Abstract
Background
Exposure-response information about particulate air-pollution constituents is needed to protect sensitive populations. Particulate matter <2.5 mm (PM2.5) components may induce oxidative stress through reactive-oxygen-species generation, including primary organics from combustion sources and secondary organics from photochemically oxidized volatile organic compounds. We evaluated differences in airway versus systemic inflammatory responses to primary versus secondary organic particle components, particle size fractions, and the potential of particles to induce cellular production of reactive oxygen species.
Methods
A total of 60 elderly subjects contributed up to 12 weekly measurements of fractional exhaled nitric oxide (NO; airway inflammation biomarker), and plasma interleukin-6 (IL-6; systemic inflammation biomarker). PM2.5 mass fractions were PM0.25 (<0.25 µm) and PM0.25–2.5 (0.25–2.5 µm). Primary organic markers included PM2.5 primary organic carbon, and PM0.25 polycyclic aromatic hydrocarbons and hopanes. Secondary organic markers included PM2.5 secondary organic carbon, and PM0.25 water soluble organic carbon and n-alkanoic acids. Gaseous pollutants included carbon monoxide (CO) and nitrogen oxides (NOx; combustion emissions markers), and ozone (O3; photochemistry marker). To assess PM oxidative potential, we exposed rat alveolar macrophages in vitro to aqueous extracts of PM0.25 filters and measured reactive-oxygen-species production. Biomarker associations with exposures were evaluated with mixed-effects models.
Results
Secondary organic markers, PM0.25–2.5, and O3 were positively associated with exhaled NO. Primary organic markers, PM0.25, CO, and NOx were positively associated with IL-6. Reactive oxygen species were associated with both outcomes.
Conclusions
Particle effects on airway versus systemic inflammation differ by composition, but overall particle potential to induce generation of cellular reactive oxygen species is related to both outcomes.
Airborne mass concentrations of ambient particulate matter <2.5 mm (PM2.5) have been associated with respiratory and cardiovascular morbidity and mortality, as well as with pulmonary and systemic inflammation in human studies.1 Experimental evidence suggests that oxidative stress may be induced by reactive oxygen species from particle components. This oxidative stress may be followed by airway and systemic inflammation when antioxidant defenses are overwhelmed.2 However, supportive evidence in human populations is limited.
Oxidative stress responses to PM2.5 exposures may be caused by direct particle surface-mediated effects or by soluble compounds, such as reactive organic chemicals that are enriched in ultrafine particles (<0.1 µm in diameter). Reactive chemicals include PM2.5 organic components such as oxygenated polycyclic aromatic hydrocarbons. Potential mechanisms of PM-induced oxidative stress include the potential of PM to directly produce reactive oxygen species and the ability of PM to stimulate cellular generation of reactive oxygen species.3
The oxidant potential of PM can be independent of mass because a large fraction of PM2.5 and PM10 mass is biologically inactive while a variable and often small fraction has the potential to induce oxidative stress.2 Therefore, underlying effects may be obscured in epidemiologic analyses of exposure-response relations based on mass alone. A sizeable mass of PM2.5 is composed of inorganic components (primarily sulfate ion, nitrate ion, and ammonium ion). However, based on a growing body of research,4,5 we hypothesized that the organic fraction of PM (comprising the majority of ultrafine particle mass) is responsible for adverse cardiovascular effects of PM2.5. Organic components of aerosols with oxidant potential are numerous and vary considerably by location and by time of day and season. In particular, effects of combustion-related primary organic aerosols and semivolatile organic compounds (eg, polycyclic aromatic hydrocarbons) may differ from effects of photochemically related secondary organic aerosols. Primary organic aerosols already exist in the particle-phase when emissions from combustion sources (eg, hot traffic exhaust) are cooled to ambient temperature. Secondary organic aerosols are typically formed when volatile reactive organic precursors are oxidized to form low-volatility products that condense to produce aerosols. Precursors originate from manufactured sources (eg, aromatics such as benzene from gasoline, diesel, and solvent vapors) and biogenic sources (eg, monoterpenes emitted from trees). Secondary organic aerosols are also partly derived from aged primary semivolatile organic compounds.6 There are few data on the importance of variations in this multipollutant characteristic of PM2.5 to respiratory or cardiovascular diseases that are strongly influenced by inflammatory mediators.
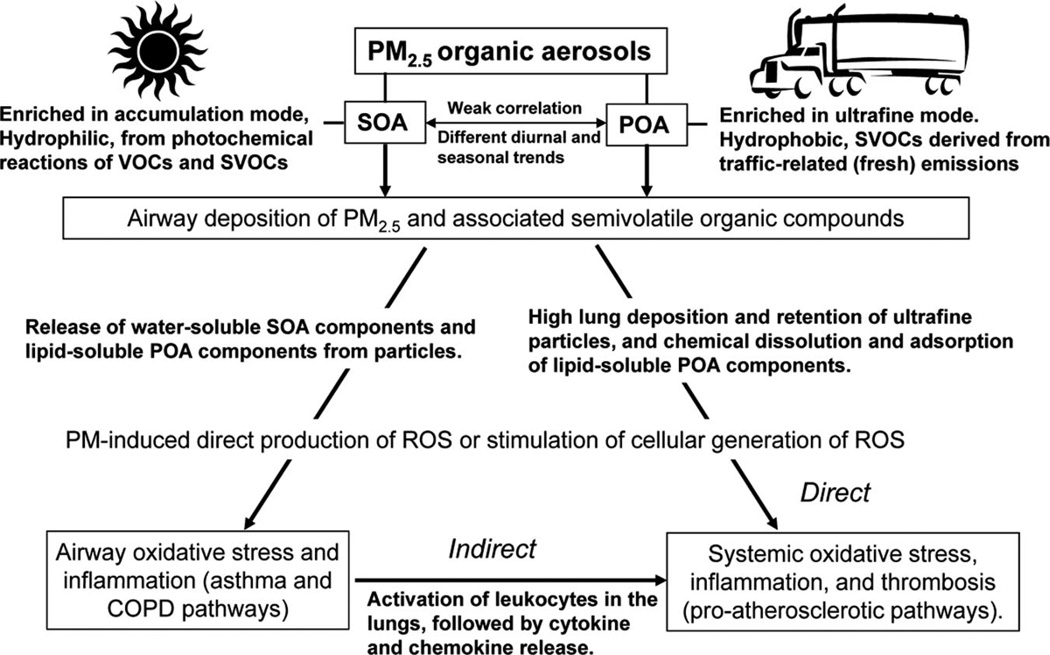
A progression from airway to systemic inflammation (and related thrombosis) by airway deposition of proinflammatory particles has been proposed to explain cardiovascular effects of PM.4,5 Although numerous studies have shown that particle exposure to the lungs is followed by systemic increases in pro-oxidative and proinflammatory mediators, this is not necessarily due to spillover from cells in the lungs (Fig. 1).5
FIGURE 1.
