Abstract
The treatment of osteoporotic women with bisphosphonates significantly reduces the incidence of bone fractures to a degree greater than can be explained by an increase in bone mineral density. In this Study, 18 month Fischer 344 rats were ovariectomized and treated with a single dose of risedronate (intravenous, iv, 500μg), zoledronic acid (iv, 100μg) or continuous raloxifene (2mg/kg, po., 3x/wk). High resolution microCT was used to measure lumbar vertebral bone microarchitecture, the degree of bone mineralization (DBM) and the distribution of mineral. Small angle x-ray scattering was used to investigate mineral crystallinity. We found prolonged estrogen deficiency, reduced trabecular bone volume, and increased micro architecture bone compression strength. lowered the degree of mineralization. Treatment with resorptive agents (bisphosphonates > raloxifene) prevented the loss of mineralization, trabecular bone volume and bone compression strength. Crystal size was not changed with OVX or with anti-resorptive treatments. In conclusion, in the aged estrogen deficient rat model, single intravenous doses of two bisphosphonates were effective in maintaining the compressive bone strength for 180 days by reducing bone turnover, and maintaining the DBM to a greater degree than with raloxifene.
Keywords: intravenous bisphosphonates, bone mineralization, compression strength, rat, OVX
INTRODUCTION
Bisphosphonates and selective estrogen receptor modulators, such as raloxifene, improve bone strength in animals and humans in estrogen deficient states in part by reducing bone turnover and by prolonging the secondary mineralization phase of bone remodeling which may improve the degree of bone mineralization (DBM) [1–4]. A number of randomized, controlled clinical trials have compared anti-resorptive agents to placebo medications and reported small increases (3–8%) in bone mineral density (BMD) of the lumbar spine. However, the changes in BMD explain only a fraction of the observed fracture risk reduction [5–7]. These observations suggest that anti-resorptive agents improve bone strength by decreasing bone turnover and by increasing intrinsic material properties of bone rather than simply affecting bone mass. Some of the intrinsic material properties of bone which relate to its fracture resistance include the degree and the homogeneity of mineral distribution, bone mineral composition and its crystallinity. The ratio of high-density bone/low-density bone had been reported to gradually increase with age [8]. In iliac crest bone biopsies from osteoporotic women, the degree of mineralization was decreased while the mineral crystal size and perfection was increased, as compared to the non-osteoporotic controls [9, 10]. Treatment of osteoporotic women with the bisphosphonate alendronate, was reported to increase the mean degree of mineralization and the homogeneity of the mineral distribution in the cancellous bone of compared to placebo treated subjects [9, 11]. Apart from affecting bone mineral distribution, bisphosphonates have non-hydrolysable P-C-P bonds with high affinities for the hydroxyapatite crystals (mineral); moreover, they have been reported to regulate crystal growth and dissolution [12]. In another study of osteoporotic women treated with either risedronate or placebo for three years, age-related increases in both mineral crystallinity and collagen cross-link ratios were reported, while risedronate prevented these changes [13]. Prevention of the age-related growth in crystallinity may be one of the mechanisms for bisphosphonates’ anti-resorptive effects. Therefore, bisphosphonates may have specific effects on the mineral crystals that may improve bone strength. In addition, the bisphosphonates may have different effects on the intrinsic material properties of the bone depending on the administration route, the dose and the duration of therapy.
In the present study, we used high-resolution imaging techniques to investigate the composition of bone matrix, bone microarchitecture, and the degree of bone mineralization in aged OVX rats treated with three anti-resorptive agents: a single intravenous dose of risedronate or zoledronic acid, and continuous dosing of the anti-resorptive agent raloxifene. The specific aim of this study was to evaluate if the changes in degree of bone mineralization affected bone strength independent of bone mass. In addition, we performed an exploratory analysis of the heterogeneity of mineral distribution using scanning probe microscopy with estrogen deficiency and anti-resorptive treatments.
MATERIAL AND METHODS
Animals and Experimental Procedures
Female Fischer 344 rats were obtained from the NIA (Bethesda, MD) and maintained on commercial rodent chow (22/5 Rodent Diet; Teklad, Madison, WI) available ad libitum with 0.95% calcium and 0.67% phosphate in a room that was maintained at 21°C with a 12-hour light/dark cycle. When the rats were 18 months of age, they were randomized into 5 experimental groups. Except for the sham-operated group animals, (Sham, n=16), all the other rats were ovariectomized and immediately treated with vehicle (normal saline 0.1ml/kg p.o., 3x/wk, n=16), or a single dose of risedronate (OVX+Ris, 500μg/kg, iv.; n=16), or a single dose of zoledronic acid (OVX+Zol, 100μg/kg, iv.; n=16) or continuous dosing of raloxifene (OVX+Ral, 1.5mg/kg p.o., 3x/wk, n=16) for 180 days. Serum and urine samples were collected every 30 days and stored at −80°C prior to the assessment of biochemical markers of bone turnover. Groups of animals (n=4) were sacrificed at days 30, 60, 120 and 180. Alizarin red (20mg/kg) and calcein (10mg/kg) were given subcutaneously at 14 and 4 days before sacrifice to assess bone formation surface. At the time of sacrifice, the right tibiae were placed in 10% phosphate-buffered formalin for 24 hours and then transferred to 70% ethanol for high resolution computed tomography (micro-CT), bone histomorphometry, scanning probe microscopy (SPM) and small angle x-ray scattering (SAXS). The 5th lumbar vertebral bodies (LVB) were scanned with micro-CT and then used for biomechanical testing. All animals were treated according to the USDA animal care guidelines with the approval of the UC Davis Committee on Animal Research.
