Both plasma FVII and FVIIa are cleared relatively fast from circulation with a half-life of 3 to 6 h in humans [1,2]. The mechanism(s) responsible for the clearance of FVII/FVIIa from circulation are unknown. Pharmacokinetic studies in mice have shown that FVII, FVIIa, active-site blocked FVIIa and pre-formed FVIIa-antithrombin complexes are cleared with similar rates, indicating that plasma elimination kinetics for FVII were independent of its activation and subsequent inactivation by plasma inhibitors [3]. Nonetheless, recent studies have implicated that a substantial fraction of pharmacologically administered FVIIa activity is inactivated by AT in humans and dogs, which could explain differences observed in FVIIa activity and antigen clearance curves in these studies [4,5]. Recent studies from our laboratory and others showed that endothelial cell protein C receptor (EPCR) acts as a true cellular receptor for FVII or FVIIa [6–8] and promotes FVIIa endocytosis in cell model systems [7,9]. Administration of murine EPCR blocking antibodies was shown to reduce FVIIa clearance from circulation, particularly in the initial phase (α-phase) of clearance, indicating that EPCR may play a role in FVII clearance in vivo [9]. However, EPCR blocking antibodies that could fully block FVIIa binding to murine EPCR prolonged the circulatory half-time of FVIIa only modestly and did not block the clearance of FVIIa from circulation [9]. Together these data suggest EPCR may play a role in the initial, rapid phase of FVIIa clearance but other mechanism(s) may be responsible for its clearance in the terminal phase. To further investigate the potential role of EPCR in FVIIa clearance in a more stringent model system, in the present study we evaluated plasma elimination kinetics of FVIIa in wild-type, EPCR-deficient and EPCR-over expressing mice. Given that human FVIIa interacts well with murine EPCR whereas murine FVIIa binds only negligibly [10,11], we have used human FVIIa in this study.
Wild-type littermate controls, EPCR-deficient mice [12] or EPCR-over expressing mice [13] were injected with 125I-labeled human FVIIa (5 µg/kg) as a single intravenous bolus via tail vein. The low concentration of FVIIa dosing was chosen to reflect elimination kinetics of FVII at its plasma concentration. All mice were bled retroorbitally at 3 min following FVIIa administration and thereafter at one or two pre-set time points. Mice were anesthetized by isoflurane gas for tail vein injection and blood sampling, and experiments were conducted in accordance with the animal welfare guidelines set forth in the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Use and Care Committee. Except in a rare instance, 3 or more mice were used per time point. Blood (45 µl) was collected into citrate anticoagulant (5 µl of 0.13 M tri-sodium citrate) and plasma was obtained by centrifugation at 4,000 × g for 5 min using a table-top Eppendorf centrifuge. FVIIa concentration in plasma was quantified by measuring radioactivity. Pharmacokinetics were evaluated by a standard non-compartmental method or fitting the data to a two-compartmental model using NONMEM modeling program (GloboMax/ICON, Ellicott City, MD, USA).
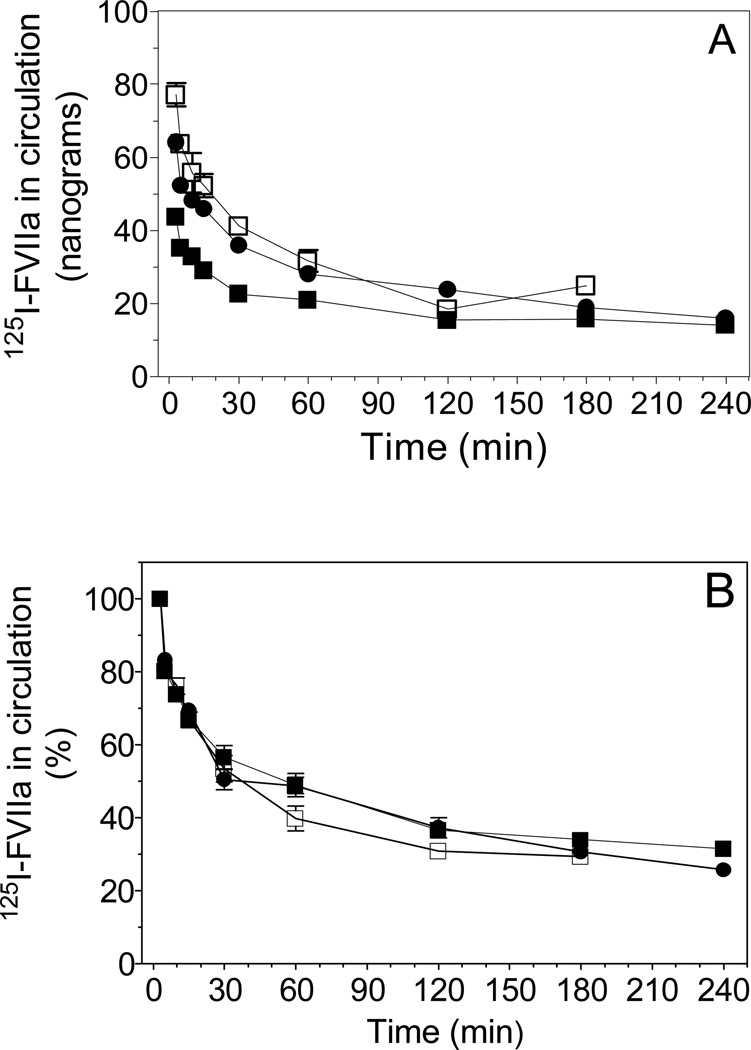
