Abstract
Tissue factor (TF) is a cellular receptor for clotting factor VII/VIIa (FVII/FVIIa). Formation of TF-FVIIa complexes on cell surfaces not only triggers the coagulation cascade but also transduces cell signaling via activation of protease-activated receptors. It is generally believed that only a small fraction of the TF found on cell surfaces is active in coagulation whereas the vast majority is cryptic (non-functional) in coagulation. It is unclear how cryptic TF differs from the coagulant active TF or potential mechanisms involved in transformation of cryptic TF to the coagulant active form. Both cryptic and coagulant active TF forms can bind FVIIa. It is generally believed that cryptic TF-FVIIa complexes fail to activate factor X as the protein substrate binding site is buried in the encrypted complex and a change in TF-FVIIa complex that exposes the substrate binding site leads to its decryption. Exposure of phosphatidylserine (PS) in response to various chemical or pathophysiological stimuli has been considered as the most potent inducer of TF decryption. In addition to PS, TF self-association and association with specialized membrane domains may also play a role in TF decryption. Recent studies suggest that cryptic form of TF contains unpaired cysteine thiols at Cys186 and Cys209 in the membrane-proximal domain, whereas the coagulant active form of TF is thought to have an oxidized Cys186-Cys209 disulfide bond. It has been suggested that protein disulfide isomerase (PDI) regulates TF decryption through its oxidoreductase activity by targeting this disulfide bond or regulating the PS equilibrium at the plasma membrane. However, this hypothesis requires further validation to become an accepted mechanism. In this article, we critically review literature on TF encryption/decryption with specific emphasis on recently published data and provide our perspective on this subject.
2. Introduction
Tissue factor (TF), a plasma membrane anchored glycoprotein, is the primary initiator of the coagulation in both physiological and pathological conditions. TF functions as the allosteric cofactor for plasma clotting protease factor VIIa (FVIIa), and TF-FVIIa complex initiates the coagulation cascade by activating clotting proteins factors IX and X through limited proteolysis. Interestingly, only a small fraction of TF expressed at the cell surface is capable of supporting the coagulation whereas majority of the TF remains non-functional, which is generally referred to as the cryptic TF. Cryptic TF could be transformed to procoagulant TF in a variety of pathophysioloigcal conditions. At present, it is not entirely clear how cryptic TF differs from coagulant active TF and mechanisms by which cryptic TF transforms to coagulant active TF. This review critically evaluates recent and key early literature on TF encryption/decryption, including the authors’ findings interjected with the authors’ perspective.
3. Tissue factor expression on cell surfaces: Evidence for cryptic tissue factor
Tissue factor is expressed constitutively on cell surfaces of many extravascular cells, including fibroblasts and pericytes in and surrounding blood vessel walls and epithelial cells [1;2]. Although cells that come in contact with blood do not typically express TF, but some of them, e.g., monocytes and endothelial cells, do express TF in response to pathologic stimuli [3;4]. As early as 1975, Maynard et al. reported that TF on cell surfaces exits in a latent form and the activity of TF on intact cells could be increased by many fold (>50-fold) by treating the cells with trypsin or other proteases [5]. Restricted formation of TF-FVIIa complex on cell surfaces is not the reason for TF latency as numerous studies performed later to that observation showed that TF expressed on a variety of cell types was capable of binding to FVII and FVIIa [6-11]. Correlation of FVIIa binding to TF and expression of TF-FVIIa procoagulant activity in fibroblasts revealed that a direct linear relationship exists between FVIIa binding to TF and TF-FVIIa procoagulant activity at low level of FVIIa binding, but with higher amounts of FVIIa bound to TF, the relationship became nonstoichiometric, i.e., less factor Xa was formed per mole of FVIIa [11]. Bach and Rifkin showed that intact bovine fibroblasts, pericytes and kidney cells manifested significantly less TF activity compared to their disrupted counter parts, supporting the hypothesis that TF activity on unperturbed cells is not fully active [12]. Discordant expression of TF protein and procoagulant activity was also described in monocytes where treatment of LPS-stimulated monocytes with cycloheximide inhibited TF protein synthesis but increased the TF procoagulant activity [10]. Le et al. [8], by correlating FVIIa binding to TF and the subsequent expression of TF-FVIIa procoagulant activity on an ovarian carcinoma cell line that constitutively express TF, provided a clear evidence for the existence of tow different populations on TF on cell surfaces. These investigators reported a striking discrepancy between the time required to express maximum TF-FVIIa coagulant activity and the time required for maximum TF-specific binding of FVIIa to monolayers. TF-FVIIa coagulant activity was fully manifested on intact monolayers after only 1 min of incubation of cells with FVIIa whereas 30 to 60 min was required for FVIIa to bind all available TF sites on the cell surface. When TF-FVIIa proteolytic activity towards factor X is fully expressed on intact monolayers at 1 min, only 10 to 15% of all available TF sites at the cell surface were occupied by FVIIa. Prolonging FVIIa exposure time to cells to allow further binding of FVIIa to remaining TF on the cell surface failed to increase TF-FVIIa coagulant activity (Fig. 1). Based on this and other supporting evidence, the authors postulated that two populations of TF-FVIIa complexes were formed on cell surfaces, one a minor population (<20%) that was formed readily within a short time of incubation with FVIIa which accounted for all TF-FVIIa activity toward factor X on intact cells, and a major population that was inactive in activating factor X on intact monolayers. The later form of the TF population constitutes cryptic TF. At present, it is not entirely clear whether cryptic TF is completely devoid of procoagulant activity or express a low procoagulant activity. Lack of antibodies or any other specific reagents that specifically blocks procoagulant TF and not the cryptic TF makes it difficult to answer this question objectively at present.
Figure 1. Lack of correlation between expression of TF-FVIIa procoagulant activity and FVIIa binding to TF on cell surfaces.
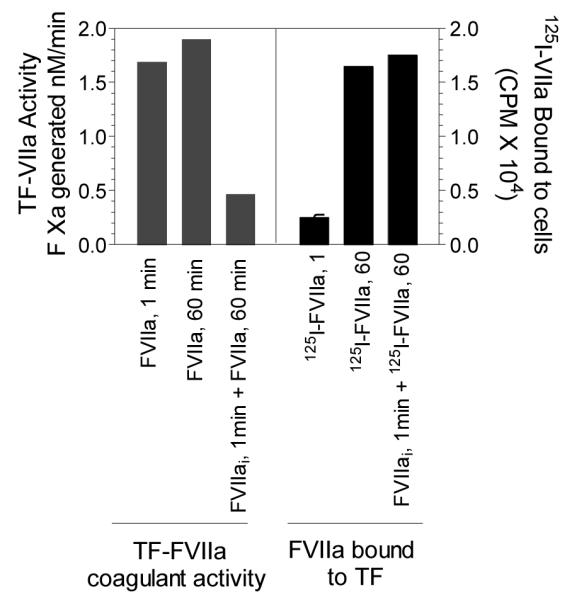
Monolayers of OC-2008 cell line expressing TF were incubated in parallel sets with 10 nM of unlabelled or 125I-labeled FVIIa. In one set, active site-inhibited FVIIa (FVIIai, 10 nM) were added for 1 min and the cells were washed quickly three times with ice-cold Ca2+-containng buffer before adding FVIIa. In all cases, at the end of specified incubation period, the cells were washed six times with ice-cold Ca2+-containing buffer and TF-FVIIa procoagulant activity at the cell surface was determined in factor X activation assay or FVIIa bound to TF was determined by measuring the radioactivity of 125I-FVIIa bound to cells (adopted from Le et al.[8]).
4. Cryptic tissue factor – the definition matters
There is a general consensus in the field that most of the TF expressed on cell surfaces is in a cryptic state and an activation step (decryption) is required for the maximum expression of TF potential procoagulant activity. However, opinions may vary on what exactly constitutes cryptic or procoagulant TF, molecular differences between these two forms, and mechanisms that are responsible for transformation from one to the other form. Some of the disagreements in the field probably stem from non-descriptive or non-discriminatory use of the terminology. Cells could express no or low TF procoagulant activity for many reasons, including lack of TF protein at the cell surface, impairment of TF to bind or to form stable complex with FVII/FVIIa or inability of TF-FVIIa complexes formed at the cell surface to activate factors X and IX. In the strict sense, only the last form of TF fits with the definition of cryptic TF. When cells are transfected with TF variants, they may exhibit low procoagulant activity because they fail to express TF protein properly and/or the expressed TF may have an impairment of binding to FVIIa. Broadening the use of term cryptic TF to describe all TF forms that exhibit low procoagulant activity, irrespective of their nature, creates unnecessary confusion and controversy in the field and hampers our progress in understanding mechanisms that regulate TF encryption and decryption. In our own studies, we adhere to the classic definition to cryptic TF, i.e., cell surface TF molecule that binds FVII or FVIIa, but the resultant TF-FVIIa complexes fail to activate its macromolecular substrates, factors IX and X [8;13]. In this definition, true determination of cryptic status of TF requires measuring TF activity at saturating concentrations of FVIIa.
