Abstract
Regulatory T cells, lymphocytes marked by expression of the transcription factor Forkhead Box Protein P3 (FoxP3), inhibit the activation of tumor-specific T cells in tumor-draining lymph nodes. Immunohistochemical analyses of sentinel lymph nodes (SLNs) from 104 breast cancer patients showed a significant association (p = .0028, Pearson correlation) between the number of FoxP3+ cells and the size of primary breast invasive ductal carcinoma. In contrast, there was no correlation between the number of FoxP3+ cells and the presence of SLN metastases, or other clinicopathological parameters. These results suggest the presence of an immune suppressive environment in SLNs of larger tumors.
Keywords: Breast cancer, Immunology/Immunobiology, Tumor immunology
INTRODUCTION
The sentinel lymph node (SLN) is the first tumor-draining node within the lymphatic system draining the breast. The recognition that metastatic tumor cells are generally present in SLNs before they reach other nodes provided the rationale for SLN biopsy, a procedure that is routinely used in the surgical management of breast cancer (1, 2). In addition to metastatic cells, SLN is also directly exposed to soluble factors released by primary tumor, such as transforming growth factor β, vascular endothelial growth factor, and other cytokines with immunosuppressive function (3–5). Studies analyzing the immune cell phenotype of SLNs and non-SLNs in breast cancer patients have provided evidence in support of the hypothesis that the immune environment of SLN is conditioned by primary tumor, while the presence of metastasis can cause further changes (6–8). The changes most commonly observed in SLNs include decreased number and/or maturation of antigen-presenting cells (APC) and decreased function of effector T cells (6–10).
Regulatory T (Treg) cells are a subset of CD4+ T cells that play a critical role in suppressing anti-tumor immune responses, as they function to maintain immunological self-tolerance (11). Treg cells accumulate within tumors in both animal models and patients with various types of malignancies, including breast cancer (11–13). In experimental models, Treg cells have been shown to suppress immune responses by cell-to-cell contact with APC such as dendritic cells (DC) and effector T cells, and by production of immunosuppressive cytokines (11). Recent evidence indicates that Treg cells inhibit the activation of tumor-specific T cells in tumor-draining lymph nodes by controlling the number of DCs (14).
Expression of the transcription factor, Forkhead Box Protein P3 (FoxP3), is required for the development and function of Treg cells in mice and human (15, 16). Although expression of FoxP3 has been reported in human T cells following activation in vitro, the ability of this marker to accurately define Treg cells with immunosuppressive function in patients with cancer has been demonstrated recently (17, 18). Several immunohistochemical (IHC) studies using FoxP3 as a marker have shown that accumulation of Treg cells within primary tumor predicts a worse prognosis (13, 19–22).
An increase in Treg cells in draining lymph nodes of breast cancer patients has been previously reported, but it remains unclear whether it is secondary to the presence of metastatic tumor cells (6, 23, 24). If the number of Treg cells in tumor-draining lymph nodes is associated with specific characteristics of primary tumor in the breast, rather than metastatic deposits in the lymph node, it would indicate the existence of an immunological cross-talk between primary tumor and its draining lymph nodes with potential biological implications.
In order to address this question, we evaluated the number of FoxP3+ Treg cells in SLNs of breast cancer patients, and determined whether it correlates with clinicopathological characteristics.
MATERIALS AND METHODS
Tissue samples
Cases were selected by searching the surgical pathology database for patients with invasive breast cancer who underwent SLN biopsy from 2005 to 2007 at NYU Langone Medical Center. One hundred twenty-one cases with available archived tissue were randomly selected to represent different nodal status (negative, isolated tumor cells (ITC), micrometastasis, and macrometastasis). Among them, 10 cases were excluded because of insufficient material. Of the remaining 111 cases, seven were excluded from the analysis because of uneven intensity of immunostaining due to technical issues. Clinicopathologic information was obtained from pathology reports, including patient’s age, tumor type, grade, and expression of hormone receptors and HER2/neu. The classification of tumors into ductal, lobular, and mixed type was based on morphological criteria. Pathological stage designation was based on criteria from the American Joint Committee on Cancer (AJCC) 7th edition Cancer Staging Manual. Estrogen receptor (ER) and progesterone receptor (PR) were evaluated by IHC performed on a Ventana Benchmark XT using clones SP1 (Labvision) and 1E2 (Ventana), respectively. ER and PR status was considered positive if ≥1% of cancer cells stained positive, based on recent American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) recommendations (25). Overexpression of HER2/neu was determined by the HercepTest™ (DAKO) and confirmed by fluorescence in situ hybridization (FISH) in equivocal cases. The research study was approved by the New York University Institutional Review Board. Waivers of informed consent and authorization were obtained.