Hypothesized pathways of inflammatory responses to primary and secondary organic aerosol exposures. Primary organic aerosols (POA) and secondary organic aerosols (SOA) have different spatial and temporal variability, and they are thus minimally correlated in the study region of the Los Angeles air basin. The organic component mix and size distribution also differs between these classes of particulate organic matter, with primary organic aerosols being the predominant mass fraction in ultrafine particles and secondary organic aerosols predominant in larger particles. Furthermore, primary organic aerosol components are more hydrophobic, and secondary organic aerosol components are more hydrophilic from the photochemical oxidation of volatile organic compounds (VOCs) and semivolatile organic compounds (SVOCs). Following airway deposition of PM2.5 and SVOCs, water-soluble SOA chemicals are likely released and induce redox reactions with constituents of the respiratory epithelial lining fluid, cell membranes, and intracellular space. This may lead to airway oxidative stress from reactive oxygen species (ROS), and then inflammation. Induction of airway inflammation is presumed to be followed by release of pro-oxidative and proinflammatory mediators into the circulation (eg, activated leukocytes and cytokines), which then lead to effects on the vasculature and on cardiac function (indirect pathway). Alternatively, adsorbed particle components (including lipid-soluble POA chemicals), and perhaps some ultrafine particles, translocate from the lungs to the circulation and then react at extrapulmonary sites to cause oxidative stress and inflammation (direct pathway). Autonomic mechanisms of effect on cardiovascular function and inflammation may involve both of these pathways but this mechanism remains unclear.4,5
In the present study, we investigate whether these 2 types of particulate organic matter and related pollutant gases are associated in similar ways with airway inflammation and systemic inflammation. Airway inflammation is represented by fractional exhaled nitric oxide (NO),7 and systemic inflammation is represented by plasma interleukin-6 (IL-6).8 Results for IL-6 in relation to primary and secondary organic aerosol markers have been previously published,9,10 and are presented here for direct comparison with new results for exhaled NO. Additionally, we investigate whether the ability of extracts of collected particle samples to stimulate in vitro cellular generation of reactive oxygen species are associated with airway and systemic inflammation.
METHODS
Population and Design
This is a longitudinal study with repeated measures, which enables within-subject comparisons. Recruited subjects came from 4 retirement communities in the Los Angeles air basin. To be eligible, a person had to be age 65 years or older, a nonsmoker, and without environmental tobacco smoke exposure at home. We recruited only subjects with a confirmed history of coronary artery disease—a population potentially susceptible to myocardial infarction from air pollution exposure.1 Of 105 volunteers, 21 were not eligible and 24 dropped out or had insufficient or invalid biomarker data, leaving 60 subjects.
Two retirement communities were studied in 2005–2006 (29 subjects) and 2 in 2006–2007 (31 subjects). Each subject was followed for up to 12 weeks with weekly measurements of exhaled NO and plasma IL-6. Each community was studied in 2 discrete 6-week seasonal periods (a warmer period followed by a cooler period) to increase the variability in primary and secondary organic aerosols. In the Los Angeles basin, primary organic aerosols come predominantly from automobile traffic.
The Institutional Review Board of the University of California Irvine approved the research protocol. Informed written consent was obtained from subjects.
Biomarkers
Both IL-6 and exhaled NO were measured on Friday afternoons of each monitored week. Exhaled NO was collected and measured using standard offline procedures (Online Supplement, http://links.lww.com/EDE/A421),7 and collected in triplicate to assess reliability. An indoor air sample was collected using the same equipment and concurrent with the breath sample to assess influence of indoor NO on exhaled NO. We retained for analysis at least 1 exhaled NO pair out of 3 weekly session measurements in each subject if mean concentration differences were ≤3 ppb or ≤10% of the larger reading. As a result, we retained 567 of 631 person-weeks of observation (90%); pairs were strongly correlated (R2 = 0.90).
After blood draws, samples were processed to obtain plasma and frozen on site within 30 minutes. Samples were assayed using 96-well immunoassay kits for the proinflammatory cytokine interleukin-6 (IL-6; Quantikine HS, R&D Systems, Minneapolis, MN). A total of 578 IL-6 measurements out of 642 samples were included in the statistical analysis (the remainder, 64, were excluded due to reported infections). We present blood biomarker results for IL-6 (we have published similar results for plasma concentrations of tumor necrosis factor [TNF]-α receptor II10 and of TNF-α and C-reactive protein9).
Exposure Measurements
We conducted outdoor air sampling at each retirement community. Over the week preceding each Friday when biomarkers were measured, we determined concentrations of the following particulate air pollutants: total particle number (Condensation Particle Counter Model 3785, TSI Inc., Shoreview, MN), PM2.5 organic carbon and elemental carbon (OC_EC Analyzer, Model 3F, Sunset Laboratory Inc., Tigard, OR), and PM2.5 black carbon (Aethalometer, Magee Scientific, Berkeley, CA). Elemental carbon and black carbon are similar but not identical markers of primary organic aerosols.
From total organic carbon, we also estimated primary organic carbon as a marker of primary organic aerosols and secondary organic carbon as a marker of secondary organic aerosols as we described elsewhere.11
We also collected size-segregated particle samples on filters daily over 5 days before biomarker measurements using the Sioutas Personal Cascade Impactors12 (SKC Inc., Eighty Four, PA).13 Three size fractions were sampled, which are as follows: particles <0.25 µm in diameter (PM0.25); accumulation mode particles, 0.25–2.5 µm in diameter (PM0.25–2.5); and coarse mode particles, 2.5–10 µm in diameter (PM2.5–10). PM0.25 is referred to here as “quasi-ultrafine” because the upper cutpoint for the ultrafine mode is considered 0.1– 0.2 µm. Daily particle mass was determined using standard gravimetric methods.
We evaluated concentrations of 5-day average organic components in PM0.25 after compositing the 5 filters. Composites were analyzed for 92 different organic compounds using Gas Chromatography/Mass Spectrometry as we described elsewhere.13,14 We grouped representative organic components into the following: low-, medium-, and high-molecular-weight polycyclic aromatic hydrocarbons, organic (n-alkanoic) acids, n-alkanes, and hopanes (eTable 1, http://links.lww.com/EDE/A421). Most polycyclic aromatic hydrocarbons are considered to be components of primary organic aerosols. Hopanes are found in the lubricant oils of diesel and gasoline vehicles and are thus tracers of primary vehicular aerosols.15,16 Composites were also analyzed for water-soluble organic carbon in aqueous extracts using a Total Organic Carbon Analyzer (GE Analytical Instruments, Boulder, CO). Water-soluble organic carbon17 and organic acids18 are tracers of secondary organic aerosols although a fraction of water-soluble organic carbon comes from biomass burning.19
We also measured US EPA-regulated criteria pollutant gases using standard federal reference methods. These included hourly NOx (NO plus nitrogen dioxide [NO2]) and CO, which serve as non-PM markers for fossil-fuel combustion, and O3, which serves as a marker for photochemistry.