5. Differences in cryptic and procoagulant TF in their abilities to bind FVIIa and TFPI
Although it is quite difficult to differentiate cryptic and procoagulant TF based solely on their interaction with FVIIa or TFPI, they do exhibit some differences on how they interact with FVIIa and TFPI. First, procoagulant TF, compared to cryptic TF, binds FVIIa relatively fast, within few seconds to a minute or two [8]. It is also likely that FVIIa binds to procoagulant TF with a higher affinity than it binds to cryptic TF. However, it is difficult to demonstrate this convincingly. Analysis of equilibrium binding studies of 125I-labeled FVIIa binding to TF on a variety of cell types failed to show that FVIIa binds to TF on cell surfaces with two different affinities [6-8;11;14;15]. Although the reported Kd values for the binding varied from 80 pM [6] to 3 nM [15], most of these studies reported a single high affinity binding site for FVIIa. It is possible that radioligand binding studies fail to detect very high affinity binding of FVIIa to procoagulant TF as this constitutes very small fraction. Binding kinetic parameters obtained with radiolabeled FVIIa largely reflects FVIIa binding to cryptic TF as this constitutes the predominant portion of TF at the cell surface. Analysis of FVIIa concentration-dependency of FX activation, which reflects FVIIa binding to procoagulant TF, generally show that FVIIa binds to procoagulant TF with very high affinity, 20 to 100 pM [16-19]. Although comparison of these data with that of radioligand binding studies suggests that procoagulant TF exhibits much higher affinity to FVIIa than cryptic TF, one should exercise caution in comparing them as there are significant differences on how the binding constants were measured in these two assays. In the radioligand binding studies, cells had to be washed multiple times to remove free FVIIa from the cell-bound FVIIa and this step is not included generally in evaluating FVIIa binding to TF in FX activation assay. This difference would markedly affect the binding measurements as we have observed recently [19]. Overall the evidence suggests that, despite the potential differences between them in their ability to interact FVIIa, both the cryptic and coagulant active TF form stable high-affinity associations with FVII and FVIIa at the plasma concentration of FVII.
A Kunitz-type protease inhibitor, tissue factor pathway inhibitor (TFPI), after forming complex with FXa, binds to TF-FVIIa and inhibits its procoagulant activity [20;21]. TFPI-FXa complex appears to distinguish between procoagulant and cryptic TF-FVIIa complexes. TFPIFXa readily binds to the procoagulant TF-FVIIa complexes with high affinity and effectively inhibits TF-FVIIa activity [8;22]. In contrast, the TFPI-FXa either fails to bind to cryptic TF-FVIIa [22] or has 10-fold lower affinity than that was noted for procoagulant TF-FVIIa [8]. At present there is no information on whether other plasma inhibitors of TF-FVIIa, such as antithrombin and PAI-1, could also differentiate between procoagulant TF-FVIIa and cryptic TF-FVIIa complexes.
6. Models of tissue factor encryption/decryption
Although the phenomenon that TF exists in two different populations, cryptic and coagulant active (nonfunctional/functional) forms is well recognized, the molecular characterization of these forms and mechanisms of their conversion from one from to other form is still elusive. It is generally believed that both cryptic and coagulant active TF bind FVII or FVIIa relatively well, below the plasma concentration of FVII; the resultant TF-FVIIa complexes are catalytically active towards small peptide substrates but not protein substrates; cryptic TF-FVIIa deso not interact with the substrates factors X and X; and the transformation of cryptic to active TF probably requires a change in TF-FVIIa complex that lead to exposure of the substrate factor X (or factor IX) binding site in the complex. In the absence of specific reagents that exclusively recognize and inhibit the function of cryptic and active forms of TF, it is not feasible to make a clear demarcation between cryptic and active TF pools. Thus, at present it is unclear the exact proportions of cryptic and active TF that exist on various cell types. This also makes it difficult to investigate potential mechanisms involved in TF decryption more objectively and obtain clear-cut data. Despite these limitations, a number of models for TF decryption have been presented, some with more convincing data than others.
(i) Phospholipid asymmetry: exposure of phosphatidylserine
Membrane phospholipid asymmetry is considered to be a general property of biological membranes. The outer leaflet of cell surface membrane bilayer is rich in choline-phospholipids, whereas anionic phospholipids are abundant in the inner leaflet. Studies with platelets and erythrocytes have shown that these asymmetries are not maintained when the cells are activated by various stimuli, and the resultant loss of membrane phospholipid asymmetry leads to increased exposure of negatively charged phosphatidylserine (PS) at the outer surface [23]. It has been known for many decades that TF procoagulant activity requires its association with phospholipids [24;25]. Studies thereafter showed that the presence of anionic phospholipids, such as PS in the phospholipid mixture greatly accelerates TF procoagulant activity [12;26-30]. Almost all stimuli that are known to increase procoagulant activity of cryptic TF on cell surfaces, i.e., freezing and thawing, sonication, nonionic detergents, proteases, phospholipases, apoptosis, complement, calcium ionophores, oxidizing agents and sulfhydryl reactive compounds, also increase invariably the exposure of PS on the outer cell surface [5;8;12;22;31-37]. Although the coincidence of PS exposure and TF decryption does not necessarily prove that the increase in PS is solely responsible for TF decryption, the critical and proven role of PS in supporting TF procoagulant activity and the observation that blocking PS by annexin V reduces the procoagulant activity of decrypted TF in many cell types strongly implicate that PS plays a critical role in TF decryption. Thus, it is now generally accepted that whether TF is cryptic or procoagulant depends primarily on the availability of anionic phospholipids in the vicinity of TF on the outer cell surface membrane, and the increased availability of anionic phospholipids at the cell surface is responsible for the transformation from cryptic TF to procoagulant TF. Although few reports have suggested that TF could undergo decryption independent of PS since annexin V treatment failed to block completely calcium ionophore-induced TF decryption [32;34], one should exercise caution in interpreting these data on their face value. Annexin V may not be very effective in blocking all PS on the cell surface and/or may require much higher concentrations to inhibit PS on cell surfaces than is needed to inhibit PS in liposomes [38]. Further, PS that is associated with TF at the cell surface may not be readily accessible to annexin V [39]. In our opinion, at present there are no convincing data in the literature that demonstrate TF could undergo activation without any increase in PS at the outer cell surface membrane.
The mechanism through which the increased anionic phospholipids convert cryptic TF to procoagulant TF is not entirely clear. The kinetics of TF-FVIIa catalyzed activation of FX on unperturbed and perturbed cells provides some insights in to the mechanism of PS effect. Bach and Rifkin [12] showed that stimulating pericytes with Ca2+ ionophore increased TF-FVIIa activation of FX and this increase stems from an increase in the Vmax and a decrease in Km of the reaction. However, the increased catalytic activity of TF-FVIIa toward FX activation following treatment of fibroblasts with sulfhydryl reactive compound N-ethylmaleimide was found to come primarily from an increase in Vmax without an accompanying decrease in the apparent Km [33]. A possible difference between these two studies is the extent of microvesicle generation, which was not investigated in these studies. Ca2+ ionophore treatment of fibroblasts was shown to induce TF bearing microvesicles [40;41]. Therefore, it is possible that the presence of microvesicles in Ca2+ ionophore stimulated cells could have accounted for the decrease in Km. The more relevant for our present discussion is the consistent finding that TF decryption increases the Vmax of TF-FVIIa activation of FX, which is very similar to that observed with inclusion of PS to TF containing phospholipid vesicles [12;33]. This suggests that formation of an increased number of catalytically active (towards factor X) TF-FVIIa complexes explains the increase in TF procoagulant activity. Despite having different binding characteristics, in the presence of excess FVIIa, the formation of TF-FVIIa complex per se is not the determinant factor in the increased number of catalytically active TF-FVIIa complexes as FVIIa binds to both encrypted and decrypted TF [8]. It is also unlikely that PS-induced conformational change in the active site of FVIIa bound to TF is involved in the decryption of TF since FVIIa catalytic site is too far away from the membrane surface [42-44].
