUV of ≥ 2 as the criterion for defining functional ER) for response to endocrine therapy were 50% (12 of 24 such patients responded) and 81% (5 of 27 such patients responded), respectively. There were 16 patients with very low FES uptake (≤ 1.0); 15 did not respond to endocrine therapy and 1 had stable disease. There was no evidence for a difference in response rate between patients who were treated with AI and fulvestrant (p = 0.075). In addition, response rate in patients who were treated with only endocrine therapy previously was not significantly different than that for the patients who received prior chemotherapy with or without endocrine therapy (p = 0.69). However, the patients with no prior therapy were more likely to respond to endocrine therapy compared with patients who had received prior therapy (p = 0.0007).
Figure 2.
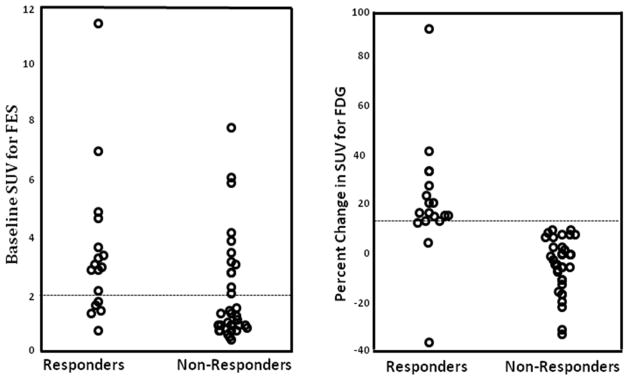
The baseline tumor FES (left) and percent change in tumor FDG (right) uptake after estradiol challenge in patients who responded and who did not respond to endocrine therapy.
Based on multivariable analysis, patients who responded to treatment had higher baseline FES than patients who did not respond. The odds of response increased by 40% (odds ratio 1.40) for each unit increase in baseline FES. The p-value associated with this effect is 0.0040. The odds of response increases by 16% (odds ratio 1.16) for each percent increase in FDG uptake. The p-value associated with this effect is 0.0011. Age, HER2 and PR receptor status, and prior chemotherapy added no information to multivariable models of response including FES or FDG SUV.
A clinical flare reaction following estradiol was suspected by the treating oncologist in only 5 patients; the disease responded to therapy in 4 of these patients (3 with osseous metastases and 1 with a chest wall recurrence) and progressed in 1 patient with osseous metastasis. All of these patients experienced worsening pain over the sites of tumor involvement within hours of initiation of estradiol. While clinical flare reactions predict for efficacy of endocrine therapy, they are rarely observed and thus of limited predictive value. In contrast, 17 women experienced an increase in tumor FDG uptake (metabolic flare) and 15 (88%) subsequently responded to therapy, suggesting that tumor FDG uptake is a more readily obtainable, and thus more practical, predictor of response.
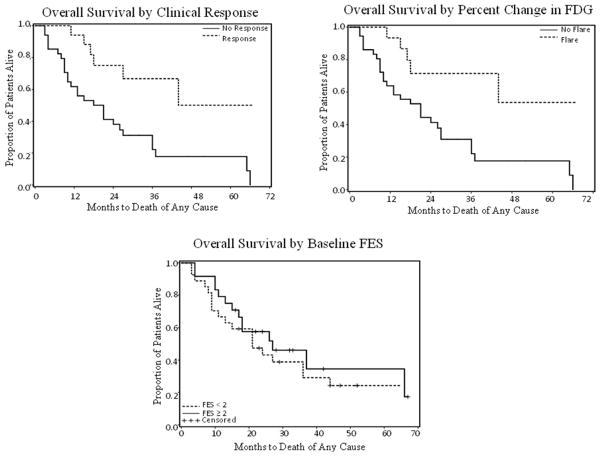
A Kaplan-Meier model of overall survival based on metabolic flare (increase in FDG uptake ≥ 12%) (Figure 3A) and clinical response (Figure 3B) showed a significantly longer survival in patients with metabolic flare than in those without flare regardless of type of endocrine therapy (p = 0.0062 and p = 0.0045, respectively) (Figure 3C), whereas baseline FES uptake was not predictive of survival (p = 0.23) (Figure 3). Figures 3A and 3B show that dichotomization of the patients by metabolic flare and by clinical response yielded virtually identical risk stratification.