Quantification of FoxP3-positive lymphocytes in SLN
Immunohistochemistry for FoxP3 was performed on formalin-fixed, paraffin-embedded lymph node tissues using mouse anti-human FoxP3, 236A/E7 clone (eBiosciences, San Diego, CA, USA). In brief, sections were deparaffinized in xylene, rehydrated through graded alcohols, and rinsed in distilled water. Heat-induced epitope retrieval was performed in 10-mM citrate buffer pH 6.0 for 20 min in a 1200-watt microwave oven at 90% power. Antibody incubations and detection were carried out at 37°C on a NEXes instrument (Ventana Medical Systems Tucson, AZ) using Ventana’s reagent buffer and detection kits. FoxP3 antibody was diluted 1:100 in phosphate buffered saline, applied, and allowed to incubate overnight at room temperature. Primary antibody was detected using a biotinylated goat anti-mouse followed by application of streptavidin–horseradish–peroxidase conjugate. The complex was visualized with 3,3 diaminobenzidene. Slides were washed in distilled water, counterstained with hematoxylin, and dehydrated and mounted with permanent media. Appropriate positive and negative controls were included with study sections.
In order to determine the number of FoxP3+ lymphocytes, IHC images of SLN were captured at 20× magnification using Olympus BX40 microscope and DP11 digital imaging system (Olympus, Melville, NY) as JPEG files. Photomicrographs were taken from interfollicular areas with a uniform distribution of FOXP3+ lymphocytes. In SLNs with metastasis the number of FoxP3+ cells was evaluated in areas of the node away from the tumor. FoxP3+ cell numbers were calculated for each sample as the mean of three 20× fields using a semi-automated system based on modification of a previously described procedure (26). The system was initially validated by comparison to manual counts obtained by two pathologists in a group of 30 cases. In brief, a three-step procedure was used to quantify the intensity of staining, using a modification of utility within Kodak Molecular Analyst software (Ver 5.0, New Haven, CT) coupled with an initial image processing step carried out in Adobe Photoshop. First, a group of three JPEG images at 20× magnification were processed to extract the stained tissue color following a procedure detailed by Matlowsky et al. (27). Second, the Photoshop image file was imported into the Kodak Molecular Analyst (ver. 2.0) program. The threshold value for the manual regions of interest (ROI) was set in order to fully encompass the perimeter of each cell and was constant for all images. Then the “density slice method” algorithm available within the Kodak Molecular Analyst software was employed for the quantification of pixel density in the ROI. Third, the “summation of intensity values” from the Kodak Molecular Analyst density table was exported and calculated using Microsoft Excel software (Microsoft Corp, Redman, Washington).
Statistical methods
The exact Mann–Whitney test was used to assess differences in the FoxP3+ lymphocyte number among subject groups defined by tumor type, tumor grade, HER2/neu status, or presence/absence of lymphovascular invasion (LVI). Pearson and Spearman rank correlations were used to evaluate the association of FoxP3+ cell numbers with each of the following: age, tumor size, and percentage of cancer cells positive for ER and PR. Least squares regression was used to assess the dependence of FoxP3+ cell numbers on specific combination of factors such as tumor type and size. Binary and nominal logistic regression analyses were used to determine whether FoxP3+ cell number could be used alone or in combination with other factors as a predictor of lymph node status.