It has been proposed that encrypted TF-FVIIa is completely inactive with respect to protein substrate (FX and FIX) hydrolysis and decryption of TF involves conversion of inactive TF-FVIIa to proteolytic and coagulant functional TF-FVIIa [13]. In this model, exposure of PS at the outer cell surface increases the number of TF-FVIIa functional and catalytic sites without changing total number of TF-FVIIa complexes formed. Data from our laboratory and others provide strong evidence for this hypothesis [8;12;22;33]. Overall, these data strongly imply that TF activation involves a direct interaction between PS and TF, which would result in structural changes in TF that exposes macromolecular substrate binding site for factors X and IX on TF (Fig. 2). At present, it is not entirely clear about what are the PS-induced structural changes in TF. One of the PS-induced proposed changes in TF structure may involve the interactions between PS polar head groups and Lys165/Lys166 in the TF extracellular domain [13;22]. It has been suggested that possible electrostatic interactions between PS polar head groups and Lys165/Lys166 could change TF quaternary structure by altering the orientation of extracellular domain relative to the membrane surface, which may facilitate the precise alignment of TF-FVIIa activity site to the scissile bonds of the membrane bound factors X and IX [13;22]. Such precise alignment could also be achieved by FVIIa binding to exposed PS on the cell surface via its Gla domain, which restricts the orientation of TF-FVIIa complex relative to the membrane surface. This interaction may complement direct interaction between TF and PS to fix the distance from the membrane surface to the catalytic site.
Figure 2. A hypothetical model for tissue factor decryption.
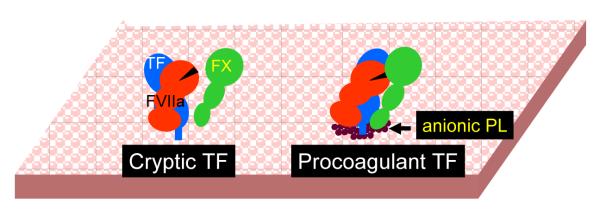
On unperturbed cells expressing TF, FVIIa binds to all available TF sites, however majority of these complexes unable to interact with substrate factor X. Upon cell perturbation that exposes to anionic phospholipids at the outer cell surface membrane, TF or TF-FVIIa complexes interact with anionic phospholipids, leading to a conformational change in TF-FVIIa that facilitate its interaction with factor X, thus transforming cryptic TF to coagulant active form.
It is possible that exposure of PS on the outer cell surface membrane could decrypt TF, independent of its interaction with cryptic TF or TF-FVIIa complexes, through the contribution of substrate factor X binding to the cell membrane. However, this seems unlikely as the increased catalytic activity of decrypted TF-FVIIa complexes stems mainly from an increase in Vmax without an accompanying decrease in the apparent Km. Further, prothrombin fragment 1, which inhibits FX binding to PS, failed to attenuate increased procoagulant activity of decrypted TF [33]. Overall, it appears that direct interaction between TF or TF-FVIIa complexes with anionic phospholipid, independent of substrate binding may play an important role in TF decryption. Further work is needed to obtain direct evidence for this and to identify potential changes in TF (or TF-FVIIa complexes) conformation following its interactions with anionic phospholipids.
(ii) Tissue factor self-association: Dimers vs. monomers
Chemical cross-linking studies showed that a minor fraction of TF on unperturbed cells exists as dimers and multimers, suggesting self-association of TF on cell surfaces [22;45]. Both the cytoplasmic and transmembrane domains appear to be necessary for optimal self-association of TF as the cross-linking is reduced in case of TF variant lacking cytoplasmic domain or eliminated entirely in case of TF variant harboring non-homologus transmembrane domain [45]. Interestingly, stimulation of TF expressing cells (HL60 cells) with Ca2+ ionophore that triggers the activation of TF also prevented the self-association of TF [22]. The failure to cross-link TF following the treatment of cells with Ca2+ ionophore was taken as evidence that calcium influx into the cytosol produces a change in TF quaternary structure [22]. Based on these and other supporting data, it had been proposed that TF exists as dimers on the surface of unperturbed cells and the dimer form of TF is the cryptic form, and calcium influx into cytosol triggers a calmodulin-dependent process, which converts inactive TF dimers to procoagulant TF monomers [22]. In this model, the FX/FIX docking site on TF are at the interfaces of homodimers, and thus not readily accessible for the substrate binding, and the dissociation of TF dimers into monomers is thought to expose the binding site on TF for FX/FIX, and thus converting cryptic TF to procoagulant TF [13].
Although this model does not exclude a role for PS in ionophore-induced TF activation, it is unclear whether exposure of PS and dissociation of TF dimers into monomers following the ionophore treatment are coordinated responses that are essential for TF activation. It is conceivable that PS direct interaction with TF could alter TF quaternary structure, but there was no direct or indirect evidence such an interaction plays a role in the dissociation of TF dimers following ionophore treatment. There are a number of caveats in the above proposed model. First, the predominant species of TF seen in every cell type that expresses TF is the monomeric form. If the cryptic TF is supposed to be in the dimeric form, then one expects to see most of the TF should exist in the dimeric form. Second, the observation that calcium ionophore treatment dissociates TF dimers to monomers was limited, as far as the authors are aware of, to a single report using one specific cell type, HL60 cells [22]. There is no information on whether other stimuli that are known to activate TF also dissociate TF dimers to monomer in these cells or the ionophore-treatment causes dissociation of TF dimers in other cell types. Third, although it is difficult to compare the data obtained with TF in solution and membrane anchored TF, TF dimerization, by attaching a leucine zipper motif to the carboxy terminus of soluble TF, was shown not to inhibit TF procoagulant activity [46]. A review article by Bach [13] provides an in-depth discussion on the evidence for and against this proposed model of TF decryption.
(iii) Protein disulfide isomerase: Disulfide bond switching
Disulfide bond formation plays an important role in the protein folding process and stabilizing the structure of subdomains [47]. Inhibition of disulfide bond formation as a consequence of cellular stress or mutations would result in misfolding of the protein, and the misfolded proteins have a tendency to form aggregates or get destroyed by the ubiquitin proteosome system [48;49]. The extracellular domain of TF contains 4 cysteines, which could form two disulfide bonds, one at the amino-terminal half (Cys49-Cys57) and one proximal to the membrane domain (Cys186-Cys209). Cys186-Cys209 disulfide bond has RHStaple configuration and thus has potential to be an allosteric disulfide bond [50]. Analysis of human TF purified from brain or transfected mammalian cells showed that four cysteines in the extracellular domain participate in forming two disulfide bonds and there were no free sulfhydryl groups exist in TF protein [51;52]. Mutagenesis studies showed that preclusion of the disulfide bond between Cys49 and Cys57 had no effect on TF function, whereas ablation of the allosteric bond between Cys186 and Cys209 severely impaired the procoagulant activity [34;53;54]. The later observation was used to hypothesize that cryptic TF contains unpaired cysteine thiols that were depleted upon activation and the mutant lacking Cys186-Cys209 disulfide bond, in fact, mimics the cryptic form of TF [34].
To investigate the above hypothesis, Chen et al. [34] treated HL60 cells with thioloxidizing agent, HgCl2, and tested for TF activation and disulfide bond formation. HgCl2 treatment increased the cell surface TF activity of HL60 cells by 15-fold. Similarly, dithiol cross-linkers also activated HL60 TF activity up to 5 to 7-fold. Monofunctional thiol alkylators that block unpaired cysteine residues reduced HgCl2-induced TF activation. Although these data were consistent with the proposed hypothesis that cryptic TF may contain unpaired cysteine thiols and the activation requires the disulfide bond formation, these investigators failed to demonstrate that TF in HL60 cells actually contains free thiols and that HgCl2 treatment facilitates the disulfide bond formation with HgCl2 [34]. Although evidence for the presence of free thiols and the depletion of free thiols following HgCl2 treatment was shown in BHK cells stably transfected with human TF, these data are not fully convincing. In these cells, either calcium ionomycin or HgCl2 treatment increased TF activity by only two-fold and depleted the free thiols to different extents. The depletion of free thiols in HgCl2-treated cells could be an artifact as this treatment not only prevented the incorporation of free thiol-directed probe into TF but also into all cellular proteins [34]. A more problematic loophole in the above study is that both calcium ionophore and HgCl2 treatments were shown to increase PS on the outer leaflet of the plasma membrane [12;22;32;55-57], a known and potent inducer of TF activation. Although Chen et al. [34] concluded that TF activation they observed in HL60 cells could not be explained by exposure of PS on the cell surface membrane, the data shown in this report does not support this conclusion convincingly. Recently we showed clearly that HgCl2 treatment in HL60 cells and other cell types induces PS exposure at the outer cell surface, and blocking PS with annexin V attenuated the HgCl2-induced TF activation [56;57].