Biochemical markers of bone turnover
Urinary levels of deoxypyridinoline cross-links and creatinine (DPD/Cr, Quidel, Mountain View, CA) and serum levels of osteocalcin (OC, Biomedical Technologies, Stoughton, MA) and TRAP5b (IDC Inc., Fountain Hills, AZ) were measured by ELISA. The manufacturer’s protocols were followed and all samples were assayed in duplicate. A standard curve was generated from each kit and the absolute concentrations were extrapolated from the standard curve. The coefficients of variations (CVs) for inter-assay and intra-assay measurements were less than 10% for all assays and were similar to the manufacturer’s references.
Bone histomorphometry
The right proximal tibial metaphyses (PTM) were dehydrated in ethanol, embedded un-decalcified in methylmethacrylate, and sectioned longitudinally with a Leica/Jung 2255 microtome at 4 and 8 μm thicknesses. Bone histomorphometry was performed using a semi-automatic image analysis Bioquant system (Bioquant Image Analysis Corporation, Nashville, TN) linked to a microscope equipped with transmitted and fluorescent light sources [14–16].
Bone histomorphometric analyses were performed in the secondary spongiosa of the right proximal tibiae that included the trabecular area between 0.1 to 3 mm distal to the growth plate and within the cortex. Bone turnover measurements included single- (sL.Pm) and double-labeled perimeter (dL.Pm), interlabel width (Ir.L.Wi) and osteoclast surface. These indices were used to calculate mineralizing surface (MS/BS) and mineral apposition rate (MAR), bone formation rate (BFR/BS) and percentage of osteoclast surface (OcS/BS), according to Parfitt et al. [17, 18]. We have reported similar methodology in other experiments in our laboratory [14–16, 19].
Micro-CT of trabecular bone microarchitecture and degree of bone mineralization
The 5th lumbar vertebral bodies (LVB) were scanned with MicroCT (VivaCT 40, Scanco Medical AG, Bassersdorf, Switzerland) at energy level of 70 kev and intensity of 85 μA with an isotropic resolution of 10.5 μm in all three spatial dimensions. Scanning for the PTM was initiated proximally at the level of growth plate and extended distally for 210 slices. Evaluations were performed on 100 slices beginning from about 0.2 mm distal to the growth plate. The entire LVB was scanned and the trabecular bone within the cranial and caudal growth plates and the cortex was evaluated. The gray-scale images were segmented using a constrained three-dimensional (3D) Gaussian filter (sigma=0.8, support=1.0, a fixed threshold of 240) to extract the structure of mineralized tissue. Since the scanner is self-calibrating before every scan, a fixed threshold provides the most reproducible result. Bone volume (BV) was calculated using tetrahedrons corresponding to the enclosed volume of the triangulated surface. Total volume was the volume of the sample that was examined. A normalized index, BV/TV, was utilized to compare samples of varying size. In order to minimize the partial volume effects, 2 surface voxels were discarded from every trabecula such that thick trabeculae (with 6 or more pixels) would be counted. The methods used for calculating connectivity density, trabecular number and trabecular thickness have been described previously [14, 15, 20]. In addition, the attenuation coefficient (cm−1) of each pixel was calculated and the histogram of bone material concentration was derived for each animal to calculate the mean degree of bone mineralization (DBM) and mineralization distribution curve. The g HA/ccm values were standardized with a manufacturer supplied phantom of 5 different HA densities embedded in soft-tissue equivalent resin. Beam hardening effects were corrected in the reconstruction process with a correction curve adapted to individual bone scans, as supplied by the manufacturer. The degree of mineralization was calculated to represent average mineralization (g/ccm) of the whole bone sample region used for architectural analyses [2, 3].
Small angle x-ray scattering of the mean crystal thickness
Small angle x-ray scattering (SAXS) data were collected with a Bruker Nanostar spectrometer using CuKa radiation (λ = 1.54Å). Trabecular bone samples from the distal femur were embedded in methylmethacrylate and sectioned to 100 μm. The samples were then oriented with the long axis of the bone (qx direction). The sample to camera distance was 104.65 mm and 2-D data were collected with a pressurized xenon gas detector (Bruker HiStar). The instrument was calibrated using a silver behenate standard. The sampled q range was 0.1 nm−1 < qx, qy < 2.1 nm−1. Two-D images were converted from the Bruker file format to 16-bit TIFF for subsequent analysis using the ImageJ software [21]. Analyses developed by Fratzl and co-workers [22] were used to calculate the average thickness and the average orientation of the crystallite along the long axis of the bone (0–100% where random orientation is 0%).