RESULTS
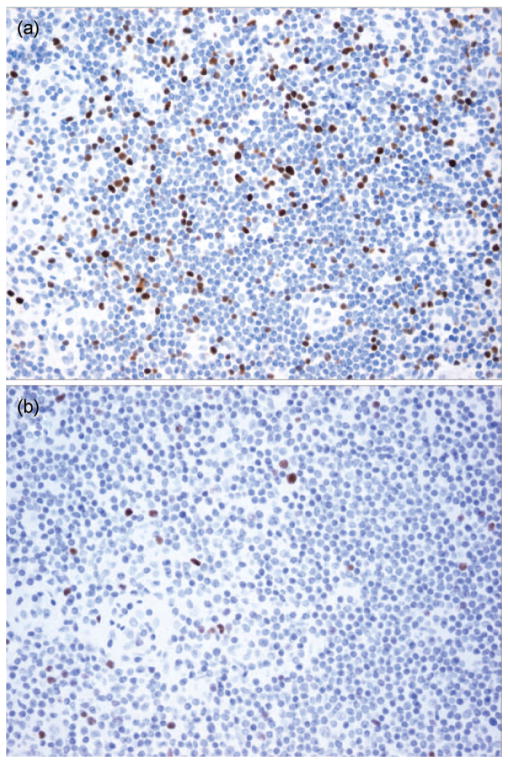
The clinicopathological characteristics of 104 breast cancer patients evaluated in the study are listed in Table 1. The majority of cancers were constituted by invasive ductal carcinoma (IDC; 82.7%) and small tumors (T1, 66%). Remaining histologies were invasive lobular carcinoma (ILC; 12.5%) and mixed, ductal, and lobular (4.8%). Approximately one-third of the patients had negative SLNs (31%) or macrometastases (31%), and the remainder had micrometastases (21%) or isolated tumor cells (ITC) (17%). IHC staining of SLN showed strong staining for FoxP3 in the nuclei of lymphocytes in the T cell areas of the lymph nodes, with variable density of positive cells in different samples (Figure 1). FoxP3+ lymphocytes were present in all samples, whereas no staining was detected in tumor cells present in SLN.
Table 1.
Patient, Tumor, and SLN characteristics
| Patients (N = 104) | Age | Mean 53 Years (Range 24 to 79 Years) |
|---|---|---|
| AJCC Stage* | IA N = 39 IB N = 15 IIA N = 26 IIB N = 17 IIIA N = 6 |
|
| Primary tumor | Histological tumor stage* | T1a N = 7 T1b N = 20 T1c N = 42 T2 N = 29 T3 N = 4 |
| Tumor type | Invasive ductal N = 86 Invasive lobular N = 13 Mixed N = 5 |
|
| Tumor grade | Well differentiated N = 18 Moderately differentiated N = 51 Poorly differentiated N = 35 |
|
| ER status* | Positive N = 86 Negative N = 16 |
|
| PR status* | Positive N = 77 Negative N = 25 |
|
| HER2/neu expression* | Over-expressed N = 22 Not over-expressed N = 80 |
|
| Lymphovascular invasion* | No N = 73 Yes N = 29 |
|
| SLN | Metastases | Negative N = 32 |
| Isolated tumor cells (≤0.2 mm) | ||
| N = 18 | ||
| Micrometastasis (≤2.0 mm) | ||
| N = 22 | ||
| Macrometastasis (≥2.0 mm) | ||
| N = 32 |
The information was not available for two cases.
Figure 1.
FoxP3 IHC staining of SLNs from breast cancer patients. FoxP3+ lymphocytes were identified by strong nuclear staining. Examples of cases with (a) high and (b) low numbers of FoxP3+ cells in the interfollicular T cell zones; magnification (×200).