As shown in Fig. 1A, relatively large differences were observed among the genotypes at the first sampling time point (3 min). At this point, approximately 20% more FVIIa was recovered in the circulation of EPCR-deficient mice compared to the wild-type littermates. In contrast, FVIIa recovery in plasma of EPCR-over expressing mice was reduced by 30% compared to wild-type mice. These data indicate that a fraction of FVIIa administered to wild-type and more so to EPCR-over expressing mice was removed from circulation almost instantaneously after dosing. This indicates that FVIIa administered to mice readily associates with EPCR on the vascular endothelium. These data also suggest that a substantial fraction of EPCR on the vascular endothelium is left unoccupied by protein C/APC at their plasma concentrations and exogenously administered FVIIa is capable of binding to unoccupied EPCR in vivo. Interestingly, when FVIIa elimination kinetics in plasma were normalized to the mean maximum concentration of FVIIa in plasma measured at 3 min, the pharmacokinetic curves were almost identical among all 3 genotypes (Fig. 1B). Analysis of the data using the standard non-compartmental method showed a very similar half-life of FVIIa, between 2.2 to 2.4 h, in all 3 genotypes but relatively large differences in the Cmax (wild-type, 60 ng/ml; EPCR-deficient, 78 ng/ml; EPCR-overexpressors, 45 ng/ml).
Fig. 1.
In vivo elimination of FVIIa in wild-type littermates, EPCR-deficient and EPCR-over expressing mice. 125I-labeled human FVIIa (5 µg/kg body weight, ~125 ng/mice) was administered to mice as a single dose intravenously via tail vein. FVIIa levels in circulation were determined by measuring radioactivity in plasma samples derived from mice at varying time periods following administration of FVIIa at time-points ranging from 3 min to 240 min. The data were shown as ng FVIIa present in circulation (A) or normalized to the percent concentration of 125I-FVIIa present in circulation at 3 min (B). The symbols denote: (●), wild-type; (□), EPCR-deficient; (■), EPCR-over expressing mice.
When the data were fitted to a two compartmental model using Nonmem modeling, it confirmed the variation in bioavailability of FVIIa among the wild-type, EPCR-deficient and EPCR-over expressing mice. The bioavailability of FVIIa in plasma was increased by about 30% in EPCR-deficient mice, compared to wild-type mice, suggesting that less FVIIa was sequestered in these mice. In EPCR-over expressing mice, the bioavailability of FVIIa in plasma was decreased by 30%, indicating that a larger fraction of FVIIa was bound to EPCR immediately after its administration. Only minor differences were found in the CL values among the wild-type (0.018 ml/min), EPCR-deficient (0.017 ml/min) and EPCR-over expressing mice (0.014 ml/min), indicating that FVIIa is cleared in a similar profile in these mice. Following the initial disparate sequestration trends in EPCR transgenic mice, FVIIa was eliminated from circulation thereafter in all three genotypes essentially with a similar half-life, t1/2α, 0.12 h and t1/2ß, 2.2 to 2.8 h. Other pharmacokinetics values, such as Q, V1 and V2, were identical among the three genotypes. Taken as a whole these data indicate that EPCR may play a role in modulation of FVII(a) levels in the circulation by sequestering it on the vascular endothelium but it is unlikely to influence the rate of FVII(a) clearance.