Finally, we assessed cellular production of reactive oxygen species induced by PM0.25 by examining the in vitro responses of rat alveolar macrophage cells (NR8383) to aqueous extracts of 5-day composited PM0.25 filters.20,21 NR8383 cells have all the normal characteristics of primary macrophages.22 Details of the assay have been presented in the validation study by Landerman et al23 and are briefly summarized in the eAppendix (http://links.lww.com/EDE/A421). A model of microbial particles, unopsonized Zymosan (a β-1,3-polysachharide of D-glucose) served as a positive control because it binds to Toll-like receptor-2 on macrophage cells, and then activates a strong respiratory burst and reactive oxygen species production. Results are reported in µg Zymosan equivalents/m3 air based the product of µg Zymosan equivalents/µg sample by 5-day average PM0.25 in µg/m3 air.
Analysis
To enable a direct comparison between exposures, associations are expressed at the interquartile ranges of each air pollutant and all analyses are based on 5-day-average air pollutant concentrations (particle composition data and in vitro reactive oxygen species were from extracts of 5-day filter composites). Analysis of relations between biomarkers and air-pollutant exposures was accomplished with linear mixed-effects models. For each model, we accounted for correlated within-individual repeated measures by estimating random effects at the subject level, nested within seasonal phase and community. We adjusted for 5-day average temperature and between-community and between-phase exposure effects by using exposures that were mean-centered across community and seasonal phase as previously described.9,10,24 There was little difference in results for IL-6 using other temperature-averaging times (temperature had a nominal effect). For exhaled NO, the regression coefficient for several pollutants was smaller by ≤17% using 5-day average temperature compared with shorter averaging times. Empirical variograms suggested an autoregressive-1 correlation structure, and this correlation structure was used throughout the analysis. Residual analyses showed 4 influential high outliers for IL-6 above 10 pg/mL that were reset to 10 pg/mL (upper limit of its standard curve).
Indoor NO was weakly associated with exhaled NO, which increased by 0.04 ppb (95% confidence interval [CI] = 0.0008 to 0.08) for each 1 ppb increase in indoor NO. This effect is presumed to be due to contamination of exhaled NO by indoor NO. Except for reactive oxygen species, as discussed in the eAppendix (http://links.lww.com/EDE/A421), there was little difference in exhaled NO associations with pollutants after adjusting for indoor NO; in some cases, associations slightly increased (secondary organic carbon, organic acids, and O3). Therefore, indoor NO was not adjusted for in the analyses.
In the eAppendix (http://links.lww.com/EDE/A421), we present exploratory analyses evaluating potential effect modification of relations between outcomes and air pollution by medications, seasonal phase (eTable 2; http://links.lww.com/EDE/A421), and for exhaled NO, by diagnoses of asthma (n = 4 subjects, eTable 3; http://links.lww.com/EDE/A421) or chronic obstructive pulmonary disease (COPD, n = 5 subjects, eTable 4; http://links.lww.com/EDE/A421).
RESULTS
Subject characteristics are given in Table 1. Exposures are presented in Table 2. Some secondary organic aerosol markers and O3 were higher in warmer periods (higher photochemistry). Some primary organic-aerosol markers, particle number, and NOx were higher in the cooler periods (higher air stagnation, lower mixing heights). Generation of macrophage-reactive oxygen species from PM0.25 was notably higher in warmer periods. PM0.25–2.5 and consequently PM2.5 were higher in warmer periods, as was PM2.5–10.
TABLE 1.
Subject Characteristics and Outcomes (n = 60)
| Variable | Valuea |
|---|---|
| Age (years); mean (SD) | 84.1 (5.6) |
| Sex | |
| Males | 34 (57) |
| Females | 26 (43) |
| Past smokersb | 19 (32) |
| Cardiovascular history | |
| Myocardial infarction | 27 (45) |
| Congestive heart failure | 13 (22) |
| Hypertension | 42 (70) |
| Hypercholesterolemia (by history) | 43 (72) |
| Other medical history | |
| Chronic obstructive pulmonary disease | 5 (8) |
| Asthma | 4 (7) |
| Diabetes | 8 (13) |
| Medications | |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists | 24 (40) |
| 3-Hydroxy-3-methylglutaryl-coenzyme reductase inhibitors (statins) | 31 (52) |
| IL-6 (pg/mL); mean (SD) | 2.42 (1.85) |
| Exhaled NO (ppb); mean (SD) | 17.2 (7.6) |
Values are number (%), unless otherwise indicated.
All subjects were nonsmokers.
SD indicates standard deviation; IL-6, interleukin-6; NO, nitric oxide.
TABLE 2.
Descriptive Statistics of Outdoor Community Air-pollutant Concentrations
| Exposurea | Warm Season | Cool Season | IQR Overallb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | IQR | Min/Max | Mean (SD) | Median | IQR | Min/Max | ||
| PM0.25 macrophage reactive oxygen species (µg Zymosan equivalents/m3) | 48.1 (41.7) | 36.0 | 66.2 | 7.77/147.2 | 24.0 (20.4) | 18.4 | 23.0 | 2.58/77.7 | 35.4 |
| PM2.5 variables | |||||||||
| PM2.5 massc | 24.0 (8.36) | 23.6 | 12.1 | 5.42/47.4 | 20.6 (15.6) | 16.0 | 17.8 | 2.47/89.3 | 17.4 |
| Elemental carbon (µg/m3) | 1.45 (0.52) | 1.41 | 0.71 | 0.53/3.01 | 1.55 (0.71) | 1.45 | 1.08 | 0.24/3.94 | 0.87 |
| Organic carbon (µg/m3) | 7.90 (4.65) | 6.47 | 4.43 | 2.32/27.3 | 9.25 (4.33) | 8.08 | 7.62 | 2.51/17.7 | 7.59 |
| Black carbon (µg/m3) | 1.59 (0.63) | 1.55 | 0.86 | 0.38/3.37 | 1.76 (0.91) | 1.58 | 1.24 | 0.30/5.11 | 1.03 |
| Primary organic carbon (µg/m3) | 4.36 (2.14) | 3.60 | 2.91 | 1.28/10.0 | 6.03 (3.53) | 4.66 | 6.25 | 0.99/13.6 | 4.04 |