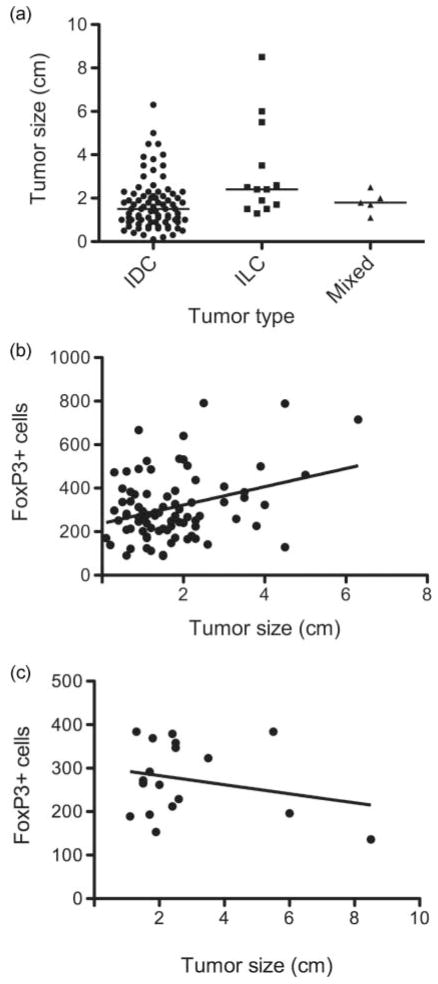
There were no significant differences in FoxP3+ cell numbers among subject groups defined by tumor type, tumor grade, HER2/neu status, lymphovascular invasion, and SLN status (exact Mann–Whitney test; Table 2). Likewise, there was no significant association between FoxP3+ cell numbers and patient age, or hormone receptor status (Pearson and Spearman rank correlation, Table 3). Regression analysis indicated a significant interaction between tumor size and tumor histology (p = .016). When stratified by tumor type, IDCs showed a significant association between FoxP3+ cell numbers and tumor size (r = 0.32, p = .0028, positive Pearson correlation), which was not observed for non-ductal invasive tumors (r = −0.24, p = .3307; Figure 2).
Table 2.
Correlation between number of FoxP3+ cells in SLN and pathological characteristics
| Parameter | FoxP3 Cell Number | p value |
|---|---|---|
| Tumor type | ||
| Ductal | 308.8 ± 151.9 | Ductal vs. lobular = NS |
| Lobular | 274.8 ± 86.1 | Ductal vs. mixed = NS |
| Mixed | 274.2 ± 86.6 | Lobular vs. mixed = NS |
| Tumor grade | ||
| Well differentiated | 314.2 ± 115.8 | Well vs. moderate = NS |
| Moderately differentiated | 301.5 ± 140.2 | Well vs. poor = NS |
| Poorly differentiated | 299.3 ± 161.3 | Moderate vs. poor = NS * Well vs. moderate + poor = NS |
| HER2/neu status | ||
| Not overexpressed | 298.2 ± 142.7 | NS |
| Overexpressed | 305.5 ± 144.0 | |
| Lymphovascular invasion | ||
| No | 302.6 ± 167.6 | |
| Yes | 304.4 ± 133.4 | NS |
| SLN status | ||
| Negative | 278.2 ± 107.2 | Neg vs. ITC = NS; ITC vs. micro = NS |
| Isolated tumor cells | 335.5 ± 178.2 | Neg vs. micro = NS; ITC vs. Macro = NS |
| Micrometastases | 296.1 ± 160.5 | Negative vs. macro = NS |
| Macrometastasis | 314.0 ± 141.1 | Micro vs. macro = NS Negative vs. ITC + Micro + Macro = NS |
Due to their similarity to each other and their relative dissimilarity with the well-differentiated tumors in terms of mean FoxP3+ cell number, the moderate and poor tumor grades were combined. Because of the low number of well-differentiated tumors, lack of significant difference may reflect low statistical power.
Table 3.
Association between FoxP3+ cell number and patient and tumor characteristics
| Parameter | Pearson Rank Correlation
|
Spearman Rank Correlation
|
||
|---|---|---|---|---|
| Correlation | p value | Correlation | p value | |
| Patients age | −0.031 | 0.7583 | 0.041 | 0.6799 |
| Tumor Size | ||||
| Total | 0.18 | 0.0778 | 0.12 | 0.2375 |
| IDC | 0.32 | 0.0028 | 0.14 | 0.1839 |
| Non-IDC | −0.24 | 0.3307 | −0.03 | 0.9027 |
| Percentage ER + tumor cells | 0.079 | 0.4304 | 0.096 | 0.3363 |
| Percentage PR + tumor cells | 0.13 | 0.2042 | 0.12 | 0.2177 |
Figure 2.