Figure 3.
A Kaplan-Meier model of overall survival based on metabolic flare (increase in FDG uptake ≥ 12%) (A) and clinical response (B) and FES uptake (C) showed that a significantly longer survival was seen in patients with metabolic flare and clinical responders than in those without flare and clinically non-responders regardless of type of hormonal therapy (p = 0.0062 and p = 0.0045, respectively). However, baseline FES uptake was not predictive of survival (p = 0.23).
With a median follow-up of 21 months, the estimated median survival was 19.5 months in non-responding patients (95% confidence interval 10 – 27 months). The median survival has not been reached among responding patients.
Discussion
The results of the baseline FES-PET analysis provide current information about tumor expression of estrogen receptors in the advanced disease setting and, therefore, may predict for clinical benefit from endocrine therapy more accurately than pathologic assessment of receptor status (10, 28). Metabolic flare assessed by FDG-PET is a marker for the activation of estrogen signaling in the tumor, and implies that the ER is functional. We have previously shown that these PET findings are predictive of response to therapy with tamoxifen in hormone-naïve women with advanced ER+ breast cancer (9, 10).
To apply FDG-PET strategy for predicting response to other endocrine therapies, such as AIs and fulvestrant, which are rarely associated with clinical flare and presumably less likely to induce a metabolic flare, we hypothesized that a “metabolic flare reaction” induced by estradiol, which is a more potent, complete and rapidly acting estrogen than tamoxifen, might be as predictive as the tamoxifen-induced metabolic flare in our prior study. Indeed, estradiol might be expected to produce an earlier and more robust response. Synthetic and natural estrogens have been used to treat advanced post-menopausal breast cancer. Mechanistically, MCF-7 cells that have been deprived of estrogen will undergo apoptosis when estradiol is subsequently introduced (29). Whether apoptosis occurs after an initial agonist effect is unknown in this model.
Hormone challenge is an old concept in the management of breast cancer (30). In the 1920s, an “estrogen challenge” was used to determine the potential benefit of oophorectomy; in women with bone metastases, elevation of urinary calcium excretion after an estrogen challenge predicted response to subsequent oophorectomy. With development of methods to assay tumor ER and PgR levels, the estrogen challenge was abandoned as a predictor of response to subsequent oophorectomy. Obviously, the presence of hormone receptors does not indicate that the receptors will be functional nor that agents affecting estrogen levels will result in tumor cell kill (16, 31). By contrast, hormone flare reactions appear to be more predictive of responsiveness to endocrine therapy, because a flare suggests that the receptor is functional, although the utility of a clinical flare is limited because many responders do not experience a flare, and flare symptoms can resemble disease progression.
To investigate this, we studied patients with ER+ breast cancer who were going to be treated with an AI or fulvestrant. Nearly 78% of these patients had previously received systemic therapy with chemotherapeutic agents, endocrine therapeutic agents, or both. We found that metabolic flare following a 1-day estrogen challenge is highly predictive of subsequent response to endocrine therapy with AI and fulvestrant (PPV = 100% and NPV = 94%). While all patients with a ≥ 12% increase in FDG uptake after estrogen challenge responded to therapy, 12% of patients with a lesser increase in FDG uptake also responded to endocrine therapy. Patients who developed metabolic flare or who were subsequently indentified as responders based on clinical evaluation had a significantly longer survival than did those without flare, regardless of type of endocrine therapy (p = 0.0045) (Figure 3A and B), similar to that of patients who were subsequently indentified as responders based on clinical evaluation. Serial FDG-PET with an estradiol challenge, which can be accomplished in as few as two days before endocrine therapy is started, discriminates likely responders from nonresponders and allows for risk stratification equivalent to that obtained by much longer clinical observation alone.