Biomechanical testing
Biomechanical testing of the 5th lumbar vertebrae (LVB) was performed using a uniaxial compression test. The relevant cross-sectional dimensions were measured using a digital caliper, after which they were subjected to unconfined compression tests along the long axis of the lumbar vertebra with a cross-head displacement rate of 0.01 mm/s. Specifically, the tests involved compression loading the samples to failure, while continuously recording the corresponding loads and displacements. Typically the lengths of the samples were twice as large as the diameters; consequently, buckling during the test was not a problem. Maximum load and cross-sectional dimensions were used to calculate the compressive strength [15, 16].
Exploratory Measurement of Elastic Modulus by Scanning Probe Microscopy
After taking three 4μm and one 8μm sections for bone histomorphometry, the remaining methylmetacrylate embedded proximal tibia blocks were further polished with different diamond pastes starting from 10 μm down to 0.1 μm diameter to obtain smooth surfaces. We used a force modulation technique called dynamic stiffness mapping (DSM) to quantitatively map dynamic nano-mechanical properties with nanometer scale resolution. The DSM is essentially the AFM which the optical head is replaced by a small transducer for force and displacement measurements [23]. Modulus maps in the form of Scanning Probe Microscopy (SPM) images were acquired using the direct force modulation operating mode of a TriboScope nanoindenter (Hysitron, Minneapolis, MN) mounted on a Multimode atomic force microscope (AFM) controlled by NanoScope IIIa electronics (Veeco, Santa Barbara, CA). In this experiment, an electrostatic operated transducer for simultaneous topographic and elastic modulus imaging replaced the conventional AFM head. The topographic contact mode was used first to locate the area of interest, usually 50×50 μm2. Subsequently the same area was scanned in force modulation mode to record the elastic modulus map. The amplitude of the modulated electrostatic force was set to 0.5 to 1.0 μN to maintain good signal-to-noise ratio, but was maintained sufficiently small to prevent plastic deformation of the sample. Voigt and Hertzian models were used to extract the elastic modulus map of 256 × 256 pixels from the amplitude and phase of displacements at each pixel [24]. One representative PTM was studied per group, 6 different areas (images) on each sample and each area (or image) has 65,536 Pixels. We obtained statistics on 393,216 points on each sample.
STATISTICAL ANALYSIS
The group means and standard deviations (SDs) were calculated for all outcome variables. Repeated measure analysis of variance was performed to analyze the changes in biochemical markers of bone turnover. The nonparametric outcome variables were tested with Kruskal-Wallis. The Wilcoxon rank sum test was then used for comparisons between groups (SPSS Version 12, SPSS Inc., Chicago, IL). Multivariate regression analyses were performed to determine the association of DBM and bone strength adjusting for BV/TV and Tb.Th, covariates. Differences were considered significant at p < 0.05.
RESULTS
A single intravenous dose of two amino bisphosphonates prevented trabecular bone loss for up to 180 days in aged estrogen-deficient rats
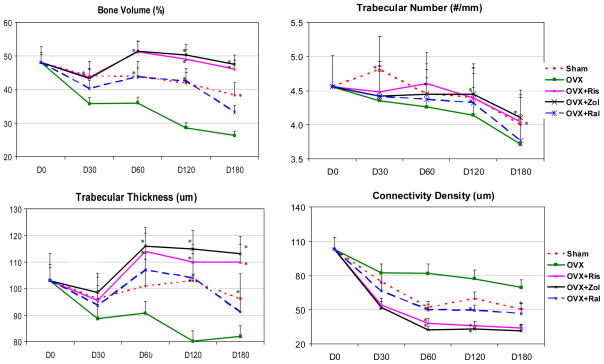
We used micro-CT to define the effects of a single intravenous dose of risedronate and zoledronic acid, as compared to long-term raloxifene treatment on bone micro-architecture. MicroCT analysis 180 days after ovariectomy (OVX) revealed a significant decrease in the lumbar vertebral trabecular bone volume of vehicle-treated OVX rats compared with sham-operated animals starting at day 30 (−18%) and was maximal at day 120 (−31%) (p < 0.05; Figure 1). OVX+Ris and OVX+Zol treatments prevented trabecular bone loss associated with OVX. At day 180, the bone volumes of the OVX+Ris and OVX+Zol groups were about 20% higher than sham-operated controls, with 70% more trabeculae than the OVX controls. In addition, trabeculae thickened by about 34% and connectivity density decreased by about 55% (all p < 0.05) in both bisphosphonate groups compared to the vehicle-treated OVX group. The OVX+Ral group trabecular bone volume was similar to the sham-operated animals from day 30 to day 180. Trabecular number, thickness and connectivity density were significantly greater than OVX at day 180 and similar to those of the sham animals.
Figure 1.
Ex-vivo micro-CT analyses of the lumbar vertebral bodies. Quantitative evaluations showed that ovariectomy (OVX) decreased bone volume, trabecular number and thickness from day 30 to day 180. Both risedronate and zoledronic acid completely prevent these changes, while raloxifene partially prevented the deterioration of trabecular bone volume, trabecular number and thickness following OVX. Lumbar vertebral connectivity increased with OVX due to perforation of the plate-like trabeculae while the anti-resorptive treatments prevented this change. Asterisks indicate significance versus OVX at the same time point at p < 0.05.