Overall, FVIIa pharmacokinetics observed in the present study were similar to that reported earlier [3]. Any minor variation in CL, V1 and V2 values reported in the present study and the earlier study could reflect variation in the methods for measuring FVIIa concentration in plasma (radioactivity versus clotting activity) and a difference in the dosage levels (5 µg/kg vs. 10 mg/kg). In an earlier study, we found that blockade of EPCR with EPCR-blocking antibody prolonged the t1/2α of FVIIa clearance from 19 min to 31 min [9]. These data were interpreted as EPCR serving a role in FVIIa clearance. However, based on the present observation that showed no significant differences in FVIIa clearance rates among wild-type, EPCR-deficient and EPCR-over expressing mice, it is unlikely that EPCR-mediated FVIIa internalization plays a significant role in FVII(a) clearance in vivo, at least at concentrations close to the endogenous plasma concentration of FVII. In our earlier study [9], the reversible nature of antibody binding to the receptor which could allow the exchange between antibody and ligand binding to EPCR coupled with potential differences in the bioavailability of FVIIa in control and EPCR blocking antibody-treated mice, might have given the impression that blockade of EPCR prolonged the initial phase of FVIIa clearance modestly but statistically significantly. Although the present data suggest that EPCR does not appear to play a significant role in the rate of FVIIa clearance from plasma, we can not completely rule out the possibility of EPCR influencing FVII clearance in humans as human EPCR may behave differently than murine EPCR. Irrespective of its role (or lack there of) in FVII clearance from plasma, EPCR still could play an important role in the continual, prolonged transport of a small but physiologically meaningful amount of FVIIa from circulation into extravascular compartments. Studies investigating this possibility are in progress within the authors’ laboratories.
Acknowledgments
This work has been partly supported by a grant from Novo Nordisk and NHLBI grants (HL 58869 and 107483).
Footnotes
Disclosure of Conflict of Interests
This work is partly supported by a grant from Novo Nordisk. One of the authors (HA) is an employee of Novo Nordisk, Denmark. UH is a consultant to Novo Nordisk A/S, Zurich, Switzerland.
References
- 1.Loeliger EA, van der Esch B, ter Haar Romney-Wachter CC, Booij HL. Factor VII; its turnover rate and its possible role in thrombogenesis. Thromb Diath Haemorrh. 1960;4:196–200. [PubMed] [Google Scholar]
- 2.Erhardtsen E. Pharmacokinetics of recombinant activated factor VII (rFVIIa) Semin Thromb Hemost. 2000;26:385–391. doi: 10.1055/s-2000-8457. [DOI] [PubMed] [Google Scholar]
- 3.Petersen LC, Elm T, Ezban M, Krogh TN, Karpf DM, Steino A, Olsen EH, Sorensen BB. Plasma elimination kinetics for factor VII are independent of its activation to factor VIIa and complex formation with plasma inhibitors. Thromb Haemost. 2009;101:818–826. [PubMed] [Google Scholar]
- 4.Agerso H, Brophy DF, Pelzer H, Martin EJ, Carr M, Hedner U, Ezban M. Recombinant human factor VIIa (rFVIIa) cleared principally by antithrombin following intravenous administration in hemophilia patients. J Thromb Haemost. 2011;9:330–838. doi: 10.1111/j.1538-7836.2010.04152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agerso H, Kristensen NR, Ostergaard H, Karpf DM, Hermit MB, Pelzer H, Petersen LC, Ezban M. Clearance of rFVIIa and NN1731 after intravenous administration of Beagle dogs. Eur J Pharm Sci. 2011;42:578–583. doi: 10.1016/j.ejps.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Preston RJ, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlback B, Lane DA. Multifunctional specificity of the protein C/activated protein C Gla domain. J Biol Chem. 2006;281:28850–28857. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S, Pendurthi UR, Steinoe A, Esmon CT, Rao LV. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J Biol Chem. 2007;282:11849–11857. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Sagaseta J, Montes R, Puy C, Diez N, Fukudome K, Hermida J. Binding of factor VIIa to the endothelial cell protein C receptor reduces its coagulant activity. J Thromb Haemost. 2007;5:1817–1824. doi: 10.1111/j.1538-7836.2007.02648.x. [DOI] [PubMed] [Google Scholar]
- 9.Nayak RC, Sen P, Ghosh S, Gopalakrishnan R, Esmon CT, Pendurthi UR, Rao LVM. Endothelial cell protein C receptor cellular localization and trafficking. Blood. 2009;114:1974–1986. doi: 10.1182/blood-2009-03-208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puy C, Hermida J, Montes R. Factor X and factor VII binding to endothelial protein C receptor differs between species. J Thromb Haemost. 2011;9:1255–1257. doi: 10.1111/j.1538-7836.2011.04295.x. [DOI] [PubMed] [Google Scholar]
- 11.Sen P, Clark CA, Gopalakrishnan R, Hedner U, Esmon CT, Pendurthi UR, Rao LVM. Factor VIIa binding to endothelial cell protien C receptor: Differences between mouse and human systems. Thromb Haemost. 2012 doi: 10.1160/TH11-09-0672. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Zheng X, Gu JM, Ferrell GL, Brady M, Esmon NL, Esmon CT. Extraembryonic expression of EPCR is essential for embryonic viability. Blood. 2005;106:2716–2722. doi: 10.1182/blood-2005-01-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Zheng X, Gu J, Hunter J, Ferrell GL, Lupu F, Esmon NL, Esmon CT. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3:1351–1359. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]