| Secondary organic carbon (µg/m3) | 3.48 (3.40) | 2.49 | 2.03 | 0.28/18.7 | 3.12 (1.62) | 2.86 | 2.50 | 0.00/6.91 | 2.43 |
| Particle number (particle number/cm3) | 10,243 (4438) | 8980 | 6526 | 1441/24,302 | 14,851 (6490) | 12,853 | 8631 | 3297/31,264 | 7354 |
| Size-fractionated PM massc | |||||||||
| PM0.25 (µg/m3) | 10.3 (3.69) | 9.76 | 4.79 | 3.16/22.8 | 9.25 (4.48) | 8.82 | 5.60 | 2.46/30.0 | 7.37 |
| PM0.25–2.5 (µg/m3) | 12.2 (6.39) | 11.8 | 9.54 | 1.64/27.8 | 10.5 (11.7) | 6.17 | 9.95 | 0.98/66.8 | 10.6 |
| PM2.5–10 (µg/m3) | 11.4 (4.65) | 11.3 | 5.32 | 1.15/23.4 | 7.25 (4.39) | 6.43 | 5.22 | 0.30/24.6 | 5.46 |
| Organic PM0.25 components | |||||||||
| Polycyclic aromatic hydrocarbon | |||||||||
| Total (ng/m3) | 0.88 (0.37) | 0.91 | 0.47 | 0.40/1.75 | 1.04 (0.61) | 0.82 | 0.73 | 0.40/2.70 | 0.56 |
| Low molecular weight (ng/m3) | 0.38 (0.15) | 0.35 | 0.20 | 0.19/0.74 | 0.33 (0.15) | 0.30 | 0.19 | 0.17/0.73 | 0.19 |
| Medium molecular weight (ng/m3) | 0.26 (0.12) | 0.27 | 0.18 | 0.09/0.50 | 0.35 (0.24) | 0.32 | 0.33 | 0.09/0.96 | 0.24 |
| High molecular weight (ng/m3) | 0.24 (0.11) | 0.20 | 0.18 | 0.11/0.50 | 0.37 (0.24) | 0.25 | 0.32 | 0.14/1.01 | 0.21 |
| Hopanes (ng/m3) | 0.27 (0.34) | 0.067 | 0.36 | 0.06/1.57 | 0.25 (0.25) | 0.079 | 0.35 | 0.06/0.83 | 0.35 |
| n-Alkanes (ng/m3) | 36.3 (23.5) | 34.1 | 43.2 | 9.9/81.2 | 54.8 (111) | 23.0 | 15.9 | 11.7/500 | 29.4 |
| Water-soluble organic carbon (µg/m3)d | 0.52 (0.23) | 0.54 | 0.31 | 0.08/1.01 | 0.38 (0.23) | 0.39 | 0.39 | 0.06/0.94 | 0.37 |
| Organic acids (µg/m3) | 0.22 (0.17) | 0.11 | 0.30 | 0.06/0.54 | 0.26 (0.22) | 0.18 | 0.26 | 0.07/0.96 | 0.29 |
| Gases | |||||||||
| NO2 (ppb) | 26.4 (12.0) | 25.7 | 19.2 | 4.52/59.8 | 28.3 (11.8) | 29.5 | 17.6 | 3.78/55.7 | 14.3 |
| NOx (ppb) | 37.2 (22.4) | 33.3 | 28.1 | 3.70/112 | 53.9 (36.1) | 47.5 | 50.9 | 4.26/188 | 41.6 |
| CO (ppm) | 0.50 (0.25) | 0.47 | 0.36 | 0.11/1.30 | 0.58 (0.35) | 0.57 | 0.52 | 0.01/1.68 | 0.51 |
| O3 (ppb) | 33.3 (11.4) | 32.1 | 15.5 | 8.04/76.4 | 20.6 (8.04) | 19.1 | 10.8 | 6.17/44.9 | 16.1 |
| Temperature (°C) | 23.1 (3.8) | 23.1 | 5.5 | 14.8/33.1 | 14.0 (3.9) | 14.2 | 5.4 | 1.46/22.7 | 8.96 |
Macrophage reactive oxygen species generation from aqueous PM0.25 extracts and organic PM0.25 components are 5-day averages; PM2.5 variables, size-fractionated PM mass, and gases are daily averages.
The overall interquartile range used to estimate expected change in the biomarker (coefficient and 95% CI) from exposure to the air pollutant.
PM2.5 mass was measured with a Beta-Attenuation Mass Monitor whereas the size-fractionated PM mass was measured with a Personal Cascade Impactor Sampler.
Water-soluble organic carbon (in µg C/m3) was multiplied by 1.8 to yield mass of organic components in µg/m3 according to Turpin and Lim (Adapted from Aerosol Sci Technol. 2001;35:602– 610).
IQR indicates interquartile range; Min, minimum; Max, maximum; SD, standard deviation.
Table 3 shows a correlation matrix of exposures. Reactive oxygen species was positively and weakly-to-moderately correlated with primary organic aerosol markers, PM0.25 mass, water-soluble organic carbon, and n-alkanes. Primary organic aerosol markers were moderately-to-strongly correlated with each other. Secondary organic aerosol markers were weakly correlated with each other, suggesting that they represent different types of secondary aerosols. Additionally, secondary organic carbon represents a different size cut (PM2.5) than water-soluble organic carbon and organic acids (PM0.25). Polycyclic aromatic hydrocarbons and hopanes showed small negative correlations with organic acids and small positive correlations with water-soluble organic carbon. This suggests that primary and secondary organic aerosols are relatively independent of each other in the study region. Ozone showed null to negative correlations with most exposures except secondary organic carbon, with which it was positively but weakly correlated.
TABLE 3.
Spearman Correlation Matrix for Outdoor Exposuresa
| Elemental Carbon |
Organic Carbon |
Black Carbon |
Primary Organic Carbon |
Secondary Organic Carbon |
Particle Number |
PM0.25 | PM0.25–2.5 | PM2.5–10 | Water-soluble Organic Carbon |
Polycyclic Aromatic Hydrocarbon Total |
Hopanes | n-Alkanes | Organic Acids |
O3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactive oxygen species | 0.31 | 0.31 | 0.40 | 0.37 | 0.08 | 0.23 | 0.41 | 0.07 | 0.09 | 0.49 | 0.39 | 0.24 | 0.38 | −0.01 | −0.23 |
| Elemental carbon | 1.00 | 0.61 | 0.89 | 0.97 | −0.03 | 0.50 | 0.54 | 0.31 | 0.36 | 0.15 | 0.48 | 0.41 | −0.08 | 0.05 | −0.39 |
| Organic carbon | 1.00 | 0.63 | 0.65 | 0.72 | 0.27 | 0.41 | 0.33 | 0.33 | 0.26 | 0.42 | 0.39 | −0.12 | 0.17 | −0.05 | |
| Black carbon | 1.00 | 0.88 | 0.07 | 0.40 | 0.52 | 0.43 | 0.44 | 0.33 | 0.51 | 0.52 | 0.00 | 0.00 | −0.38 | ||
| Primary organic carbon | 1.00 | 0.00 | 0.47 | 0.55 | 0.33 | 0.36 | 0.16 | 0.44 | 0.41 | −0.08 | 0.05 | −0.36 | |||
| Secondary organic carbon | 1.00 | −0.08 | 0.09 | 0.16 | 0.15 | 0.33 | 0.18 | 0.19 | −0.03 | 0.20 | 0.26 | ||||
| Particle number | 1.00 | 0.36 | −0.12 | 0.06 | 0.02 | 0.33 | 0.08 | −0.03 | −0.28 | −0.38 | |||||
| PM0.25 | 1.00 | 0.17 | 0.35 | 0.25 | 0.45 | 0.31 | 0.17 | −0.18 | 0.01 | ||||||
| PM0.25–2.5 | 1.00 | 0.60 | 0.01 | 0.17 | 0.06 | 0.13 | 0.11 | 0.08 | |||||||
| PM2.5–10 | 1.00 | 0.08 | 0.13 | 0.09 | −0.07 | −0.03 | 0.06 | ||||||||
| Water-soluble organic carbon | 1.00 | 0.39 | 0.31 | 0.15 | 0.09 | −0.13 | |||||||||
| Polycyclic aromatic hydrocarbons total | 1.00 | 0.54 | 0.15 | −0.19 | −0.52 | ||||||||||
| Hopanes | 1.00 | 0.08 | −0.26 | −0.21 | |||||||||||
| n-Alkanes | 1.00 | −0.06 | −0.01 | ||||||||||||
| Organic acids | 1.00 | 0.11 |
All exposures are mean-centered by community and seasonal phase.