We used the same cutoff value (SUV ≥ 2) for baseline FES uptake to identify patients with tumor ER expression as in our previous tamoxifen study. However, in our current patient population, SUV ≥ 2 was less successful (PPV = 50% and NPV = 81% vs. PPV = 79% and NPV = 88%) in predicting responsiveness to endocrine therapy with either AIs or fulvestrant. This difference between the current study and our previous tamoxifen study is likely related to the differences in the patient populations; previously, we included only hormone-naïve patients, while currently only 25% of patients were hormone naïve. This may have affected the ER bioavailability and/or affected ER interaction with growth factor-related pathways that determine tumor responsiveness to endocrine therapy. In recent years significant advances in the understanding of ER biology have revealed an increasingly complex process of ER signaling that includes interaction of ER with a multitude of cellular coregulator proteins, extensive post-translational modifications of these proteins, as well as an interdependence and interaction of estrogen signaling with other growth factor signaling pathways in the cancer cell (32). Knowledge of the existence of the crosstalk between ER and other growth factor signaling pathways may help us to better investigate the causes of endocrine resistance in patients with breast cancer.
In this study, pretreatment FES uptake was a weak predictor of response. Those with high FES were more likely to benefit from endocrine therapy, while an FES uptake with SUV ≤ 1 was highly predictive of non-responsiveness to endocrine therapy. Linden et al. (28) performed FES-PET in a group of patients with metastatic breast cancer, many of whom were already undergoing endocrine therapy, predominantly with AI agents, and similarly found that patients with absent FES uptake did not benefit from endocrine therapy. The association between qualitative FES-PET results and response was not significant (p = 0.14). They also found that quantitative FES uptake and response were significantly associated; none of 15 patients with tumor SUV < 1.5 responded to endocrine therapy, compared with 11 of 32 patients (34%) with SUV higher than 1.5 (p < .01) (27). Many of the patients in Linden’s study were begun on endocrine therapy before FES-PET. We previously performed serial FES-PET in women treated with tamoxifen and found that clinical benefit correlates with a decrease in FES uptake following therapy (10). Therefore, it is of concern that the endocrine therapy administered prior to FES imaging in Linden’s study may have produced falsely low FES uptake values.
Our study provides additional support for our hypothesis that metabolic flare on FDG-PET can be documented within 24 hours of treatment with an estrogen agonist and is predictive of benefit from endocrine therapy. By comparison, determining the effectiveness of endocrine therapy clinically may not be possible for several months after starting treatment. The abilities to predict clinical benefit from endocrine therapy expeditiously and to distinguish clinical flare reaction from disease progression have major health care implications. Objective confirmation of treatment efficacy allows patients to continue less-toxic endocrine therapies, thus delaying initiation of more costly and morbid cytotoxic therapy. Our findings suggest that a prospective multicenter trial is warranted to confirm the clinical value of the PET-based estradiol challenge for guiding systemic therapy in women with advanced breast cancer
Acknowledgments
This work was supported by Komen Foundation, AstraZeneca, Department of Energy DE FG02 84ER60218 and PHS R01 CA25836.
References
- 1.Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 2.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann M, Bajetta E, Dirix LY, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol. 2000;18:1399–1411. doi: 10.1200/JCO.2000.18.7.1399. [DOI] [PubMed] [Google Scholar]
- 4.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 5.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–48. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 6.McGuire AH, Dehdashti F, Siegel BA, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. J Nucl Med. 1991;32:1526–1531. [PubMed] [Google Scholar]
- 7.Dehdashti F, Mortimer JE, Siegel BA, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36:1766–1774. [PubMed] [Google Scholar]