The hypothesis that status of Cys186-Cys209 disulfide bond dictates whether TF is cryptic or coagulant active and the disulfide switching regulates TF procoagulant activity and signaling has gained much attention from studies published by Ruf and colleagues [54]. These investigators demonstrated by mutational studies that the formation of Cys186-Cys209 disulfide is required to generate TF with high affinity for FVIIa and to express full coagulant activity but is not necessary for TF-FVIIa-mediated cell signaling via PAR2. These studies also revealed that protein disulfide isomerase (PDI), an enzyme capable of catalyzing all of the reactions involved in disulfide bond formation, cleavage and isomerization [58], associates with TF extracellular domain on the cell surface of HaCaT keratinocytes under growth conditions that lead to low TF procoagulant activity. Further studies showed that exposure of cells to HgCl2, the oxidizing agent that was shown to activate TF, dissociated PDI from TF. Inhibition of PDI expression by siRNA was found to increase TF procoagulant activity. Overall these studies indicate that disulfide bond cleavage by PDI acts allosterically to switch TF function. The hypothesis that PDI activates TF by disulfide isomerization was further supported by Reinhardt et al. [59]. These investigators detected the presence of free thiols in soluble and cell surface TF, and exposure of soluble TF to supernatants from activated platelets or recombinant PDI was shown to decrease number of thiols. These studies also suggest that cysteine 209 of TF was constitutively glutathionylated, which maintains TF in a cryptic state, and PDI converts cysteines from glutathionylated to disulfide state.
The hypothesis that PDI regulates TF activation received further support from in vivo thrombotic models. A time-dependent increase in PDI was observed in murine thrombus following injury, and infusion of blocking monoclonal antibody against PDI or the PDI inhibitor bacitracin inhibited platelet thrombus formation and fibrin generation in different murine models of thrombus formation [59;60]. Although these studies provide strong evidence that PDI plays a critical role in local thrombin generation following the vascular injury, they do not show that PDI promotes coagulation by switching disulfide bond in TF. There was no direct evidence that TF actually exists in the reduced form in vivo, that PDI decrypts TF by forming the Cys186-Cys209 disulfide bond, and that the decrypted TF is responsible for thrombus formation. PDI may influence thrombin generation and fibrin formation by its known actions on platelet activation [61] and integrin ligation [62].
Although the proposed mechanism of PDI-mediated disulfide isomerization regulating the activity status of TF is novel and very interesting, and on the face value the published data appear to provide strong support for this hypothesis, at present these data remain at best circumstantial. A main draw back in these studies is that they failed to provide unequivocally convincing evidence that disulfide switching and not other potential changes, such as exposure of anionic phospholipids following perturbation of cells to activate TF in vitro or after vessel wall injury in vivo, are responsible for observed activation of TF. The primary evidence for the postulate that activation of TF involves the disulfide bond formation of Cys186-Cys209 comes from two key observations. First, the observation that TF mutant, in which Cys186-Cys209 disulfide bond formation was precluded by site-specific mutagenesis, exhibits very low or no TF coagulant activity [34;53;54]. Second, TF contains free thiols and the thiol oxidizing agent HgCl2 or oxidoreductase enzyme PDI facilitates the disulfide bond formation and increase TF procoagulant activity. We have critically evaluated the validity of these two postulates.
Careful analysis of HgCl2-mediated TF activation in a variety of cells (MDA-MB-231, fibroblasts, stimulated endothelial cells and HL60 cells) showed that increased availability of PS at the cell surface, and the PS is responsible for the increased TF activity following TF activation [56;57]. No evidence was found for the presence of free thiols in TF expressed at the cell surface [57]. Further, there was no evidence even for the presence of PDI let alone an association of TF with PDI at the cell surface [57]. Even if there is undetectable amount of PDI present on cell surfaces and this PDI associates with TF, the PDI levels would be far below the stoichiometric levels of TF, thus it would have no significant consequences on the status of the disulfide bond of TF. PDI is predominantly localized in ER, and in the absence of a classical membrane attachment site, it would seem probable that, at best, only traces of PDI would be present on cell surfaces [63]. Although a number of earlier reports, including a recent report suggest that PDI is secreted from platelets and endothelial cells upon their activation [64], other studies found no evidence for secretion of PDI [65-67]. In a recent study, PDI in platelets was shown to be exclusively located in the dense tubular system and remains confined to the intracellular dense tubular system and neither released nor targeted to the cell surface following platelet activation by various stimuli [65]. Similar results were obtained in endothelial cells and other cell types [65-67] (Fig. 3). Finally, inhibition of PDI by PDI neutralizing antibodies or gene silencing, or addition of rPDI exogenously had no effect on TF coagulant activity [57]. Overall these findings undermine the hypothesis that PDI-mediated disulfide exchange plays a role in regulating TF procoagulant and cell signaling functions. To explain variance between our data [57] and the earlier published data [34;54], Liang and Hogg [68] suggested that some cell lines, such as MDA-MB 231 cells are not appropriate for studying TF decryption as they believe TF is constitutively active in this cell line. However, this suggestion may not be valid. Although it is possible that the amount of active TF could be higher in MDA-MB231 cells compared to other cell types, as in other cell types the majority of TF on MDA-MB231 cells also appears to be in the cryptic form [69].
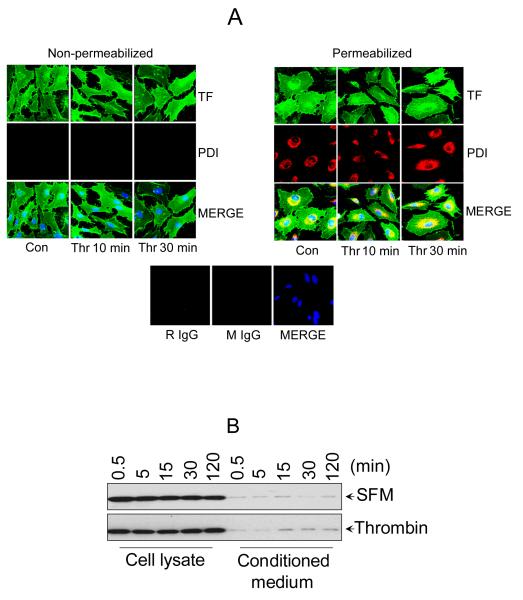
Figure 3. PDI is localized intracellularly in endothelial cells and not secreted in response to thrombin.
(A) Cellular localization of PDI by immunofluorescence confocal microscopy. HUVEC transfected with TF adenovirus were stimulated with thrombin for 10 or 30 min (0.5 U/ml) or left unperturbed. After the treatment, the cells were fixed and permeabilized with 0.25% Triton X-100. Both the intact and permeabilized cells were immunostained with rabbit anti-human TF IgG and monoclonal PDI antibody (RL90), followed by Oregon Green-labeled anti-rabbit IgG and Rhodamine Red-conjugated anti-mouse IgG as secondary reporter antibodies. The bottom panel shows staining with isotytpe controls IgG (B) HUVEC cultured in 24-well plate were treated with 500 μl of control serum-free medium or serum-free medium containing thrombin (0.5 U/ml) for varying time periods. At the end of the treatment, the whole supernatant medium was collected and concentrated by 25 times (to ~20 μl) by ultrafiltration and the cells were lysed in 100 μl of lysis buffer. Equal volumes (10 μl) of cell lysates and the concentrated supernatant media were subjected to SDS-PAGE and immunoblotted with anti-PDI antibodies.
Recently, we also evaluated critically the concept that the status of Cys186-Cys209 disulfide bond dictates whether TF is cryptic or coagulant active and the formation of Cys186-Cys209 disulfide bond is essential for its decryption [56]. We found, as reported earlier [34;54;70] that cells transfected with TF mutants that preclude Cys186-Cys209 bond formation expressed very little procoagulant activity at the cell surface. However, this study also revealed that very little TF protein was present at the cell surface in these transfected cells. Detailed analysis of FVIIa and TF activity in HUVEC expressing similar levels of wild-type TF and TFC186S/C209S showed that the TF mutant had severe impairment in binding to FVIIa, but once it binds to FVIIa, the mutant TF-FVIIa complexes exhibited a similar coagulant activity as that of wild-type TF-FVIIa complexes. As discussed in the preceding section, cryptic TF forms a stable high-affinity association with FVII/FVIIa well below plasma concentrations of FVII, increasing the FVIIa concentration does not transform cryptic TF to procoagulant TF. TF disulfide mutants that exhibit low procoagulant activity because they have diminished affinity for FVIIa do not fit with this definition and therefore should not be viewed that the TF mutant lacking Cys186-Cys209 disulfide bond as a mimic of cryptic TF. More importantly, our data also revealed that treatment of human umbilical vein endothelial cells (HUVEC) expressing TFC186S/C209S with the thiol oxidizing agent HgCl2 or ionomycin increased the cell surface TF activity to the same extent as that of the wild-type TF. These data clearly illustrate that although the Cys186-Cys209 disulfide bond is critical for TF synthesis/processing and for FVIIa binding, it is not essential for TF decryption per se. As with natural and wild-type TF, TF disulfide mutant expressed on cell surfaces also exist in two forms, cryptic and coagulant active. However the disulfide mutated coagulant active TF exhibits low procoagulant activity because it binds FVIIa poorly, but the procoagulant activity of this form could be restored completely using high concentrations of FVIIa. Cryptic disulfide mutant TF could undergo decryption process in same manner as that of wild-type TF.