A single intravenous dose of risedronate and zoledronic acid inhibited bone turnover
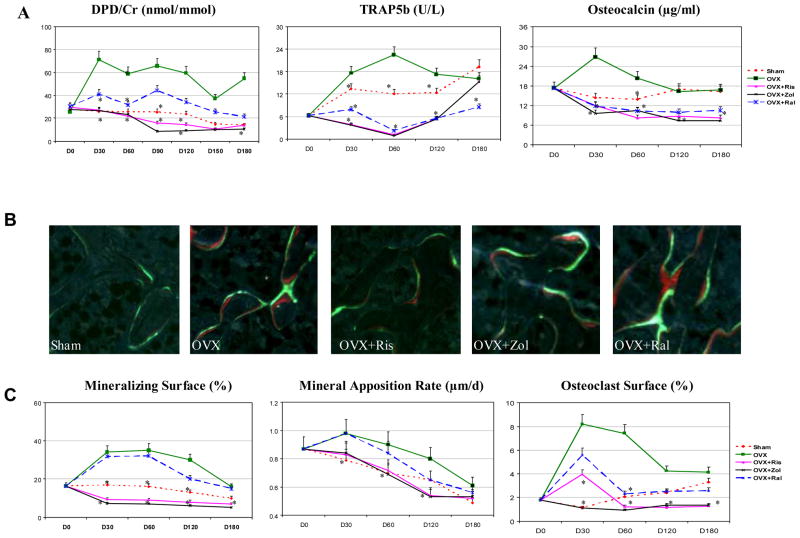
After a single intravenous dose of risedronate or zoledronic acid, elevations of urine DPD/Cr, serum TRAP5b, and serum osteocalcin (Figure 2A) at days 30–120 were completely prevented. Raloxifene partially prevented the elevations of these bone turnover markers following OVX. DPD/Cr remained suppressed by risedronate and zoledronic acid at day 180, while TRAP5b and osteocalcin returned to the sham-operated levels at day 180 (Figure 2A).
Figure 2.
Bone turnover evaluated by bone markers and bone histomorphometry. (A) The increases of marker of osteoclast maturation (TRAP5b), resorption marker (DPD/Cr) and the formation marker (osteocalcin) after OVX were suppressed by risedronate = zoledronic acid > raloxifene. (B) Un-decalcified sections taken from day 30 groups. OVX and OVX+Ral had similar bone forming surface (double labeling surface) while OVX+Ris and OVX+Zol had less double labeling surface. (C) Mineralizing surface, mineral apposition rate and osteoclast surface were elevated after OVX and were suppressed by risedronate = zoledronic acid > raloxifene. Asterisks indicate significance versus OVX at the same time point at p < 0.05.
Bone turnover measured by bone histomorphometric analysis of trabecular bone in the PTM confirmed the findings obtained with the bone markers. OVX caused a 110% increase in mineralized surface, a 24% increase in mineral apposition rate and a six fold increase in osteoclast surface (Figures 2B–C), as compared with the d30-sham group (p < 0.05). Raloxifene treatment of OVX rats had similar mineralized surface and mineral apposition rate at days 30–60 as the OVX rats; whereas OVX+Ris and OVX+Zol significantly lower bone formation parameters (p < 0.05) and suppressed osteoclast surface, as compared to OVX alone at days 30–180 (p < 0.05; Figure 2B).
A single intravenous dose of risedronate and zoledronic acid improved trabecular bone material and mechanical properties
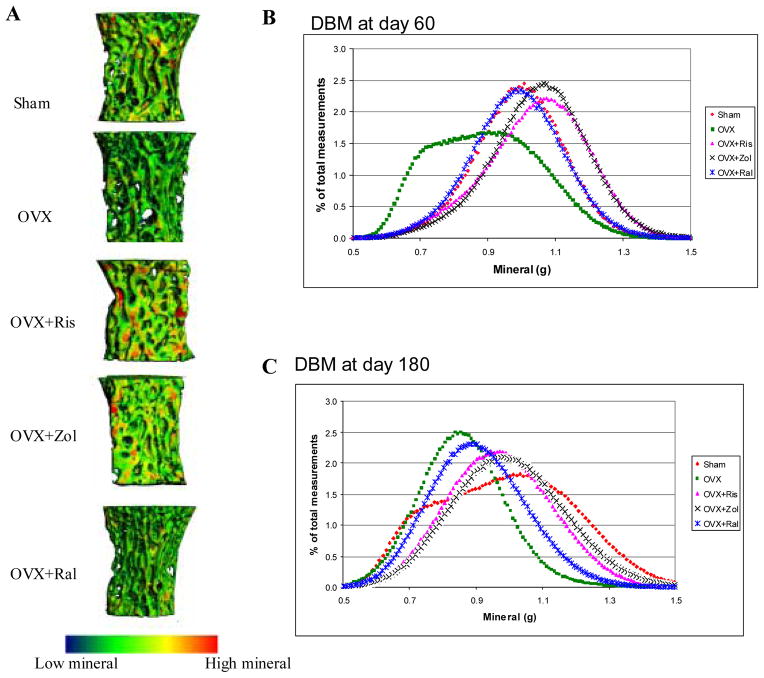
We next ascertained if a single intravenous dose of either risedronate or zoledronic acid affected bone material properties during estrogen deficiency in comparison to long-term raloxifene treatment. High-resolution micro-CTs of the lumbar vertebral bodies were used to determine the degree and distribution of bone mineralization (Figure 3). The overall mineral after 180 days of estrogen deficiency was significantly reduced, as compared to the sham-operated group. After 180 days of treatment, risedronate and zoledronic acid increased the overall mineralization (Figure 3A). The mean mineral density for OVX was about 0.8g HA/ccm. The mineralization distribution curves were shifted to the left with OVX at days 60 (Figures 3B) and 180 (Figures 3C), indicating that the OVX group had a higher percentage of bone with low mineral concentration. The OVX+Ris and OVX+Zol had similar distributions of mineralization, with mean mineral density values of 1–1.1g HA/ccm. Raloxifene treated rats have intermediate values between bisphosphonate treated and OVX from day 60 onward. We utilized scanning probe microscopy mapping to generate an elastic modulus map of the trabecular surface from one PTM specimen in each d180 treatment group. We observed a broad distribution in trabecular surface elastic modulus in the OVX alone and OVX+Ral groups compared to the OVX+Ris and OVX+ Zol and sham groups (data not shown).