PM indicates particulate matter.
We assessed the relation between the 2 biomarker outcomes to test the hypothesis that airway inflammation is positively related to systemic inflammation. On the contrary, exhaled NO was negatively associated with IL-6 in a mixed model. IL-6 decreased by 0.022 pg/mL (95% = CI −0.042 to −0.002) per 1 ppb increase in exhaled NO. This association was relatively unchanged after removing 5 subjects with COPD or 4 subjects with asthma.
Table 4 shows results from the mixed regression models of the relation between biomarkers of inflammation and air pollution. Both IL-6 and exhaled NO were positively associated with the ability of PM0.25 particle extracts to induce macrophage-reactive oxygen species production in vitro. We found that exhaled NO was positively associated with PM0.25–2.5 mass as well as PM2.5 mass, but not PM0.25 mass. Exhaled NO was also positively associated with total organic carbon and markers of secondary organic aerosols in PM2.5 (secondary organic carbon) and in PM0.25 (water-soluble organic carbon and organic acids), but not with markers of primary organic aerosols. An unexpected negative association between exhaled NO and particle number was observed. Exhaled NO was positively associated with O3.
TABLE 4.
Associations of Exhaled NO and IL-6 With 5-day Average Outdoor Community Air Pollutants
| Air Pollutant | IL-6 (pg/mL) Regression Coefficient (95% CI)a |
Exhaled NO (ppb) Regression Coefficient (95% CI)a |
|---|---|---|
| Macrophage reactive oxygen species | 0.21 (0.08 to 0.34) | 0.71 (0.09 to 1.32) |
| Hourly PM mass and markers | ||
| PM2.5 mass | −0.25 (−0.48 to −0.03) | 1.57 (0.59 to 2.54) |
| Marker of primary and secondary organic aerosols | ||
| Organic carbon | −0.022 (−0.53 to 0.49) | 2.11 (0.17 to 4.06) |
| Markers of primary organic aerosols | ||
| Elemental carbon | 0.29 (0.07 to 0.50) | −0.17 (−1.18 to 0.83) |
| Black carbon | 0.21 (−0.02 to 0.45) | 0.89 (−0.19 to 1.96) |
| Primary organic carbon | 0.48 (−0.06 to 1.02) | 0.28 (−1.96 to 2.51) |
| Marker of secondary organic aerosols | ||
| Secondary organic carbon | −0.13 (−0.35 to 0.09) | 1.01 (0.20 to 1.83) |
| Particle number | 0.22 (−0.04 to 0.47) | −2.27 (−3.62 to −0.92) |
| Size-fractionated PM mass | ||
| PM0.25 (enriched in primary organic aerosols) | 0.26 (−0.06 to 0.57) | −0.02 (−1.49 to 1.44) |
| PM0.25–2.5 (enriched in secondary organic aerosols) | −0.19 (−0.36 to −0.02) | 1.16 (0.43 to 1.88) |
| PM2.5–10 | 0.01 (−0.15 to 0.17) | 0.04 (−0.65 to 0.72) |
| Organic PM0.25 components | ||
| Markers of primary organic aerosols | ||
| Polycyclic aromatic hydrocarbons | ||
| Total | 0.27 (0.10 to 0.44) | 0.32 (−0.53 to 1.17) |
| Low molecular weight | 0.22 (0.05 to 0.39) | 0.72 (−0.15 to 1.59) |
| Medium molecular weight | 0.30 (0.12 to 0.48) | −0.16 (−1.04 to 0.72) |
| High molecular weight | 0.26 (0.07 to 0.44) | 0.49 (−0.44 to 1.42) |
| Hopanes | 0.06 (−0.08 to 0.20) | 0.23 (−0.40 to 0.86) |
| Markers of secondary organic aerosols | ||
| Water-soluble organic carbon | −0.08 (−0.27 to 0.10) | 0.91 (0.13 to 1.69) |
| Organic acids | −0.23 (−0.39 to −0.06) | 1.50 (0.76 to 2.23) |
| n-Alkanes | 0.01 (−0.03 to 0.05) | −0.01 (−0.18 to 0.16) |
| Hourly gases | ||
| Markers of primary emissions | ||
| NO2 | 0.23 (−0.03 to 0.50) | 0.62 (−0.67 to 1.91) |
| NOx | 0.42 (0.18 to 0.66) | 0.18 (−1.04 to 1.40) |
| CO | 0.54 (0.27 to 0.80) | 0.73 (−0.60 to 2.06) |
| Marker of photochemistry | ||
| O3 | −0.14 (−0.44 to 0.17) | 1.41 (0.01 to 2.81) |
Regression coefficients and 95% confidence intervals are for the expected change in the biomarker associated with an interquartile range change in the air pollutant (Table 2), mean-centered by community and seasonal phase, and adjusted for temperature.
CI indicates confidence interval, NO, nitric oxide; PM, particulate matter; CO, carbon monoxide; IL-6, interleukin-6.
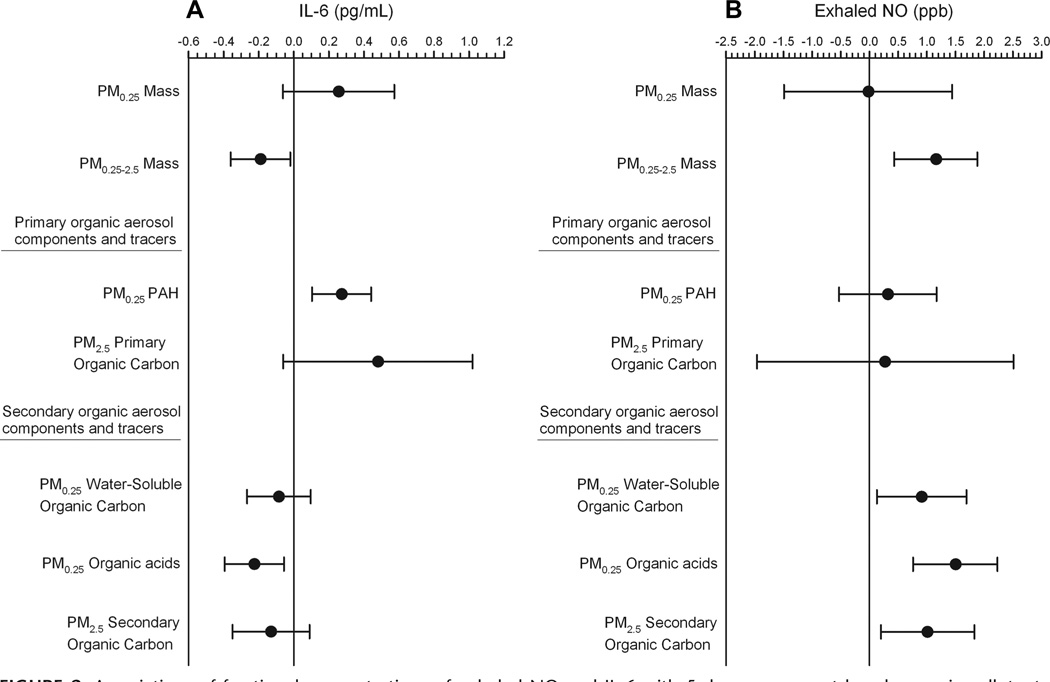
In contrast to exhaled NO, we found that IL-6 was positively associated with particle number and with several markers of primary organic aerosols in PM2.5 (elemental carbon, black carbon, and primary organic carbon), and in PM0.25 (total polycyclic aromatic hydrocarbons and all molecular-weight classes of polycyclic aromatic hydrocarbons) (Table 4). IL-6 was positively but weakly associated with PM0.25 mass and negatively associated with PM0.25–2.5 mass. Key contrasting associations of particle measurements with IL-6 and exhaled NO are shown in Figure 2. IL-6 was positively associated with NOx and CO as well. For measurements where additional averaging time other than the 5-day average were available (elemental carbon, organic carbon, black carbon, PM2.5, CO, NOx, O3), results were generally consistent with the 5-day average including, shorter (1 through 4 days) and longer averaging times (up to 9 days) for IL-6 as previously reported10 and for exhaled NO (eTable 5, http://links.lww.com/EDE/A421). In general, associations for both IL-6 and exhaled NO were strongest for 3–5-day averages or 7-day averages.