In addition to the above experimental data that raised critical questions on validity of the hypothesis that assign a critical role for disulfide switching for TF encryption and decryption processes, other investigators raised theoretical concerns on the validity of this hypothesis [71;72]. Bach and Monroe [72] points out that the half-cystines are buried deep within complex when FVIIa binds to the extracellular domain of TF and therefore, Cys186 and Cys209 are not available to interact with PDI or even participate in the proposed reactions. In light of several experimental and theoretical inconsistencies, further support and clarification are needed to validate the hypothesis that PDI-mediated disulfide switching induces TF decryption.
(iv) Protein disulfide isomerase: Regulation of TF activity by modulation of lipid environment
As discussed earlier in this chapter, exposure of PS in response to a variety of stimuli has been considered the primary mechanism responsible for TF decryption. PS is normally sequestered in the inner leaflet of the plasma membrane. The asymmetric distribution of PS in the plasma membrane of quiescent cells is maintained by ATP-dependent aminophospholipid translocase (catalyzes the transport of PS from outer to inner membrane leaflet) and Ca2+-dependent phospholipid scramblase (catalyzes rapid transbilayer movement of phospholipids between membrane leaflets) [73-75]. The translocase activity is dependent on a free thiol group and thus modification of the thiol group by alkylation or oxidation by the sulfhydryl-reactive agents such as NEM or diamide results in cell surface exposure of PS [76-78]. Thiolation also significantly lowers the threshold for Ca2+ required to activate outward PS transport by the scramblase and may directly activate a stress response that leads to a surge in Ca2+ into the cytosol [78]. Therefore, it is possible that oxidoreductase, such as PDI, could modulate changes in PS dynamics. Consistent with this possibility, Popescu et al. [79] showed recently using an endothelial cell model system that inhibition of cell surface PDI by PDI mAb induced a marked increase in TF procoagulant function. Increased TF procoagulant activity in cells treated with anti-PDI antibody was correlated with the increased prothrombinase activity and inhibited by annexin V, suggesting exposure of PS at the cell surface upon treatment with the antibody. PS exposure was confirmed by direct annexin V binding and the dynamics of fluorescent analogs of PS. Overall these data suggest that PDI affects coagulation at least in part through changes in lipid asymmetry of the plasma membrane and PDI acts as a negative regulator of coagulation. In our own studies, we have observed a small increase in TF procoagulant activity in cells treated with PDI mAb, the same isotype used by Popescu et al. [79]. However, we also found that the effect of the antibodies was lost if the antibodies were dialyzed before using them in the treatment. Initial analysis suggests that glycerol present in the antibody stock could have contributed to the increased PS exposure (Fig. 4), but this needs to be confirmed independently. Additional studies utilizing different cell model systems are required to validate PDI-induced changes in lipid environment plays a role in TF decryption.
Figure 4. The effect PDI mAb, before and after dialysis, on tissue factor procoagulant activity in endothelial cells.
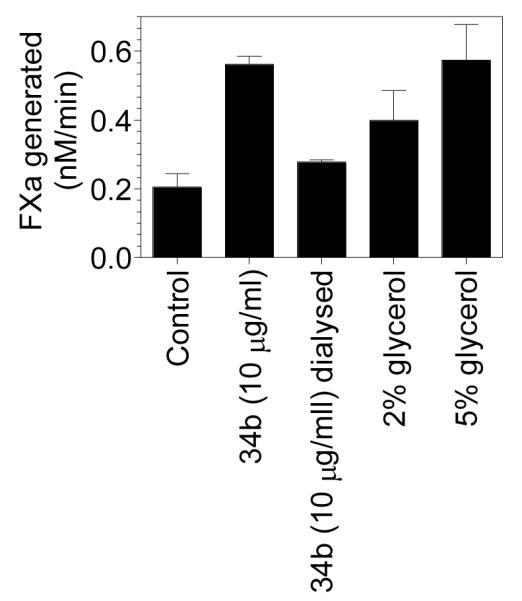
PDI mAb (clone #34b) was obtained from BD Biosciences, which was supplied at 250 μg/ml concentration in glycerol. A portion of the antibody was dialyzed overnight against 10 mM Hepes buffer to remove the glycerol. Confluent monolayers of HUVEC were treated with pre and post-dialyzed PDI mAb (10 μg/ml) for 30 min at room temperature. In parallel wells, HUVEC were also treated with glycerol (2 and 5%). At the end of 30 min treatment, FVII (10 nM) and FX (175 nM) was added to cells and the rate of FX activation was measured in a chromogenic assay.
(v) Protein disulfide isomerase: Regulation of TF activity by chaperone function
Versteeg and Ruf [80] reported that PDI selectively acts, independent of its oxidoreductase activity, upon cryptic TF to facilitate ternary complex formation and macromolecular substrate turnover. These investigators showed that PDI enhances the activity of FVIIa-dependent activation of factor X in the presence of wild-type, oxidized soluble TF (sTF) but not TF mutants that contain an unpaired Cys186 or Cys209. PDI was also shown to enhance TF coagulant activity on microvesicles shed from cells PDI-accelerated factor Xa generation was blocked by bacitracin. However, inhibition of vicinal thiols, reduction of PDI or other modifications that specifically abolish PDI oxidoreductase activity but not chaperone activity failed to block PDI-mediated enhancement of FX activation. Based on these data it had been suggested that PDI plays a role as an activating chaperone for circulating cryptic TF. Interestingly, PDI had no effect on full-length TF that had been reconstituted in negatively charged phospholipids or cell surface TF [80]. In contrast to these data, we found no significant effect of PDI on the activity of sTF [81]. The main difference between these two studies was that the earlier study employed commercially obtained purified bovine PDI whereas we used recombinant human PDI. Comparative studies with purified bovine PDI and recombinant human PDI revealed that PS contaminant in the bovine PDI reagent could have contributed to the enhancing effect of PDI. These observations have been confirmed independently by others [82]. Thus, it appears that PDI had no stimulatory chaperone effect on FX activation by sTF-FVIIa and the effect observed with bovine PDI could have been the result of contaminant PS-like material in the bovine PDI. However, in a recent study Raturi and Ruf [83] reported that extraction of bovine PDI with acetone to remove any potential contaminant did not diminish the potency of the rate-enhancing effect of PDI in the soluble TF system whereas depletion of the bPDI preparation with anti-PDI antibody completely abolished the rate-enhancing effect of bPDI. It is possible that PS or an unknown component that structurally bound PDI, which is responsible for the rate enhancing effect of soluble TF, could have been depleted along with PDI when bovine PDI was passed through the anti-PDI antibody column. Although it is unlikely that the chaperone activity of PDI plays a role in TF decryption, in light of the recent observations [83], further studies are needed to determine that PDI actually increases TF activity via its chaperone activity.
(vi) Caveolae, lipid rafts and cholesterol: Modulation of tissue factor procoagulant activity
Distribution of TF on cell surfaces is not a random process. Ultrastructural localization of TF in a variety of cell types showed that a fraction of the TF in these cells is associated with caveolae, a specialized membrane domain rich in cholesterol- and sphingolipid-rich rafts. [84-87]. Mulder et al. [84] speculated that the caveolae-associated TF represents the encrypted form, which can rapidly be activated at sites in which vessel wall integrity is lost. Ruf and colleagues [88] demonstrated that TF translocates to caveolae following ternary complex formation with FVIIa, TFPI, FXa. TF translocated to caveolae exhibited a lower procoagulant activity even in the presence of anti-TFPI antibodies. These data are consistent with the hypothesis that TF localized in caveolae is less active or cryptic. Further supporting this hypothesis, treatment of HEK293 cells or dermal fibroblasts with methyl ß-cyclodextrin (mßCD), which disrupts caveolar structure by removing membrane cholesterol, increased the TF procoagulant activity by 2 to 3-fold [89]. However, opposite results were reported in other cell model systems. Disruption of caveolae by removal of cholesterol with mßCD treatment was shown to decrease TF procoagulant activity in fibroblasts and tumor cells [87;90]. Interestingly, filipin treatment, which also disrupts caveolae, increased TF procoagulant activity [90]. Disruption of caveolae by silencing of caveloin-1 had no effect on TF coagulant activity at the cell surface [87]. Overall these data indicate that caveolae are not negative regulators of TF procoagulant activity, as previously thought. It is possible that the increased TF-FVIIa procoagulant activity observed in cells following caveolae disruption may arise independent of caveolae. For example, increased TF procoagulant activity associated with filipin treatment of fibroblasts [90] could have been the result of the increased concentration of cholesterol in membrane patches due to the fact that filipin sequesters the membrane cholesterol by forming complex with it [91]. The observation that depletion of membrane cholesterol reduces the cell surface TF activity and increasing the cholesterol concentration in the membrane by loading the cells with cholesterol increases the cell surface TF activity [90] fits with the concept that cholesterol regulates TF activity. Cholesterol modulation of TF activity on cell surfaces appears to be independent of PS. FVIIa binding studies suggest that membrane cholesterol may modulate TF affinity to FVIIa [90]. At present it is unclear whether cholesterol modulates TF procoagulant activity by direct interaction between cholesterol and TF or as a critical component necessary to form lipid rafts. Interestingly, del conde et al. [92] found that TF-bearing microvesicles selectively arise from lipid raft regions from the membrane, and these vesicles readily fuse with the activated platelets and thus increasing the catalytic efficiency of TF-FVIIa procoagulant activity. Further studies are needed to sort out the importance of caveolae, lipid rafts, and cholesterol in TF encryption and decryption.