The number of FoxP3+ lymphocytes in SLNs correlates with the tumor size of invasive ductal carcinomas. (a) Comparison of the tumor size distribution between invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), and carcinomas with mixed features. Horizontal bars indicate the median in each group. (b) and (c) Scatter plots of FoxP3+ cell numbers versus tumor size for (b) ductal and (c) non-ductal (ILC and mixed) carcinomas. Regression lines shown are given by the equation: (b) predicted FoxP3 cell number = 237.9 + 42.14 × tumor size; (c) predicted FoxP3 cell number = 303.9 – 10.49 × tumor size.
The nominal and binary logistic regression analyses indicated that FoxP3+ cell numbers were not predictive of SLN status irrespective of whether or not other parameters were included in the prediction model. These analyses identified tumor size as the only predictor of SLN status in agreement with previous findings (28).
DISCUSSION
In this study we demonstrate that the number of FoxP3+ lymphocytes in SLNs of breast cancer patients increased in parallel with the size of the primary breast tumor when the latter was of ductal type. In contrast, for non-IDC tumors, mainly ILC, there was no association between tumor size and FoxP3+ cell numbers, suggestive of a different biology of ILC regarding interaction between tumor and host immunity. Although this observation needs to be confirmed in a larger cohort of patients, it is in concordance with genome expression analyses, demonstrating that ILC are molecularly distinct entities from ER+ IDC tumors (29, 30).
Few studies have investigated Treg cells in SLNs of breast cancer patients. Matsuura et al. (6) in a study of 58 patients reported higher expression of FoxP3 mRNA in metastasis-positive compared to metastasis-negative SLNs. In contrast, we did not find a significant difference in FoxP3+ lymphocyte numbers between metastasis-negative and metastasis-positive SLNs, irrespective of whether negative SLNs were compared to SLNs with ITC, micro- or macro-metastases. This discordance may be because of the method of FoxP3 detection; we employed IHC, which allows the simultaneous histological evaluation to ensure that FoxP3 is expressed by lymphocytes. Breast cancer cells have been recently reported to express FoxP3 (31, 32), raising the possibility that metastatic deposits in SLNs could contribute to the increased FoxP3 signal detected by RT-PCR. In another study, Mansfield et al. (24) reported a higher proportion of FoxP3+ cells, detected by IHC, in SLNs with metastases as compared with negative SLNs. The study population consisted of 11 node-negative and 36 node-positive patients with invasive cancer. However, the relationship between FoxP3+ cell numbers and tumor size was not investigated. As tumor size has been shown to correlate with the incidence of axillary lymph node metastases (28), it should be ruled out that the increased FoxP3+ cell numbers in SLNs with metastases were influenced by larger tumors in this group as compared to node-negative patients.
Recently, Nakamura et al. (23) analyzed 129 patients with node-negative breast cancer by IHC for FoxP3 and showed that patients with high FoxP3+ cell numbers (defined as ≥60/HPF) in SLNs had a shorter relapse-free survival compared with patients with low FoxP3+ cell numbers. Thus, in the absence of metastases, increased numbers of Treg cells in SLNs may have a negative influence on prognosis.
The correlation between SLN Treg cell numbers and primary tumor size but not presence of metastases in SLN suggests that soluble factors produced by the primary tumor are likely to be responsible for increase in Treg cells. Although no study, to our knowledge, has compared Treg cell numbers in SLN and axillary non-SLN, Matsuura et al. (6) have reported decreased maturation marker expression in DC present in SLN but not in non-SLN, suggesting that the immunological environment of SLNs, which are directly exposed to tumor-derived factors, is uniquely affected. Another study reported that changes in T cells and DC were detectable in non-SLNs, but were different from changes seen in SLNs (7). Therefore, there may be a gradient of alterations in lymph nodes (LN) draining the breast. It remains to be determined if the extent and nature of changes in LNs that are downstream to SLNs are independent from, or secondary to, the alterations in the immunological environment of SLN.