Figure 3.
(A), Mineralized tissue distribution map of the 5th lumbar vertebral bodies at day180 for each experimental group generated by high-resolution microCT. Blue-green represents lower mineral while yellow-red represents higher mineral. Risedronate and zoledronic acid treatments had higher mineral content. (B, C), Distributions of bone mineralization at day 60 and day 180. The distribution of mineralization has shifted to the left with OVX, with lower values of DBM. Raloxifene had intermediate values for DBM distribution between bisphosphate treated and sham animals from day 60 until day 180. After risedronate or zoledronic acid treatment, the distribution curves shifted the distribution to the right, consisting of higher mineral at day 60. At day 180, OVX+Ris or Zol overlapped with those of sham at day 180.
We also evaluated the SAXS patterns of the crystals within the bone matrix to characterize the size and arrangement of the mineral particles (crystal) in the trabecular bone. We found the size of the mineral particles was not different between sham-operated, OVX and antiresorptive-treated groups at day 180 (Table 1). It appeared that single intravenous doses of bisphosphonates did not affect the size of the mineral crystals.
Table 1.
Mean crystal thickness at day 180 (nm)
| Treatment | Mean | SD |
|---|---|---|
| Sham | 1.852 | .113 |
| OVX | 1.950 | .111 |
| OVX+Ris | 1.990 | .053 |
| OVX+Zol | 1.930 | .028 |
| OVX+Ral | 1.905 | .117 |
Mechanical compression testing of the 4th lumbar vertebrae indicated that OVX caused significant reductions in compression strength (Figure 4, days 60 and 180) and stiffness (data not shown), as compared with those in sham-operated groups from days 30 to180 (p < 0.05). The compression strength was about 20%–25% higher in OVX+Ris and OVX+Zol treatment groups, as compared to the OVX controls at days 30–180 (p < 0.05). OVX+Ral had similar compression strength compared to the OVX group at all time points. The compression strength and the DBM were negatively correlated with bone turnover variables including osteoclast surface, mineralized surface, and bone formation rate (Table 2). Lumbar compression strength was significantly correlated with BV/TV and Tb.Th. However, the Tb.N was not significantly correlated with compression strength (Table 3). BV/TV was not related to DBM (R=0.306, p=0.1). Since BV/TV and Tb.Th are confounding variables for our independent and dependent variables, DBM and bone strength, we performed a multiple regression to determine the association of DBM and bone strength that was adjusted for BV/TV and Tb.Th. We found that 39% of the variation in bone compression strength could be explained by DBM alone (p=0.001) and 60% of the variation in bone compression strength could be explained by DBM, BVTV and Tb.Th together (p=0.004) (Table 3). Based on a multiple regression analysis, for each unit increase in the DBM controlling for BV/TV and Tb.Th, we would expect a 25 MPa increase in lumbar vertebral compression strength. When the analysis was performed for individual treatment groups, bone volume was the strongest predictor for compressive bone strength in OVX group while DBM was the strongest predictor for compressive bone strength in OVX+Zol group (Table 4).
Figure 4.
