Abstract
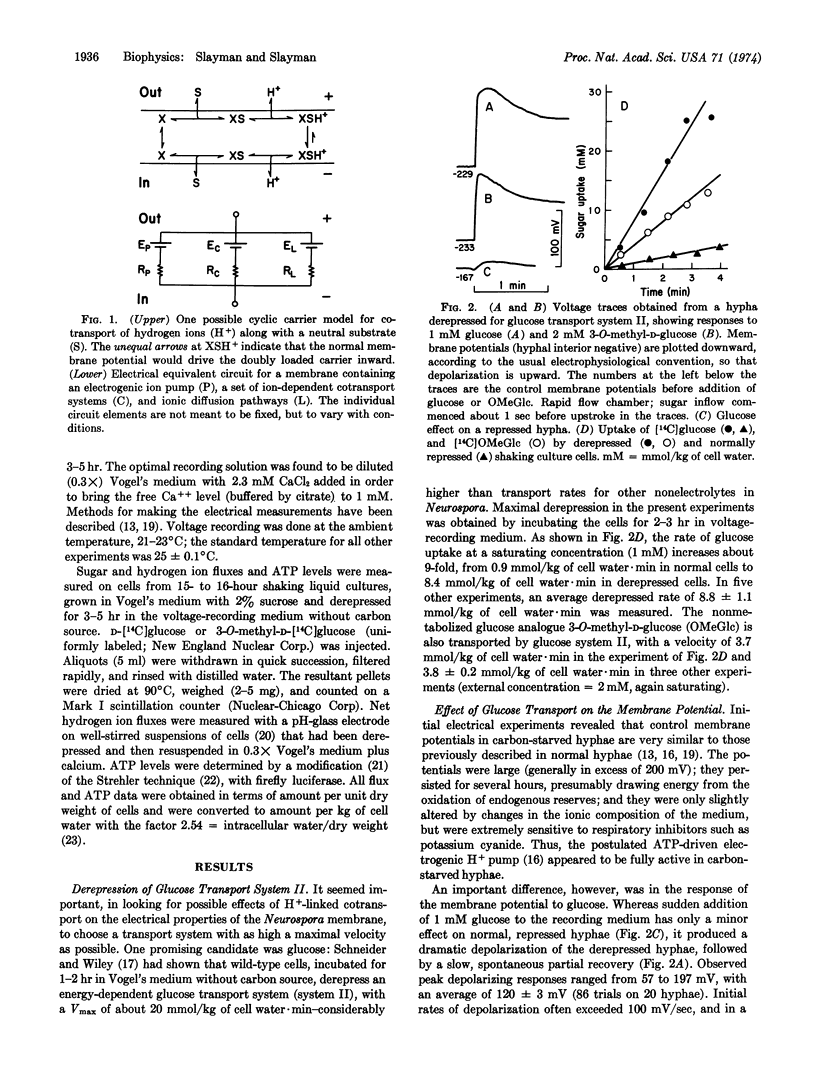
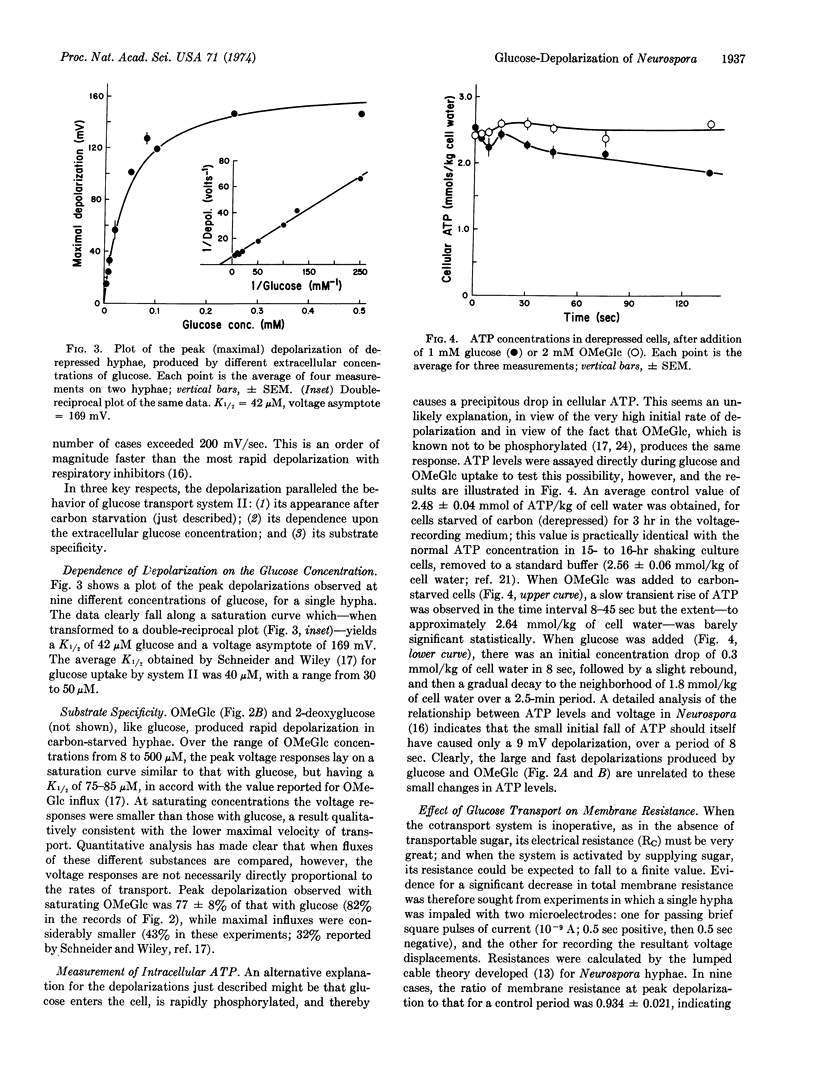
Intracellular microelectrodes were used to measure the effects of glucose transport on membrane voltage and membrane resistance in Neurospora crassa. Sudden activation of glucose uptake, via the high-affinity, derepressible system II, results in a very large depolarization of the plasma membrane. At saturating concentrations of glucose, the depolarization averages 120 mV; it is diphasic in time, with an initial shift at rates of 100-200 mV/sec followed by a slow, spontaneous, partial repolarization. Changes in intracellular ATP concentration are small and could account for only 10 mV of the initial depolarization, while the rest appears to depend upon the transport process itself. A plot of peak depolarization against the extracellular glucose concentration gives a saturation curve which is half-maximal at 42 μM, in good agreement with the K1/2 reported for glucose transport via system II. The nonmetabolized analogue 3-O-methyl-D-glucose also causes depolarization, and in addition leads to a pulsed alkalinization of the medium occurring at approximately the same rate as 3-O-methyl-D-glucose uptake. The membrane resistance falls only slightly during glucose depolarization, a fact which requires the transport system itself to have a high internal resistance, or the membrane current-voltage relationship in glucose-starved cells to be quite nonlinear. All of the data support Mitchell's notion that sugar and hydrogen ions are contransported under the influence of the membrane potential, and lead to values for H+:glucose stoichiometry of 0.8 to 1.4.
Keywords: chemiosmotic hypothesis, membrane potential, electrogenic pumps, microelectrodes