Accumulating evidence supports the critical role of Treg cells in suppressing the anti-tumor immune response in cancer patients (19). In breast cancer, Bates et al. (13) have shown an association between high FoxP3+ Treg cell numbers in the primary tumor and risk of relapse after 5 years. A recent study in which Treg cells were isolated from fresh breast cancer tissue confirmed their immune suppressive function (33). Together, these data suggest that FoxP3+ Treg cells contribute to an immune suppressive environment in breast cancer. Although the use of archived paraffin-embedded tissue in our study precludes functional studies, the immune suppressive function of the FoxP3+ Treg cells present in tumor-draining lymph nodes has been demonstrated in colorectal cancer patients (34), confirming that Treg cells present in this compartment are also functionally suppressive. It has become increasingly evident that T cell-mediated anti-tumor immunity plays an important role in the response of breast cancer patients to chemo- and radio-therapy, leading to improved disease-free and overall survival (35–39). Recent studies have investigated the lymphocytic infiltrate in primary breast tumors and its association with response to neoadjuvant chemotherapy (40, 41), but the composition of SLN has not been investigated in this context. Increased accumulation of Treg cells in SLN, which constitute the primary site of activation of anti-tumor T cells (5), might limit the efficacy of pre-operative or adjuvant therapies by inhibiting the activation of tumor-specific T cells (14). Strategies to deplete Treg cells in cancer patients are being tested in clinic (42–44), and should be evaluated in combination with chemotherapy and/or radiotherapy to improve the outcomes of breast cancer patients.
CONCLUSION
We show that the number of FoxP3+ Treg cells in SLNs of breast cancer patients correlates with the size of primary breast tumor but not with the presence of lymph node metastases. In addition, association between tumor size and the number of Treg cells is significant only for IDC, suggesting that the interaction between the tumor and the immune system may be different depending on the type of breast cancer.
Acknowledgments
We thank Ryan Pennell and the personnel of the NYU Cancer Institute’s Shared Resources Experimental Pathology and Clinical Laboratory Cores for expert assistance.
Footnotes
DECLARATION OF INTEREST
The authors report no conflict of interest. This work was supported by a grant from the Chemotherapy Foundation (to S. Demaria). NYU Cancer Institute Core facilities are supported by NIH/NCI 5P30CA016087-31.
References
- 1.Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, Feldman S, Kusminsky R, Gadd M, Kuhn J, Harlow S, Beitsch P. The sentinel node in breast cancer—a multicenter validation study. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Eladoumikdachi F. Optimal surgical treatment of breast cancer: implications for local control and survival. J Surg Oncol. 2010;101:677–686. doi: 10.1002/jso.21502. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 5.Kim R, Emi M, Tanabe K, Arihiro K. Immunobiology of the sentinel lymph node and its potential role for antitumour immunity. Lancet Oncol. 2006;7:1006–1016. doi: 10.1016/S1470-2045(06)70975-5. [DOI] [PubMed] [Google Scholar]
- 6.Matsuura K, Yamaguchi Y, Ueno H, Osaki A, Arihiro K, Toge T. Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer. 2006;106:1227–1236. doi: 10.1002/cncr.21729. [DOI] [PubMed] [Google Scholar]
- 7.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang RR, Wen DR, Guo J, Giuliano AE, Nguyen M, Offodile R, Stern S, Turner R, Cochran AJ. Selective modulation of paracortical dendritic cells and T-lymphocytes in breast cancer sentinel lymph nodes. Breast J. 2000;6:225–232. doi: 10.1046/j.1524-4741.2000.98114.x. [DOI] [PubMed] [Google Scholar]
- 9.Schüle J, Bergkvist L, Håkansson L, Gustafsson B, Håkansson A. Down-regulation of the CD3-zeta chain in sentinel node biopsies from breast cancer patients. Breast Cancer Res Treat. 2002;74:33–40. doi: 10.1023/a:1016009913699. [DOI] [PubMed] [Google Scholar]