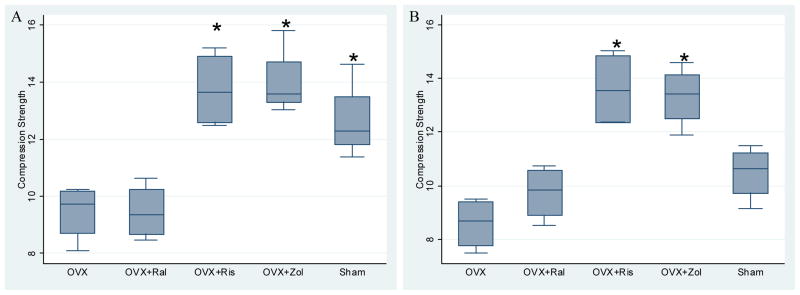
Lumbar vertebral compression strength at day 60 (A) and day 180 (B) are shown in box plots. (A) At day 60, lumbar vertebral compression strength was decreased with OVX and OVX+Ral, as compared to the other groups. (p < 0.05). (B). At day 180, lumbar compression strength was higher than OVX alone only in the OVX+Ris and OVX+Zol groups. Asterisks indicate significance versus OVX at p < 0.05.
Table 2.
Correlations between bone turnover measurements by histomorphometry and lumbar vertebral compression bone strength/degree of bone mineralization
| Parameters | Compression Strength | Degree of Bone Mineralization |
|---|---|---|
| Osteoclast surface | −.288(*) | −.368(*) |
| Mineralized surface | −.567(**) | −.319(*) |
| Bone formation rate/BS | −.312(*) | −.323(*) |
Numbers represent R value.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 3.
The association of bone compression strength with bone structural parameters and the degree of bone mineralization measured in the 5th lumbar vertebral body from all treatment groups
| Parameters | Compression Strength (MPa) | ||
|---|---|---|---|
| R-Square | Coefficient | P | |
| BV/TV | 0.384 | 28.64 | 0.0179 |
| Th.Th | 0.254 | 194.64 | 0.0236 |
| Tb.N | 0.136 | 6.45 | 0.6221 |
| DBM | 0.393 | 23.90 | 0.0013 |
| DBM adjusted for BV/TV, Tb.Th | 0.603 | 24.51 | 0.0004 |
DBM, degree of bone mineralization; BV/TV, bone volume; Tb.Th, trabecular thickness.
Table 4.
The association of bone compression strength with bone volume or the degree of bone mineralization measured in the 5th lumbar vertebral body from individual treatment group
| Treatment groups | BV/TV | DBM | |||
|---|---|---|---|---|---|
| n | R-Square | p | R-Square | p | |
| Sham | 16 | 0.091 | 0.314 | 0.018 | 0.772 |
| OVX | 16 | 0.450 | 0.017 | 0.211 | 0.540 |
| OVX+Ris | 16 | 0.091 | 0.395 | 0.376 | 0.377 |
| OVX+Zol | 16 | 0.203 | 0.091 | 0.757 | 0.050 |
| OVX+Ral | 16 | 0.011 | 0.734 | 0.626 | 0.091 |
DBM, degree of bone mineralization; BV/TV, bone volume.
DISCUSSION
The present Study demonstrated that a single intravenous dose of risedronate and zoledronic acid in aged estrogen deficient rats maintained bone compression strength by reducing bone turnover, improving the degree of bone mineralization and maintaining bone microarchitecture. Our results suggest that the degree of bone mineralization appears to be an independent predictor for bone compression strength.
Estrogen deficiency elevated bone turnover with resorption markers increasing until 60 days post-OVX, and over the next 120 days, returned to the level of aged estrogen-competent animals. In accordance with previous findings [25], substantial trabecular bone loss occurred predominantly by a reduction of the number of trabeculae, and loss of trabecular connectivity density. Estrogen-deficient animals treated for 180 days with either an intravenous dose of risedronate or zoledronic acid had significantly higher trabecular bone volumes as compared with age-matched controls. Treatment with raloxifene maintained bone microarchitecture as compared to the sham-control level. Mineral apposition rate, an index for the amount of new matrix that was added during the treatment period, was suppressed with bisphosphate treatment to levels that were similar to the sham-operated group and crystal thickness was not different between the groups. However, the degree and distribution of bone mineralization, measured by high resolution micro-CT, were maintained with a single intravenous dose of either bisphosphonate. Consequently, our findings suggest that a single dose of an iv. bisphosphonate, either zoledronic acid or risedronate maintained the degree of mineralization with long-term estrogen deficiency.
In normal aging population, the variation in bone mineralization is small and this variable is probably not a significant predictor of either localized or whole bone strength. [26]. However, prevention of OVX induced bone loss with prolonged bisphosphonates treatment, appeared to increase the DBM, and was associated with improved bone strength [1, 3, 19]. Postmenopausal women were treated with oral risedronate or placebo for 5 years, and the average degree of bone mineralization, estimated from iliac crest biopsies, increased by 4.2% after 3 years of treatment, which was sustained for another 2 years [27]. Risedronate also prevented the age-associated and estrogen deficiency-related elevation in mineral maturity/crystallinity and collagen cross-link ratios [13]. In a study on human calcanei, Follet et al. [4] found that the degree of bone mineralization is a determinant of bone strength after adjustment for bone volume and other bone microarchitecture parameters. We also found that in OVX rats treated with all three anti-resorptive agents (risedronate, zoledronic acid and raloxifene), the degree of mineralization was a strong predictor of compression bone strength in the lumbar vertebral body, independent of bone volume or trabecular thickness. To further determine if the strength-degree of mineralization or strength-bone volume fraction relations are altered by a specific treatment [28], we performed additional regression tests for individual treatment groups. We found that trabecular bone volume fraction was the most significant predictor for compressive bone strength following estrogen deficiency, while DBM was the most important factor in increasing bone strength after zoledronic acid treatment. Our analyses suggested that with estrogen deficiency, the large reduction in bone volume (−54%) accounted for the decrease in bone compression strength. On the other hand, with zoledronic acid treatment, bone was more density packed so that the degree of mineralization became the most important factor to maintain bone strength.