- 8.Mortimer JE, Dehdashti F, Siegel BA, et al. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res. 1996;2:933–939. [PubMed] [Google Scholar]
- 9.Dehdashti F, Flanagan FL, Mortimer JE, et al. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51–56. doi: 10.1007/s002590050359. [DOI] [PubMed] [Google Scholar]
- 10.Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RE, Jessiman AG, Moore FD. Severe exacerbation of cancer of the breast after oophorectomy and adrenalectomy: report of four cases. N Engl J Med. 1958;258:312–317. doi: 10.1056/NEJM195802132580702. [DOI] [PubMed] [Google Scholar]
- 12.Plotkin D, Lechner JJ, Jung WE, et al. Tamoxifen flare in advanced breast cancer. Jama. 1978;240:2644–2646. [PubMed] [Google Scholar]
- 13.Vogel CL, Schoenfelder J, Shemano I, et al. Worsening bone scan in the evaluation of antitumor response during hormonal therapy of breast cancer. J Clin Oncol. 1995;13:1123–1128. doi: 10.1200/JCO.1995.13.5.1123. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RE, Mashiter G, Whitaker KB, et al. Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988;29:1354–1359. [PubMed] [Google Scholar]
- 15.Coleman RE, Whitaker KB, Moss DW, et al. Biochemical prediction of response of bone metastases to treatment. Br J Cancer. 1988;58:205–210. doi: 10.1038/bjc.1988.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legha SS, Powell K, Buzdar AU, et al. Tamoxifen-induced hypercalcemia in breast cancer. Cancer. 1981;47:2803–2806. doi: 10.1002/1097-0142(19810615)47:12<2803::aid-cncr2820471208>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881–894. doi: 10.1200/JCO.2001.19.3.881. [DOI] [PubMed] [Google Scholar]
- 18.Brodack JW, Kilbourn MR, Welch MJ, et al. Application of robotics to radiopharmaceutical preparation: controlled synthesis of fluorine-18 16 alpha-fluoroestradiol-17 beta. J Nucl Med. 1986;27:714–721. [PubMed] [Google Scholar]
- 19.Toorongian SA, Mulholland GK, Jewett DM, et al. Routine production of 2-deoxy-2-[18F]fluoro-D-glucose by direct nucleophilic exchange on a quaternary 4-aminopyridinium resin. Int J Rad Appl Instrum B. 1990;17:273–279. doi: 10.1016/0883-2897(90)90052-3. [DOI] [PubMed] [Google Scholar]
- 20.Wienhard K, Dahlbom M, Eriksson L, et al. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;18:110–118. [PubMed] [Google Scholar]
- 21.Wienhard K, Eriksson L, Grootoonk S, et al. Performance evaluation of the positron scanner ECAT EXACT. J Comput Assist Tomogr. 1992;16:804–813. doi: 10.1097/00004728-199209000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Adam LE, Zaers J, Ostertag H, et al. Performance evaluation of the whole-body PET scanner ECAT EXAT HR+ following the IEC standard. IEEE Transactions on Nuclear Science. 1997;44:1172–1179. [Google Scholar]
- 23.Xu M, Luk WK, Cutler PD, et al. Local threshold for segmented attenuation correction of PET imaging of the thorax. IEEE Transactions on Nuclear Science. 1994;41:1532–1537. [Google Scholar]
- 24.Furman E, Rushkin E, Margalit R, et al. Tamoxifen induced changes in MCF7 human breast cancer: in vitro and in vivo studies using nuclear magnetic resonance spectroscopy and imaging. J Steroid Biochem Mol Biol. 1992;43:189–195. doi: 10.1016/0960-0760(92)90207-y. [DOI] [PubMed] [Google Scholar]
- 25.Shinkarenko L, Kaye AM, Degani H. 13C NMR kinetic studies of the rapid stimulation of glucose metabolism by estrogen in immature rat uterus. NMR Biomed. 1994;7:209–217. doi: 10.1002/nbm.1940070503. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Kubota K, Matsuzawa T, Ito M, et al. Lung tumor imaging by positron emission tomography using C-11 L-methionine. J Nucl Med. 1985;26:37–42. [PubMed] [Google Scholar]
- 28.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 29.Jordan VC, Lewis JS, Osipo C, et al. The apoptotic action of estrogen following exhaustive antihormonal therapy: a new clinical treatment strategy. Breast. 2005;14:624–630. doi: 10.1016/j.breast.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Nathanson IT, Kelley RM. Hormonal treatment of cancer. N Engl J Med. 1952;246:135–145. doi: 10.1056/NEJM195201242460405. [DOI] [PubMed] [Google Scholar]
- 31.Kimmick G, Muss HB. Current status of endocrine therapy for metastatic breast cancer. Oncology (Williston Park) 1995;9:877–886. 889–890. discussion 892–874. [PubMed] [Google Scholar]
- 32.Schiff R, Massarweh S, Shou J, et al. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9:447S–454S. [PubMed] [Google Scholar]