7. Cryptic TF and TF-FVIIa signaling
The formation of TF-FVIIa complexes on cell surfaces not only triggers the coagulation cascade but also transduces cell signaling via activation of protease-activated receptors (PARs) [93;94]. Interestingly, in contrast to sub-nanomolar concentration of FVIIa needed to from TF-FVIIa coagulant active complexes maximally, a much higher concentration of FVIIa (5 to 10 nM) were needed to express TF-FVIIa signaling complexes [17;54;93]. The FVIIa concentration required for TF-FVIIa cell signaling is quite similar to that of FVIIa binding to all the available TF sites on cell surfaces [95], suggesting that cryptic TF-FVIIa complexes are capable of activating PAR2. The observation that a TF monoclonal antibody that presumably binds to cryptic TF inhibited the TF-FVIIa signaling without inhibiting the TF procoagulant activity has provided further evidence for that cryptic TF-FVIIa is capable of activating PAR2 [54]. Ahamed et al. [54] showed that Cys186-Cys209 disulfide bond is not required for TF-FVIIa-induced PAR2-mediated cell signaling as a TF mutant (TF C209A) with an unpaired Cys186 retains TF-FVIIa signaling. Further it had been shown that TF associates with PDI extracellularly and disulfide/thiol exchange is required for TF-PAR2 complex formation and TF-FVIIa signaling [54]. Based on these data, it has been postulated that TF-FVIIa-mediated coagulation and cell signaling involves two distinct cellular pools of TF - the TF with intact Cys186-Cys209 disulfide bond is the coagulant active/cell signaling inactive and the TF with the reduced Cys186-Cys209 disulfide bond is cryptic/cell signaling active. The disulfide exchange mediated by PDI acts as a regulatory mechanism that switches TF function from coagulation to signaling and vice versa.
The notion that cryptic TF is functional in TF-FVIIa signaling seems valid and consistent with early observations that showed FVIIa binds to cryptic TF and the resultant TF-FVIIa complexes are catalytically active [8]. It also correlates well with dose-dependent kinetics of FVIIa binding to TF and TF-FVIIa signaling [95]. However, it is doubtful at present whether the status of Cys186-Cys209 bond actually determines whether TF can be active either in coagulation or cell signaling and PDI-mediated disulfide isomerization switches TF from coagulation to cell signaling or vice versa. There is no compelling evidence that only cryptic TF and not the coagulant active TF supports the TF-FVIIa-mediated cell signaling. Further, as discussed earlier, PDI association with TF and its effect on either TF-FVIIa signaling or the coagulation is not evident in all cell types [57].
8. TF encryption and FVII activation
As plasma contains only traces of FVIIa [96], most of the complexes formed, either intravascularly (if any), or extravascularly, are TF-FVII complexes. Therefore, FVII bound to TF has to be converted to FVIIa first before it could activate factors X and IX. FVII bound to TF can be converted to FVIIa via an autocatalytic reaction [97]. Since plasma already contains traces of FVIIa, traces of TF-FVIIa will be formed on cell surfaces along TF-FVII, and if both the complexes are close enough to interact TF-FVIIa can convert FVII bound to TF to FVIIa [98;99]. Alternatively traces of FVIIa bound to coagulant active TF can activate traces of FX to FXa, and this FXa can readily activate FVII bound to TF [28]. Activation of TF bound FVII by FXa is much more efficient than autocatalytic activation of FVII [100]. It is unclear at present whether FVII bound to cryptic TF could be converted to FVIIa either autocatalytically or by FXa or TF had to undergo decryption process before it could support FVII activation. We are not aware of any studies in the literature that examined this directly. Since the encrypted TF-FVIIa does not interact efficiently with substrates FX/FIX [8;13;22], it is logical to believe that FXa also does not bind well to encrypted TF-FVII. Therefore, FVII bound to encrypted TF would be resistant to activation by FXa. However, analysis of FVII activation bound to cell surface TF showed that FVII bound to TF was readily activated by FXa [100]. Although these studies were not designed specifically to address whether FXa activates the FVII bound to cryptic TF, since most of the FVII bound to TF is converted to FVIIa in these experiments, it is likely that FVII bound to cryptic TF may also activated by FXa. However, further experiments are needed to validate this possibility. Whether encrypted or decrypted TF-FVIIa can autocatalytically activate encrypted TF-FVII or decryption of TF-FVII is required before it is activated by decrypted TF-FVIIa remains unknown. Either case, since both the substrate TF-FVII and the enzyme TF-FVIIa are anchored to the membrane, the rate of autoactivation would be highly dependent on surface density of TF and the membrane mobility [13]. As the activation of FVII bound to TF is an essential step in the initiation of TF-dependent blood coagulation [101] and there were no specific studies that examined the role of TF encryption and decryption in regulating FVII activation, further studies are warranted in this direction.
9. Conclusions
Although at present it is not entirely clear how cryptic TF differs from procoagulant TF and mechanisms responsible for TF decryption, the predominant evidence in the literature point out that availability of anionic phospholipids, such as PS, on the membrane is the critical modulator of TF procoagulant activity. There is a wider acceptance for the hypothesis that exposure of PS on the outer surface of cell membrane decrypts TF. However, a role for PS does not automatically exclude the involvement of other mechanisms in TF decryption. However, if these other mechanisms also result in exposure of PS, then it is fair to scrutinize them more critically and vigorously to determine whether the proposed mechanism(s) and not the exposure of PS are responsible for TF decryption. Validating the potential mechanism in several cell types with different activating stimuli would be required to establish it as a general regulatory mechanism for TF decryption. The most challenging task for future is to decipher how PS transforms cryptic TF to procoagulant TF. This may require development of specific reagents that recognize different forms of TF, development of new technologies and innovative experimental approaches.
Acknowledgements
This work was supported by grants from the National Institute of Health HL58869 and HL65500. The authors thank Curtis Clark for proofreading the manuscript.