The uniformity of the localized mineral distribution may affect the localized material properties of bone. At least two other studies have evaluated bone mineral heterogeneity. Goldman et al. reported there was a significant increase in mineral heterogeneity in the mid-femoral cortex among 45–64 year old females and males [29]. Other investigators reported an overall reduction in the mineralization, crystallinity and type-B carbonation in males aged 60 years or older, indicating a reduction in heterogeneity with aging [30]. In chronically estrogen deficient old female rats we observed an overall reduction in the degree of bone mineralization, with a higher percentage of bone with lower mineralization in our estrogen deficient rats than age similar control animals. In addition, these estrogen deficient rats had a very broad distribution of elastic modulus on the trabecular surface with a higher percentage of trabecular surfaces with low elastic modulus and high elastic modulus. On the other hand, treatments with both risedronate and zoledronic acid maintained the degree of bone mineralization. In addition, OVX+ bisphosphonates treated animals appeared to have more uniform elastic modulus across the trabecular bone surface. Although the mechanism that underlies these observations is unclear, the bisphosphonates’ ability to slow bone turnover may allow the concentric lamellae of mineralized collagen fibers to be more densely packed compared to the alternating loose and dense pattern that occurs in the estrogen-deficient animals. In addition to the overall improvements in the degree of mineralization, the improvements in the localized mineral distribution may be responsible for the improvements in whole bone strength. Additional studies are needed to assess if the mineral distribution within bone matrix is associated with crack propagation. One might hypothesize that a bone matrix with adjacent regions of high and low mineralization may allow for a crack to propagate. Additional studies are now underway to test these hypotheses.
Although it was reported that there was an age-related increases in mineral crystallinity and that risedronate prevented this change in post-menopausal women [13], we did not detect difference in the crystal size between the aged OVX animals and those of age-match sham animals. Bisphosphonate treatments also did not seem to alter the crystal size. However, our observation on crystal size was attained at one time point (180 days of treatment), we were not able to detect age-related changes or time-dependent changes with any of the three anti-resorptive agents in the one dose studied.
While our study has a number of strengths including the measurement of the crystal size at nanoscale levels and precise estimates of the degree of trabecular bone mineralization, it also some of weaknesses. First, we obtained most of our study endpoints only at day 180 (SPM, crystal size), which made it difficult for us to differentiate between primary and secondly effects of the anti-resorptive treatments and the time course of the changes. Second, the animal numbers in each treatment group might not be large enough to truly determine the associations with bone strength in individual treatment groups. Finally, trabecular bone volume fracture is 40–50% in the Fischer 344 rats which is significantly higher than in postmenopausal women, therefore these results may not be generalizable to clinical populations.
In conclusion, we found that a single intravenous dose of two bisphosphonates in aged, estrogen-deficient rats was more effective than raloxifene in their ability to prevent loss of bone mineralization and compressive bone strength. The reduction in bone turnover, coupled with a prolonged phase of mineralization may explain part of this effect. The increased degree of bone mineralization was associated with increased bone compression strength, especially with zoledronic treatment.
Acknowledgments
This work was funded by National Institutes of Health grants nos. R01 AR043052-07, 1K12HD05195801, and a research grant from Procter and Gamble Pharmaceuticals to Dr. Nancy Lane. Support for Kurt Koester, Joel Ager and Robert O. Ritchie was provided by the Laboratory Directed Research and Development Program of Lawrence Berkeley National Laboratory under contract no. DE-AC02-05CH11231 from the U.S. Department of Energy.