FIGURE 2.
Associations of fractional concentrations of exhaled NO and IL-6 with 5-day average outdoor home air pollutants: differences by particle-size fraction and primary versus secondary organic aerosol components and tracers. A, exhaled NO; B, IL-6. Expected change in the biomarker (adjusted coefficient and 95% CI) corresponds to an interquartile-range increase in the air-pollutant concentration (Table 2), adjusted for temperature.
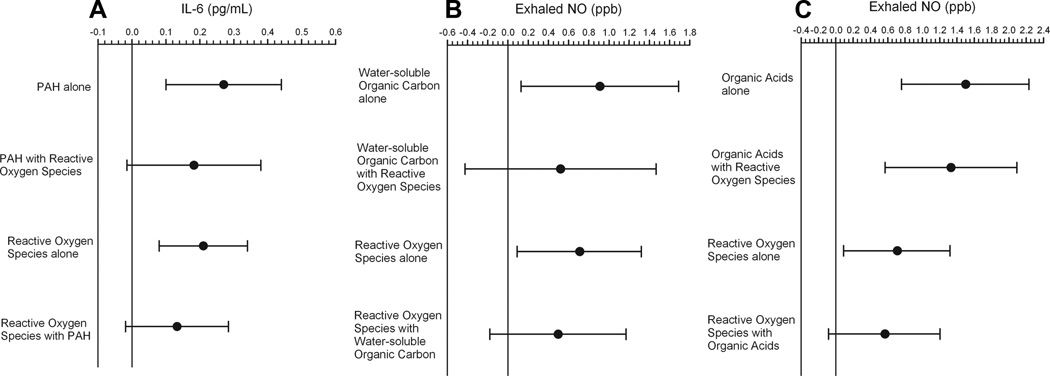
Two pollutant models were tested to assess whether associations of IL-6 and exhaled NO with macrophage reactive oxygen species generation explained associations with particle components (Fig. 3). The magnitude of associations decreased similarly for reactive oxygen species and for the components. These findings suggest that chemical components incorporate important additional information beyond that contained in the in vitro reactive oxygen species data, and vice versa. In addition, 2-pollutant models of PM0.25 with ROS production suggested that oxidative potential explains most of the association of IL-6 with PM0.25 mass (eFigure 2, http://links.lww.com/EDE/A421). Results may have been influenced by differences in measurement error between the 2 pollutants, but not multicollinearity (variance inflation factors less than 2.0).
FIGURE 3.
Associations of IL-6 and fractional concentrations of exhaled NO with PM0.25 chemical components coregressed with macrophage production of reactive oxygen species from PM0.25 aqueous extracts. A, IL-6 with polycyclic aromatics hydrocarbons (PAH) and reactive oxygen species. B, Exhaled NO with water-soluble organic carbon and reactive oxygen species. C, Exhaled NO with organic acids and reactive oxygen species. Expected change in the biomarker (adjusted coefficient and 95% CI) corresponds to an interquartile-range increase in the air-pollutant concentration (Table 2), adjusted for temperature.
DISCUSSION
We found compelling evidence that particle effects on airway inflammation compared with systemic inflammation differ by particle composition. As previously reported,9,10 IL-6 was positively associated with primary organic aerosol markers, especially carbonaceous markers in PM2.5 (elemental carbon, black carbon, and primary organic carbon) and specific organic chemicals in PM0.25 (all polycyclic aromatic hydrocarbon molecular weight classes), but IL-6 was not positively associated with secondary organic aerosol markers. An opposite pattern was evident with exhaled NO, which was associated with the secondary organic aerosol marker in PM2.5 (secondary organic carbon) and the secondary organic aerosol markers in PM0.25 (water-soluble organic carbon and organic acids), but not primary organic aerosol markers. This does not mean primary organics do not affect airway inflammation; a larger sample size would be necessary to establish this with more certainty. Nevertheless, different chemical reactions, solubility, and interstitial transport of aerosol components may determine varying effects of organics on pulmonary versus extrapulmonary target sites. The particle findings are supported by associations of IL-6 with NOx and CO (markers of combustion sources in the Los Angeles air basin), whereas exhaled NO was associated with O3, an unambiguous marker of pollutant photochemical activity. Our contrasting findings for particle components do not support the hypothesized progression from airway to systemic inflammation by airway deposition of particles.4,5 Furthermore, exhaled NO was negatively associated with IL-6—a finding we cannot explain. A cohort study of 1000 adults showed no association between exhaled NO and C-reactive protein or fibrinogen.25
We also found that both outcomes are related to the potential of soluble components in particle extracts to induce generation of cellular reactive oxygen species in vitro. From these results, we hypothesize that oxidative stress or redox signaling may be involved in effects of air pollutants on both exhaled NO and IL-6. However, the hypothesis of shared mechanisms would need to be tested in experimental models. For example, the production of reactive oxygen species may simply have been a nonspecific reflection of cell injury and stress in response to the in vitro challenge, reflecting both particle concentration and chemical toxicity of the extract. Nevertheless, our hypothesis is conceptually consistent with established knowledge that oxidative stress is a driver of cytokine expression, and based upon our control experiments (eAppendix, http://links.lww.com/EDE/A421), we believe that the measurements of reactive oxygen species activity were not biased by cell injury.