11. Reference List
- 1.Fleck RA, Rao LVM, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–37. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 2.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 3.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nature Medicine. 1996;2:209–15. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 4.Osterud B, Flaegstad T. Increased thromboplastin activity in monocytes of patients with meningococcal infection: Related to an unfavorable prognosis. Thromb Haemost. 1983;49:5–7. [PubMed] [Google Scholar]
- 5.Maynard JR, Heckman CA, Pitlick FA, Nemerson Y. Association of tissue factor activity with the surface of cultured cells. J Clin Invest N Y. 1975;55:814–22. doi: 10.1172/JCI107992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broze GJ., Jr Binding of human factor VII and VIIa to monocytes. J Clin Invest N Y. 1982;70:526–35. doi: 10.1172/JCI110644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fair DS, MacDonald MJ. Cooperative interaction between factor VII and cell surface-expressed tissue factor. J Biol Chem. 1987;262:11692–8. [PubMed] [Google Scholar]
- 8.Le DT, Rapaport SI, Rao LVM. Relations between factor VIIa binding and expression of factor VIIa/tissue factor catalytic activity on cell surfaces. J Biol Chem. 1992;267:15447–54. [PubMed] [Google Scholar]
- 9.Sakai T, Lund-Hansen T, Paborsky L, Pedersen AH, Kisiel W. Binding of human factors VII and VIIa to a human bladder carcinoma cell line (J82) implications for the initiation of the extrinsic pathway of blood coagulation. J Biol Chem. 1989;264:9980–8. [PubMed] [Google Scholar]
- 10.Walsh JD, Geczy CL. Discordant Expression of Tissue Factor Antigen and Procoagulant Activity on Human Monocytes Activated with LPS and Low Dose Cycloheximide. Thromb Haemost. 1991;66:552–8. [PubMed] [Google Scholar]
- 11.Ploplis VA, Edgington TS, Fair DS. Initiation of the extrinsic pathway of coagulation. Association of factor VIIa with a cell line expressing tissue factor. J Biol Chem. 1987;262:9503–8. [PubMed] [Google Scholar]
- 12.Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990;87:6995–9. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–61. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 14.Drake TA, Ruf W, Morrissey JH, Edgington TS. Functional tissue factor is entirely cell surface expressed on lipopolysaccharide-stimulated human blood monocytes and a constitutively tissue factor-producing neoplastic cell line. J Cell Biol. 1989;109:389–95. doi: 10.1083/jcb.109.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai T, Lund-Hansen T, Paborsky L, Pedersen AH, Kisiel W. Binding of human factors VII and VIIa to a human bladder carcinoma cell line (J82) J Biol Chem. 1989;264:9980–8. [PubMed] [Google Scholar]
- 16.Sorensen BB, Persson E, Freskgard P-O, Kjalke M, Ezban M, Williams T, Rao LVM. Incorporation of an active site inhibitor in factor VIIa alters the affinity for tissue factor. J Biol Chem. 1997;272:11863–8. doi: 10.1074/jbc.272.18.11863. [DOI] [PubMed] [Google Scholar]
- 17.Petersen LC, Albrektsen T, Hjorto GM, Kjalke M, Bjorn SE, Sorensen BB. Factor VIIa/tissue factor-dependent gene regulation and pro-coagulant activity: effect of factor VIIa concentration. Thromb Haemost. 2007;98:909–11. [PubMed] [Google Scholar]
- 18.Ghosh S, Ezban M, Persson E, Pendurthi U, Hedner U, Rao LV. Activity and regulation of factor VIIa analogs with increased potency at the endothelial cell surface. J Thromb Haemost. 2006;5:336–46. doi: 10.1111/j.1538-7836.2007.02308.x. [DOI] [PubMed] [Google Scholar]
- 19.Sen P, Ghosh S, Ezban M, Pendurthi UR, Vijaya Mohan RL. Effect of glycoPEGylation on factor VIIa binding and internalization. Haemophilia. 2010;16:339–48. doi: 10.1111/j.1365-2516.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 20.Broze GJ. The role of tissue factor pathway inhibitor in a revised coagulation cascade. Semin Hematol. 1992;29:159–69. [PubMed] [Google Scholar]
- 21.Rapaport SI, Rao LVM. Initiation and regulation of tissue factor-dependent blood coagulation. Arterioscler Thromb. 1992;12:1111–21. doi: 10.1161/01.atv.12.10.1111. [DOI] [PubMed] [Google Scholar]
- 22.Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89:3270–6. [PubMed] [Google Scholar]
- 23.Zwaal RF, Bevers EM, Comfurius P, Rosing J, Tilly RH, Verhallen PF. Loss of membrane phospholipid asymmetry during activation of blood platelets and sickled red cells; mechanisms and physiological significance. Mol Cell Biochem. 1989;91:23–31. doi: 10.1007/BF00228075. [DOI] [PubMed] [Google Scholar]
- 24.Chargaff E. Remarks on the role of lipids in blood coagulation. Arch Sci Physiol. 1948;2:269–71. [Google Scholar]
- 25.Nemerson Y. The phospholipid requirement of tissue factor in blood coagulation. J Clin Invest. 1968;47:72–80. doi: 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjorklid E, Storm E. Purification and Some Properties of the Protein Component of Tissue Thromboplastin from Human Brain. Biochem J. 1977;165:89–96. doi: 10.1042/bj1650089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnaswamy S, Field KA, Edgington TS, Morrissey JH, Mann KG. Role of the membrane surface in the activation of human coagulation factor X. J Biol Chem. 1992;267:26110–20. [PubMed] [Google Scholar]
- 28.Rapaport SI, Rao LVM. The tissue factor pathway: How it has become a “prima ballerina”. Thromb Haemost. 1995;74:7–17. [PubMed] [Google Scholar]
- 29.Neuenschwander PF, Bionco-Fisher E, Rezaie AR, Morrissey JH. Phosphatidylethanolamine augments factor VIIa-tissue factor activity: Enhancement of sensitivity to phosphatidylserine. Biochem. 1995;34:13988–93. doi: 10.1021/bi00043a004. [DOI] [PubMed] [Google Scholar]
- 30.Shaw AW, Pureza VS, Sligar SG, Morrissey JH. The local phospholipid environment modulates the activation of blood clotting. J Biol Chem. 2007;282:6556–63. doi: 10.1074/jbc.M607973200. [DOI] [PubMed] [Google Scholar]
- 31.Carson SD, Archer PG. Tissue factor activity in Hela cells measured with a continuous chromogenic assay and elisa reader. Thromb Res. 1986;41:185–95. doi: 10.1016/0049-3848(86)90228-8. [DOI] [PubMed] [Google Scholar]
- 32.Wolberg AS, Monroe DM, Roberts HR, Hoffmann MR. Tissue factor de-encryption:ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coag Fibrinol. 1999;10:201–10. [PubMed] [Google Scholar]
- 33.Le DT, Rapaport SI, Rao LVM. Studies of the mechanism for enhanced cell surface factor VIIa/tissue factor activation of factor X in fibroblast monolayers after their exposure to N-ethylmalemide. Thromb Haemost. 1994;72:848–55. [PubMed] [Google Scholar]
- 34.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochem. 2006;45:12020–8. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 35.Carson SD, Johnson DR. Consecutive Enzyme Cascades: Complement Activation at the Cell Surface Triggers Increased Tissue Factor Activity. Blood. 1990;76:361–7. [PubMed] [Google Scholar]
- 36.Carson SD. Manifestation of cryptic fibroblast tissue factor occurs at detergent concentrations which dissolve the plasma membrane. Blood Coag Fibrin. 1996;7:303–13. doi: 10.1097/00001721-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Otnaess AB, Prydz H, Bjorklid E, Berre A. Phospholipase C from Bacillus cereus and its use in studies of tissue thromboplastin. Eur J Biochem. 1972;27:238–43. doi: 10.1111/j.1432-1033.1972.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 38.Ravanat C, Archipoff G, Beretz A, Freund G, Cazenave JP, Freyssinet JM. Use of annexin-V to demonstrate the role of phosphatidylserine exposure in the maintenance of haemostatic balance by endothelial cells. Biochem J. 1992;282:7–13. doi: 10.1042/bj2820007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao LVM, Tait JF, Hoang AD. Binding of annexin V to a human ovarian carcinoma cell line (OC-2008). Contrasting effects on cell surface factor VIIa/tissue factor activity and prothrombinase activity. Thromb Res. 1992;67:517–31. doi: 10.1016/0049-3848(92)90013-z. [DOI] [PubMed] [Google Scholar]
- 40.Carson SD, Perry GA, Pirruccello SJ. Fibroblast tissue factor: Calcium and ionophore induce shape changes, release of membrane vesicles, and redistribution of tissue factor antigen in addition to increased procoagulant activity. Blood. 1994;84:526–34. [PubMed] [Google Scholar]
- 41.Rao LVM, Pendurthi UR. Tissue factor on cells. Blood Coag Fibrin. 1998;9:S27–S35. [PubMed] [Google Scholar]
- 42.Banner DW, D’Arcy A, Chene C, Winkler FM, Guha A, Konigsberg WH, Nemerson Y, Kirchhofer D. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–6. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 43.McCallum CD, Hapak RC, Neuenschwander PF, Morrissey JH, Johnson AE. The location of the active site of blood coagulation factor VIIa above the membrane surface and its reorientation upon association with tissue factor. A fluorescence energy transfer study. J Biol Chem. 1996;271:28168–75. doi: 10.1074/jbc.271.45.28168. [DOI] [PubMed] [Google Scholar]
- 44.McCallum CD, Su B, Neuenschwander PF, Morrissey JH, Johnson AE. Tissue factor positions and maintains the factor VIIa active site far above the membrane surface even in the absence of the factor VIIa Gla domain. J Biol Chem. 1997;272:30160–6. doi: 10.1074/jbc.272.48.30160. [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Paborsky LR, Vehar GA. Self-association of tissue factor as revealed by chemical crosslinking. J Biol Chem. 1991;266:4665–8. [PubMed] [Google Scholar]