- 10.Schüle JM, Bergkvist L, Håkansson L, Gustafsson B, Håkansson A. CD28 expression in sentinel node biopsies from breast cancer patients in comparison with CD3-zeta chain expression. J Transl Med. 2004;2:45. doi: 10.1186/1479-5876-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 12.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 13.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 14.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, Powell DJJ, Wunderlich JR, Merino MJ, Rosenberg SA. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, Redman B, Welling TH, Chang A, Zou W. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 19.Yakirevich E, Resnick MB. Regulatory T lymphocytes: pivotal components of the host antitumor response. J Clin Oncol. 2007;25:2506–2508. doi: 10.1200/JCO.2007.11.3191. [DOI] [PubMed] [Google Scholar]
- 20.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DHJ, Patz EFJ. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura R, Sakakibara M, Nagashima T, Sangai T, Arai M, Fujimori T, Takano S, Shida T, Nakatani Y, Miyazaki M. Accumulation of regulatory T cells in sentinel lymph nodes is a prognostic predictor in patients with node-negative breast cancer. Eur J Cancer. 2009;45:2123–2131. doi: 10.1016/j.ejca.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Mansfield AS, Heikkila PS, Vaara AT, von Smitten KA, Vakkila JM, Leidenius MH. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer. 2009;9:231. doi: 10.1186/1471-2407-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton AL, Eder JP, Pavlick AC, Clark JW, Liebes L, Garcia-Carbonero R, Chachoua A, Ryan DP, Soma V, Farrell K, Kinchla N, Boyden J, Yee H, Zeleniuch-Jacquotte A, Wright J, Elliott P, Adams J, Muggia FM. Proteasome inhibition with bortezomib (PS-341): a phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J Clin Oncol. 2005;23:6107–6116. doi: 10.1200/JCO.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 27.Matkowskyj KA, Schonfeld D, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem. 2000;48:303–312. doi: 10.1177/002215540004800216. [DOI] [PubMed] [Google Scholar]
- 28.Reger V, Beito G, Jolly PC. Factors affecting the incidence of lymph node metastases in small cancers of the breast. Am J Surg. 1989;157:501–502. doi: 10.1016/0002-9610(89)90645-4. [DOI] [PubMed] [Google Scholar]
- 29.Gruel N, Lucchesi C, Raynal V, Rodrigues MJ, Pierron G, Goudefroye R, Cottu P, Reyal F, Sastre-Garau X, Fourquet A, Delattre O, Vincent-Salomon A. Lobular invasive carcinoma of the breast is a molecular entity distinct from luminal invasive ductal carcinoma. Eur J Cancer. 2010;46:2399–2407. doi: 10.1016/j.ejca.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J, Srovnal J, Hajduch M, Murray P, Kolar Z. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, Liu JQ, Love R, Liu CG, Godfrey V, Shen R, Huang TH, Yang T, Park BK, Wang CY, Zheng P, Liu Y. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–1286. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Mènard S, Tagliabue E, Balsari A. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–1752. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 33.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Ménétrier-Caux C. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 34.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 36.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, André F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 37.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Génin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, André F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 38.Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams S, Chakravarthy AB, Donach M, Spicer D, Lymberis S, Singh B, Bauer JA, Hochman T, Goldberg JD, Muggia F, Schneider RJ, Pietenpol JA, Formenti SC. Preoperative concurrent paclitaxel radiation in locally advanced breast cancer: pathologic response correlates with five-year overall survival. Breast Cancer Res Treat. 2010;124:723–724. doi: 10.1007/s10549-010-1181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hornychova H, Melichar B, Tomsova M, Mergancova J, Urminska H, Ryska A. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest. 2008;26:1024–1031. doi: 10.1080/07357900802098165. [DOI] [PubMed] [Google Scholar]
- 41.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 42.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann NY Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 44.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, Jaffee EM. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]