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Eddy A. A., Nowacki J. A. Stoicheiometrical proton and potassium ion movements accompanying the absorption of amino acids by the yeast Saccharomyces carlsbergensis. Biochem J. 1971 May;122(5):701–711. doi: 10.1042/bj1220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYMAN C. W., TATUM E. L. POTASSIUM TRANSPORT IN NEUROSPORA. I. INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS, AND CATION REQUIREMENTS FOR GROWTH. Biochim Biophys Acta. 1964 Nov 29;88:578–592. [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. J Biol Chem. 1970 Apr 10;245(7):1694–1698. [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Kinetic characteristics of the two glucose transport systems in Neurospora crassa. J Bacteriol. 1971 May;106(2):479–486. doi: 10.1128/jb.106.2.479-486.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Seaston A., Inkson C., Eddy A. A. The absorption of protons with specific amino acids and carbohydrates by yeast. Biochem J. 1973 Aug;134(4):1031–1043. doi: 10.1042/bj1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L. Adenine nucleotide levels in Neurospora, as influenced by conditions of growth and by metabolic inhibitors. J Bacteriol. 1973 May;114(2):752–766. doi: 10.1128/jb.114.2.752-766.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Effects of external cations on the intracellular potential. J Gen Physiol. 1965 Sep;49(1):69–92. doi: 10.1085/jgp.49.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Respiration and the intracellular potential. J Gen Physiol. 1965 Sep;49(1):93–116. doi: 10.1085/jgp.49.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Lu C. Y., Shane L. Correlated changes in membrane potential and ATP concentrations in Neurospora. Nature. 1970 Apr 18;226(5242):274–276. doi: 10.1038/226274a0. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Movement of ions and electrogenesis in microorganisms. Am Zool. 1970 Aug;10(3):377–392. doi: 10.1093/icb/10.3.377. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Net uptake of potassium in Neurospora. Exchange for sodium and hydrogen ions. J Gen Physiol. 1968 Sep;52(3):424–443. doi: 10.1085/jgp.52.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973 Mar;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]