We found positive associations of exhaled NO with total PM2.5 mass, consistent with other studies of nonasthmatic adults.26–28 We also found associations of exhaled NO with PM2.5 secondary organic carbon, which to our knowledge has not been previously reported. This was consistent with novel associations of exhaled NO with secondary organic aerosol components in the quasi-ultrafine fraction, even though a greater mass of secondary organic-aerosol components are expected in particles larger than 0.25 µm. These exposures represent secondary organic-aerosol chemicals that are mostly water soluble and highly oxygenated. Therefore, it is possible that these chemicals dissolve after being deposited on the airway epithelium, and then react rapidly with extracellular macromolecules and cell constituents. An experimental study found that organic acid concentrations in urban PM, but not total organic matter, were positively associated with inflammatory activity in the lungs of mice after particle instillation.29
We did not measure many potentially important hydrophilic organic components in secondary organic aerosols derived from the photochemical oxidation of semivolatile organic compounds from fossil-fuel combustion.4 For example, reactive chemicals may include PM2.5 organic components such as quinones that are themselves oxidizing species and possibly correlated with the measured secondary organic aerosol variables. This may explain, in part, the low correlation between the various secondary organic aerosol markers. Furthermore, the lack of a positive association between IL-6 and the secondary organic aerosol markers does not mean that cardiovascular outcomes will not be affected by these aerosols. For example, we recently reported positive associations of systolic and diastolic blood pressure with secondary PM2.5 organic carbon in the present panel cohort, although the magnitude of association was smaller by around half compared with associations of blood pressure with primary PM2.5 organic carbon.30
Effects of primary organic aerosol components on systemic inflammation may occur as follows. Polycyclic aromatic hydrocarbons initially bound to PM upon airway deposition can induce oxidative stress responses after biotransformation to quinones by cytochrome P-450 1A1.31 Polycyclic aromatic hydrocarbons and other lipid-soluble components may thus become bioavailable after deposition followed by systemic distribution of unmetabolized chemicals.32 This direct pathway does not require an intermediate step of airway inflammation to induce systemic inflammation. Although some of these particle-bound toxic components may be translocated into the circulation on ultrafine particles, the high retention of ultrafine particles found in the lungs of human subjects33 is likely to lead to multiday exposure effects on systemic inflammation through the sustained transfer of chemicals (including polycyclic aromatic hydrocarbons) to the circulation. Multiday and lag-day effects on systemic inflammation are supported by findings of several air pollution panel studies including this one.9,34–36
Our results may be compared with human experimental findings of Mills et al.37 They exposed human subjects to concentrated ambient particles that were low in combustion-derived particles. They found that particles did not affect markers of systemic inflammation and thrombosis or vasomotor function. On the other hand, the same particle exposure led to increased 8-isoprostane concentrations in exhaled breath condensates (a biomarker of airway oxidative stress). Authors contrasted these findings with their similarly designed previous studies that exposed human subjects to dilute diesel exhaust, leading to systemic inflammation, impaired endogenous fibrinolysis, vasomotor dysfunction, and myocardial ischemia.38–40 These differences in findings suggest that particle composition is important in the different effects of PM on the vasculature and on airways.
Limitations to our study include a lack of exposure data on composition and oxidant potential for PM size fractions larger than 0.25 µm, or more time-resolved than 5-day averages. However, results for daily exposures to the continuously measured air pollutants were largely consistent with results for 5-day average (eTable 5, http://links.lww.com/EDE/A421). In addition, although we measured air pollutants in the outdoor environment of each subject’s retirement community, personal exposures may have differed.
The ability of particles or particle extracts to induce the generation of reactive oxygen species in cells such as macrophages is likely important. However, we did not assess the inherent ability of particles to generate reactive oxygen species and reactive nitrogen species. We do not know what the underlying mechanisms of particle-induced reactive-oxygen-species generation are in the observed macrophage data. We speculate that the reaction is induced by ultrafine particles in the aqueous extract that pass through the 0.22-µm pore-size filter (due in part to water insoluble primary organic aerosols adherent to these particles), as well as by water-soluble secondary organic and inorganic components that are directly redox active. PM species may be directly redox active, leading to the formation of cellular reactive oxygen species. However, in responding to and phagocytizing particles, macrophages generate excess reactive oxygen species in the oxidative burst, and then initiate cytokine cascades that result in additional reactive oxygen species and inflammation. Future studies should include measurements of the reactive-oxygen-species generating potential of different PM size fractions (ultrafine, accumulation, and coarse modes), and a detailed assessment of particles and components in the aqueous extract.
Additional limitations are that our 2 biomarker outcomes offer a limited view of inflammation. For example, the tracer of vehicular combustion (hopanes) was not associated with IL-6. However, we previously reported that hopanes were associated with another systemic biomarker of inflammation, TNF-α receptor II.10 Other biochemical processes besides inflammation might increase NO release and alter NO half-life in the lungs. Furthermore, IL-6 concentrations can be influenced by many host factors and is produced by several cell types, including leukocytes, hepatocytes, endothelial cells, and adipocytes.8 Unmeasured factors may have influenced the unexpected negative association of IL-6 with PM0.25–2.5 and organic acids that contrasts the positive association of exhaled NO with these exposures. Finally, the external validity of the findings is limited to elderly subjects living in retirement communities of the selected regions of Los Angeles.
Our findings may have clinical relevance in that IL-6 has been associated with cardiovascular disease risk,8 and exhaled NO is related to the presence of large airway inflammation in asthma.7 Our observations suggest that the effects of particles on airway inflammation and on systemic inflammation vary by particle composition, with components related to the primary combustion of fossil fuel having a greater impact on systemic inflammation and components related to secondary photochemical ageing of particles having a greater impact on airway inflammation. These findings are supported by our data using criteria air pollutant gases. On the other hand, the oxidant potential of particles to induce cellular reactive oxygen species generation is related to both inflammatory outcomes, supporting the role of either shared or different oxidative stress pathways. Both primary and secondary organic aerosols have particle components with oxidant potential, but the target sites in the body may differ because of particle solubility in water compared with lipids, and because of differing toxicokinetics, including the need for systemic bioactivation by phase I enzymes. This has importance to the regulation of particles based on total mass concentration (PM2.5 and PM10) as the mass fraction of toxic components with oxidative potential may be small.2
Supplementary Material
ACKNOWLEDGMENTS
We thank staff from the Department of Epidemiology and General Clinical Research Center, University of California, Irvine, Department of Civil and Environmental Engineering, University of Southern California, Wisconsin State Laboratory of Hygiene, the California Air Resources Board, and the South Coast Air Quality Management District.
Supported by the National Institute of Environmental Health Sciences grant ES12243, from the National Heart, Lung, and Blood Institute grant HL070645, and from the National Center for Research Resources grant MO1 RR00827, U.S. National Institutes of Health, the California Air Resources Board contract number 03–329, and the U.S. Environmental Protection Agency STAR grant number RD83241301 to the University of California, Los Angeles.