The authors acknowledge the use of the Advanced Light Source at the Lawrence Berkeley National Laboratory, which is also supported by the Department of Energy under Contract No. DE-AC02-05CH11231. The authors additionally thank Fred Tileston for his excellent editorial assistance and Andres Laib in Scanco Medical AG for his assistance in attaining DBM from high-resolution micro-CT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boivin G, Lips P, Ott SM, Harper KD, Sarkar S, Pinette KV, Meunier PJ. Contribution of raloxifene and calcium and vitamin D3 supplementation to the increase of the degree of mineralization of bone in postmenopausal women. J Clin Endocrinol Metab. 2003;88:4199–205. doi: 10.1210/jc.2002-022020. [DOI] [PubMed] [Google Scholar]
- 2.Borah B, Dufresne TE, Ritman EL, Jorgensen SM, Liu S, Chmielewski PA, Phipps RJ, Zhou X, Sibonga JD, Turner RT. Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: Sequential triple biopsy studies with micro-computed tomography. Bone. 2006;39:345–52. doi: 10.1016/j.bone.2006.01.161. [DOI] [PubMed] [Google Scholar]
- 3.Borah B, Ritman EL, Dufresne TE, Jorgensen SM, Liu S, Sacha J, Phipps RJ, Turner RT. The effect of risedronate on bone mineralization as measured by microcomputed tomography with synchrotron radiation: correlation to histomorphometric indices of turnover. Bone. 2005;37:1–9. doi: 10.1016/j.bone.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34:783–9. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 6.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Jama. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 7.Riggs BL, Melton LJ., 3rd Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res. 2002;17:11–4. doi: 10.1359/jbmr.2002.17.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Reid SA, Boyde A. Changes in the mineral density distribution in human bone with age: image analysis using backscattered electrons in the SEM. J Bone Miner Res. 1987;2:13–22. doi: 10.1002/jbmr.5650020104. [DOI] [PubMed] [Google Scholar]
- 9.Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–94. doi: 10.1016/s8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 10.Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R. Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int. 2005;16:2031–8. doi: 10.1007/s00198-005-1992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–91. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 12.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 13.Durchschlag E, Paschalis EP, Zoehrer R, Roschger P, Fratzl P, Recker R, Phipps R, Klaushofer K. Bone material properties in trabecular bone from human iliac crest biopsies after 3- and 5-year treatment with risedronate. J Bone Miner Res. 2006;21:1581–90. doi: 10.1359/jbmr.060701. [DOI] [PubMed] [Google Scholar]
- 14.Lane NE, Kumer J, Yao W, Breunig T, Wronski T, Modin G, Kinney JH. Basic fibroblast growth factor forms new trabeculae that physically connect with pre-existing trabeculae, and this new bone is maintained with an anti-resorptive agent and enhanced with an anabolic agent in an osteopenic rat model. Osteoporos Int. 2003;14:374–82. doi: 10.1007/s00198-003-1374-7. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE, Yao W, Kinney JH, Modin G, Balooch M, Wronski TJ. Both hPTH(1–34) and bFGF increase trabecular bone mass in osteopenic rats but they have different effects on trabecular bone architecture. J Bone Miner Res. 2003;18:2105–15. doi: 10.1359/jbmr.2003.18.12.2105. [DOI] [PubMed] [Google Scholar]
- 16.Yao W, Hadi T, Jiang Y, Lotz J, Wronski TJ, Lane NE. Basic fibroblast growth factor improves trabecular bone connectivity and bone strength in the lumbar vertebral body of osteopenic rats. Osteoporos Int. 2005;16:1939–47. doi: 10.1007/s00198-005-1969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parfitt AM. Bone histomorphometry: standardization of nomenclature, symbols and units (summary of proposed system) Bone. 1988;9:67–9. doi: 10.1016/8756-3282(88)90029-4. [DOI] [PubMed] [Google Scholar]
- 18.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 19.Yao W, Balooch G, Balooch M, Jiang Y, Nalla RK, Kinney J, Wronski TJ, Lane NE. Sequential treatment of ovariectomized mice with bFGF and risedronate restored trabecular bone microarchitecture and mineralization. Bone. 2006 doi: 10.1016/j.bone.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Kinney JH, Haupt DL, Balooch M, Ladd AJ, Ryaby JT, Lane NE. Three-dimensional morphometry of the L6 vertebra in the ovariectomized rat model of osteoporosis: biomechanical implications. J Bone Miner Res. 2000;15:1981–91. doi: 10.1359/jbmr.2000.15.10.1981. [DOI] [PubMed] [Google Scholar]
- 21.Fratzl P, Schreiber S, Klaushofer K. Bone mineralization as studied by small-angle x-ray scattering. Connect Tissue Res. 1996;34:247–54. doi: 10.3109/03008209609005268. [DOI] [PubMed] [Google Scholar]
- 22.Fratzl P, Paris O, Klaushofer K, Landis WJ. Bone mineralization in an osteogenesis imperfecta mouse model studied by small-angle x-ray scattering. J Clin Invest. 1996;97:396–402. doi: 10.1172/JCI118428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balooch G, Marshall GW, Marshall SJ, Warren OL, Asif SA, Balooch M. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J Biomech. 2004;37:1223–32. doi: 10.1016/j.jbiomech.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, Kinney JH, Bonewald LF. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466–76. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wronski TJ, Cintron M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988;43:179–83. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- 26.Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci. 1984;304:509–18. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 27.Zoehrer R, Roschger P, Paschalis EP, Hofstaetter JG, Durchschlag E, Fratzl P, Phipps R, Klaushofer K. Effects of 3- and 5-year treatment with risedronate on bone mineralization density distribution in triple biopsies of the iliac crest in postmenopausal women. J Bone Miner Res. 2006;21:1106–12. doi: 10.1359/jbmr.060401. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39:1173–81. doi: 10.1016/j.bone.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman HM, Bromage TG, Boyde A, Thomas CD, Clement JG. Intrapopulation variability in mineralization density at the human femoral mid-shaft. J Anat. 2003;203:243–55. doi: 10.1046/j.1469-7580.2003.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yerramshetty JS, Lind C, Akkus O. The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone. 2006;39:1236–43. doi: 10.1016/j.bone.2006.06.002. [DOI] [PubMed] [Google Scholar]