- 46.Donate F, Kelly CR, Ruf W, Edgington TS. Dimerization of tissue factor supports solution-phase autoactivation of factor VII without influencing proteolytic activation of factor X. Biochem. 2000;39:11467–76. doi: 10.1021/bi000986p. [DOI] [PubMed] [Google Scholar]
- 47.Mamathambika BS, Bardwell JC. Disulfide-linked protein folding pathways. Annu Rev Cell Dev Biol. 2008;24:211–35. doi: 10.1146/annurev.cellbio.24.110707.175333. [DOI] [PubMed] [Google Scholar]
- 48.Hampton RY. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002;14:476–82. doi: 10.1016/s0955-0674(02)00358-7. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt B, Ho L, Hogg PJ. Allosteric disulfide bonds. Biochem. 2006;45:7429–33. doi: 10.1021/bi0603064. [DOI] [PubMed] [Google Scholar]
- 51.Bach R, Konigsberg WH, Nemerson Y. Human tissue factor contains thioester-linked palmitate and sterate on the cytoplasmic half-cystine. Biochem. 1988;14:4227–31. doi: 10.1021/bi00412a004. [DOI] [PubMed] [Google Scholar]
- 52.Paborsky LR, Harris RJ. Post-Translational Modifications of Recombinant Human Tissue Factor. Thromb Res. 1990;60:367–76. doi: 10.1016/0049-3848(90)90219-3. [DOI] [PubMed] [Google Scholar]
- 53.Rehemtulla A, Ruf W, Edgington TS. The integrity of the cystein 186-cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–9. [PubMed] [Google Scholar]
- 54.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaneko H, Kakkar VV, Scully MF. Mercury compounds induce a rapid increase in procoagulant activity of monocyte-like U937 cells. Br J Haematol. 1994;87:87–93. doi: 10.1111/j.1365-2141.1994.tb04875.x. [DOI] [PubMed] [Google Scholar]
- 56.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–83. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–8. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–22. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–31. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Essex DW, Li M, Miller A, Feinman RD. Protein disulfide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochem. 2001;40:6070–5. doi: 10.1021/bi002454e. [DOI] [PubMed] [Google Scholar]
- 62.Lahav J, Jurk K, Hess O, Barnes MJ, Farndale RW, Luboshitz J, Kehrel BE. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood. 2002;100:2472–8. doi: 10.1182/blood-2001-12-0339. [DOI] [PubMed] [Google Scholar]
- 63.Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol. 2002;193:154–63. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 64.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116:4665–74. doi: 10.1182/blood-2010-04-278184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Nispen Tot, Pannerden HE, van Dijk SM, Du V, Heijnen HF. Platelet protein disulfide isomerase is localized in the dense tubular system and does not become surface expressed after activation. Blood. 2009;114:4738–40. doi: 10.1182/blood-2009-03-210450. [DOI] [PubMed] [Google Scholar]
- 66.Kothari H, Kaur G, Sahoo S, Idell S, Rao LV, Pendurthi U. Plasmin enhances cell surface tissue factor activity in mesothelial and endothelial cells. J Thromb Haemost. 2009;7:121–31. doi: 10.1111/j.1538-7836.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajaj MS, Ghosh M, Bajaj SP. Fibronectin-adherent monocytes express tissue factor and tissue factor pathway inhibitor whereas endotoxin-stimulated monocytes primarily express tissue factor: physiologic and pathologic implications. J Thromb Haemost. 2007;5:1493–9. doi: 10.1111/j.1538-7836.2007.02604.x. [DOI] [PubMed] [Google Scholar]
- 68.Liang HPH, Hogg PJ. Critical importance of the cell system when studying tissue factor de-encryption. Blood. 2008;112:912–3. doi: 10.1182/blood-2008-05-158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pendurthi UR, Rao LVM. Tissue factor de-encyrption: the cell model sytem. Blood. 2008;112:913. [Google Scholar]
- 70.Rehemtulla A, Ruf W, Edgington TS. The integrity of the cysteine 186-cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–9. [PubMed] [Google Scholar]
- 71.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–96. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bach RR, Monroe D. What is wrong with the allosteric disulfide bond hypothesis? Arterioscler Thromb Vasc Biol. 2009;29:1997–8. doi: 10.1161/ATVBAHA.109.194985. [DOI] [PubMed] [Google Scholar]
- 73.Bevers EM, Smeets EF, Comfurius P, Zwaal RFA. Physiology of membrane lipid asymmetry. Lupus. 1994;3:235–40. doi: 10.1177/096120339400300406. [DOI] [PubMed] [Google Scholar]
- 74.Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971–88. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost. 2001;86:266–75. [PubMed] [Google Scholar]
- 76.Fadeel B, Gleiss B, Hogstrand K, Chandra J, Wiedmer T, Sims PJ, Henter JI, Orrenius S, Samali A. Phosphatidylserine exposure during apoptosis is a cell-type-specific event and does not correlate with plasma membrane phospholipid scramblase expression. Biochem Biophys Res Commun. 1999;266:504–11. doi: 10.1006/bbrc.1999.1820. [DOI] [PubMed] [Google Scholar]
- 77.van Engeland M, Kuijpers HJ, Ramaekers FC, Reutelingsperger CP, Schutte B. Plasma membrane alterations and cytoskeletal changes in apoptosis. Exp Cell Res. 1997;235:421–30. doi: 10.1006/excr.1997.3738. [DOI] [PubMed] [Google Scholar]
- 78.Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282:18357–64. doi: 10.1074/jbc.M700202200. [DOI] [PubMed] [Google Scholar]
- 79.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116:993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–24. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 81.Kothari H, Sen P, Pendurthi UR, Rao LV. Bovine protein disulfide isomerase-enhanced tissue factor coagulant function: is phospholipid contaminant in it the real culprit? Blood. 2008;111:3295–6. doi: 10.1182/blood-2007-12-129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Persson E. Protein disulfide isomerase has no stimulatory chaperone effect on factor X activation by factor VIIa-soluble tissue factor. Thromb Res. 2008;123:171–6. doi: 10.1016/j.thromres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Raturi A, Ruf W. Effect of protein disulfide isomerase chaperone activity inhibition on tissue factor activity. J Thromb Haemost. 2010;8:1863–5. doi: 10.1111/j.1538-7836.2010.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mulder AB, Smit JW, Bom VJJ, Blom NR, Ruiters MHJ, Halie R, van der Meer J. Association of smooth muscle cell tissue factor with caveolae. Blood. 1996;88:1306–13. [PubMed] [Google Scholar]
- 85.Mulder AB, Smit JW, Bom VJJ, Blom NR, Halie MR, van der Meer J. Association of endothelial tissue factor and thrombomodulin with caveolae. Blood. 1996;88:3667–3670M. [PubMed] [Google Scholar]
- 86.Mandal SK, Pendurthi UR, Rao LVM. Cellular localization and trafficking of tissue factor. Blood. 2006;107:4746–53. doi: 10.1182/blood-2005-11-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Awasthi V, Mandal SK, Papanna V, Rao LV, Pendurthi UR. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler Thromb Vasc Biol. 2007;27:1447–55. doi: 10.1161/ATVBAHA.107.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sevinsky JR, Rao LVM, Ruf W. Ligand induced protease receptor translocation into caveolae: A mechanism regulating cell surface proteolysis of the tissue factor dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dietzen DJ, Page KL, Tetzloff TA. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood. 2004;103:3038–44. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- 90.Mandal SK, Iakhiaev A, Pendurthi UR, Rao LV. Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol. Blood. 2005;105:153–60. doi: 10.1182/blood-2004-03-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–15. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 93.Rao LVM, Pendurthi UR. Tissue factor-factor VIIa signaling. Arterioscler Thromb Vasc Biol. 2005;25:47–56. doi: 10.1161/01.ATV.0000151624.45775.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–50. doi: 10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 95.Pendurthi UR, Allen KE, Ezban M, Rao LVM. Factor VIIa and thrombin induce the expression of Cyr61 and connective tissue growth factor, extracellular matrix signaling proteins that could act as possible downstream mediators in factor VII.tissue factor-induced signal transduction. J Biol Chem. 2000;275:14632–41. doi: 10.1074/jbc.275.19.14632. [DOI] [PubMed] [Google Scholar]
- 96.Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81:734–44. [PubMed] [Google Scholar]
- 97.Neuenschwander PF, Fiore MM, Morrissey JH. Factor VII autoactivation proceeds via interaction of distinct protease-cofactor and zymogen-cofactor complexes. J Biol Chem. 1993;268:21489–92. [PubMed] [Google Scholar]
- 98.Nakagaki T, Foster DC, Berkner KL, Kisiel W. Initiation of the extrinsic pathway of blood coagulation: Evidence for the tissue factor dependent autoactivation of human coagulation factor VII. Biochem. 1991;30:10819–24. doi: 10.1021/bi00109a001. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto M, Nakagaki T, Kisiel W. Tissue factor dependent autoactivation of human blood coagulation factor VII. J Biol Chem. 1992;267:19089–94. [PubMed] [Google Scholar]
- 100.Rao LVM, Williams T, Rapaport SI. Studies of the activation of factor VII bound to tissue factor. Blood. 1996;87:3738–48. [PubMed] [Google Scholar]
- 101.Rao LVM, Rapaport SI. Activation of factor VII bound to tissue factor: A key early step in the tissue factor pathway of blood coagulation. Proc Natl Acad Sci U S A. 1988;85:6687–91. doi: 10.1073/pnas.85.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]