Footnotes
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 2.Ayres JG, Borm P, Cassee FR, et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—a workshop report and consensus statement. Inhal Toxicol. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- 3.Shinyashiki M, Eiguren-Fernandez A, Schmitz DA, et al. Electrophilic and redox properties of diesel exhaust particles. Environ Res. 2009;109:239–244. doi: 10.1016/j.envres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Rajagopalan S, Pope CA, III, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 5.Mills NL, Donaldson K, Hadoke PW, et al. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- 6.Robinson AL, Donahue NM, Shrivastava MK, et al. Rethinking organic aerosols: semivolatile emissions and photochemical aging. Science. 2007;315:1259–1262. doi: 10.1126/science.1133061. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society (ATS) and European respiratory Society (ERS) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 8.Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ. Cardiovascular biology of interleukin-6. Curr Pharm Des. 2009;15:1809–1821. doi: 10.2174/138161209788186290. [DOI] [PubMed] [Google Scholar]
- 9.Delfino RJ, Staimer N, Tjoa T, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delfino RJ, Staimer N, Tjoa T, et al. Association of biomarkers of systemic effects with organic components and source tracers in quasi-ultrafine particles. Environ Health Perspect. 2010;118:756–762. doi: 10.1289/ehp.0901407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polidori A, Arhami M, Delfino RJ, Allen R, Sioutas C. Indoor-outdoor relationships, trends and carbonaceous content of fine particulate matter in retirement communities of the Los Angeles basin. J Air Waste Manage Assoc. 2007;57:366–379. doi: 10.1080/10473289.2007.10465339. [DOI] [PubMed] [Google Scholar]
- 12.Misra C, Singh M, Shen S, Sioutas C, Hall PM. Development and evaluation of a personal cascade impactor sampler (PCIS) J Aerosol Sci. 2002;33:1027–1104. [Google Scholar]
- 13.Arhami M, Minguillón MC, Polidori A, Schauer JJ, Delfino RJ, Sioutas C. Organic compound characterization and source apportionment of indoor and outdoor quasi-ultrafine PM in retirement homes of the Los Angeles basin. Indoor Air. 2010;20:17–30. doi: 10.1111/j.1600-0668.2009.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone EA, Snyder DC, Sheesley RJ, Sullivan AP, Weber RJ, Schauer JJ. Source apportionment of fine organic aerosol in Mexico City during the MILAGRO experiment 2006. Atmos Chem Phys. 2008;8:1249–1259. [Google Scholar]
- 15.Schauer JJ, Rogge WF, Hildemann LM, Mazurek MA, Cass GR. Source apportionment of airborne particulate matter using organic compounds as tracers. Atmos Environ. 1996;30:3837–3855. [Google Scholar]
- 16.Schauer JJ, Cass GR. Source apportionment of wintertime gas-phase and particle-phase air pollutants using organic compounds as tracers. Environ Sci Technol. 2000;34:1821–1832. [Google Scholar]
- 17.Weber RJ, Sullivan AP, Peltier RE, et al. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J Geophys Res. 2007;112:D13302. [Google Scholar]
- 18.Rogge WF, Mazurek MA, Hildemann LM, Cass GR. Quantification of urban organic aerosols at a molecular level: identification, abundance and seasonal variation. Atmos Environ. 1993;27A:1309–1330. [Google Scholar]
- 19.Docherty KS, Stone EA, Ulbrich IM, et al. Apportionment of primary and secondary organic aerosols in Southern California during the 2005 Study of Organic Aerosols in Riverside (SOAR-1) Environ Sci Technol. 2008;42:7655–7662. doi: 10.1021/es8008166. [DOI] [PubMed] [Google Scholar]
- 20.Hu S, Polidori A, Arhami M, et al. Redox activity and chemical speciation of size-fractionated PM in the communities of the Los Angeles-Long Beach harbor. Atmos Chem Physics. 2008;8:6439–6451. [Google Scholar]
- 21.Verma V, Polidori A, Schauer JJ, Shafer MM, Cassee FR, Sioutas C. Physicochemical and toxicological profiles of particulate matter (PM) from the October 2007 Southern California Wildfires. Environ Sci Technol. 2009;43:954–960. doi: 10.1021/es8021667. [DOI] [PubMed] [Google Scholar]
- 22.Lane KB, Egan B, Vick S, Abdolrasulnia R, Shepherd VL. Characterization of a rat alveolar macrophage cell line that expresses a functional mannose receptor. J Leukoc Biol. 1998;64:345–350. doi: 10.1002/jlb.64.3.345. [DOI] [PubMed] [Google Scholar]
- 23.Landerman AP, Shafer MM, Hemming JC, Hannigan MP, Schauer JJ. A macrophage-based method for the assessment of the oxidative stress activity of atmospheric particulate matter (PM) and application to routine (daily 24-hour) aerosol monitoring studies. Aerosol Sci Technol. 2008;42:946–957. [Google Scholar]
- 24.Janes H, Sheppard L, Shepherd K. Statistical analysis of air pollution panel studies: an illustration. Ann Epidemiol. 2008;18:792–802. doi: 10.1016/j.annepidem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland TJ, Taylor DR, Sears MR, et al. Association between exhaled nitric oxide and systemic inflammatory markers. Ann Allergy Asthma Immunol. 2007;99:534–539. doi: 10.1016/S1081-1206(10)60383-3. [DOI] [PubMed] [Google Scholar]
- 26.Adamkiewicz G, Ebelt S, Syring M, et al. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax. 2004;59:204–209. doi: 10.1136/thorax.2003.006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adar SD, Adamkiewicz G, Gold DR, Schwartz J, Coull BA, Suh H. Ambient and microenvironmental particles and exhaled nitric oxide before and after a group bus trip. Environ Health Perspect. 2007;115:507–512. doi: 10.1289/ehp.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Amsterdam JG, Verlaan BP, Van Loveren H, et al. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Arch Environ Health. 1999;54:331–335. doi: 10.1080/00039899909602496. [DOI] [PubMed] [Google Scholar]
- 29.Happo MS, Hirvonen MR, Halinen AI, et al. Chemical compositions responsible for inflammation and tissue damage in the mouse lung by coarse and fine particulate samples from contrasting air pollution in Europe. Inhal Toxicol. 2008;20:1215–1231. doi: 10.1080/08958370802147282. [DOI] [PubMed] [Google Scholar]
- 30.Delfino RJ, Tjoa T, Gillen D, et al. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21:396–404. doi: 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonvallot V, Baeza-Squiban A, Baulig A, et al. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am J Respir Cell Mol Biol. 2001;25:515–521. doi: 10.1165/ajrcmb.25.4.4515. [DOI] [PubMed] [Google Scholar]
- 32.Gerde P, Muggenburg BA, Lundborg M, Dahl AR. The rapid alveolar absorption of diesel soot-adsorbed benzo[a]pyrene: bioavailability, metabolism and dosimetry of an inhaled particle-borne carcinogen. Carcinogenesis. 2001;22:741–749. doi: 10.1093/carcin/22.5.741. [DOI] [PubMed] [Google Scholar]
- 33.Möller W, Felten K, Sommerer K, et al. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- 34.Delfino RJ, Staimer N, Tjoa T, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with urban air pollution in elderly subjects with a history of coronary artery disease. Environ Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rückerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 37.Mills NL, Robinson SD, Fokkens PH, et al. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ Health Perspect. 2008;116:709–715. doi: 10.1289/ehp.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills NL, Tornqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 39.Mills NL, Tornqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 40.Tornqvist H, Mills NL, Gonzalez M, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.