Summary
Recent in vitro studies have shown that the zymogen and activated form of FVII bind to endothelial cell protein C receptor (EPCR). At present, there is no evidence that FVIIa binds to EPCR on vascular endothelium in vivo in the presence of circulating protein C, a primary ligand for EPCR. The present study was carried out to investigate the interaction of murine and human ligands with murine EPCR both in vivo and in vitro. Measurement of endogenous plasma levels of FVII in wild-type, EPCR-deficient and EPCR-over expressing mice showed slightly lower levels of FVII in EPCR-over expressing mice. However, infusion of high concentrations of competing ligands, either human APCi or FVIIai, to EPCR-over expressing mice failed to increase plasma levels of mouse FVII whereas they increased the plasma levels of protein C by 2 to 3-fold. Examining the association of exogenously administered mouse FVIIa or human FVIIa by immunohistochemistry revealed that human, but not murine FVIIa, binds to the murine endothelium in an EPCR-dependent manner. In vitro binding studies performed using surface plasmon resonance and endothelial cells revealed that murine FVIIa binds murine EPCR negligibly. Human FVIIa binding to EPCR, particularly to mouse EPCR, is markedly enhanced by availability of Mg2+ ions. In summary, our data show that murine FVIIa binds poorly to murine EPCR, whereas human FVIIa binds efficiently to both murine and human EPCR. Our data suggest that one should consider the use of human FVIIa in mouse models to investigate the significance of FVIIa and EPCR interaction.
Keywords: Endothelial cell protein C receptor, factor VIIa, protein C
Introduction
Endothelial cell protein C receptor (EPCR) is the cellular receptor for protein C (PC) and activated protein C (APC). In addition to regulating coagulation by modulating the PC/APC-mediated anticoagulant pathway, EPCR has been shown to be involved in the protective mechanisms exerted by APC (1). Recent studies from our laboratory (2) and others (3;4) have shown that FVII/FVIIa also binds to EPCR in a true ligand fashion. Additional studies suggest that FVIIa binding to EPCR may facilitate FVIIa transport from blood to extravasculature (5), as well as TF-independent FVIIa-induced cell signaling and endothelial barrier protection (6). Given that FVII/FVIIa binds to EPCR with a similar affinity as that of PC/APC, it is believed that the amount of each ligand associated with EPCR in vivo would be approximately proportional to their respective concentrations in plasma (2). Recently, it has been reported that FX and FXa also bind to EPCR and that these interactions may play a role in regulating TF-FVIIa-FXa or FXa-mediated cell signaling via activation of protease activated receptors (PARs) (7;8). However, studies from others have failed to show any significant binding of hFX or hFXa to hEPCR (9;10). This is consistent with sequence homology data that show that the EPCR binding site in the Gla-domain of PC is conserved in hFVII but not in hFX or other human vitamin K-dependent clotting factors (3). Interestingly, the Gla region involved in EPCR binding is also not fully conserved in either mFVII or mPC, indicating the possibility that FVII and PC binding to EPCR may differ between species. Limited in vitro studies performed on mFVIIa binding to mEPCR yielded conflicting data as to whether or not mFVIIa binds to mEPCR (8;9).
Calcium binding to the Gla domain is essential for the interaction of vitamin K-dependent clotting proteins with phospholipids and their respective receptors. Ca2+ is an obligatory cofactor, but other divalent cations such as Mg2+ and Zn2+ also bind to vitamin K-dependent clotting proteins and modulate their binding affinities and functions (11–15). In the absence of metal ions, the Gla domain is highly disordered and unstructured whereas cation binding stabilizes the protein structure thereby facilitating its interaction with phospholipids and receptors (16–18). The crystal structure of active-site inhibited FVIIa bound to sTF revealed that FVIIa binds seven Ca2+ ions in the Gla domain, one in the EGF1 domain and one in the protease domain (15). Inclusion of Mg2+ with Ca2+ results in displacement of three of the Ca2+ ions by Mg2+ in the Gla domain. Mg2+ binding, in the presence of Ca2+, further stabilizes the omega loop of FVIIa (15). At the physiologic concentration of Ca2+, Mg2+ increases the binding of FVIIa to TF and enhances FXa generation (15). Presently, there is no information on whether and how Mg2+ influences FVIIa binding to EPCR. Potential differences in Mg2+ binding between mFVIIa and hFVIIa may contribute to differential binding of mFVIIa and hFVIIa to EPCR. In addition to variation in the Gla region of ligands involved in EPCR binding, subtle differences in the structure of mEPCR and hEPCR may also contribute to differences in FVIIa binding to EPCR between human and mouse systems. The structure and net charge of hEPCR and bovine EPCR are quite similar, but differ in mEPCR (19). mEPCR has an electronegative 106 loop, which is missing in hEPCR (19). This difference may also contribute to differences in ligand binding to mEPCR and hEPCR.
In order to properly perform and interpret in vivo studies investigating the significance of the interaction between FVIIa and EPCR in human physiology and pathophysiology, it is absolutely imperative to characterize the interaction of mFVIIa with mEPCR compared to that of hFVIIa and hEPCR. Data presented in this report demonstrate that mFVIIa binds poorly to mEPCR, whereas hFVIIa on the other hand binds to mEPCR and hEPCR with a similar affinity. The present data suggest that use of hFVIIa instead of mFVIIa in murine models may more closely represent the human system in investigating the potentially important functions of FVIIa-EPCR in hemostasis and inflammation.
Methods
Reagents
Recombinant human FVIIa was from Novo Nordisk A/S (Maaloev, Denmark) and recombinant activated human protein C (Xigris) was from Eli Lilly (Indianapolis, IN). Recombinant mouse FVIIa and soluble mouse TF were provided kindly by Lars C. Petersen, Novo Nordisk, Denmark. Generation of blocking monoclonal antibodies against hEPCR and mEPCR is described earlier (20;21). Alexa Fluor-488 (AF488) microscale protein labeling kit and antibodies specific to AF488 were obtained from Molecular Probes (Invitrogen Corporation, Carlsbad, CA). Antigen retrieval solution, secondary antibodies and substrates used for immunohistochemistry were obtained from Dako. FuGENE HD transfection reagent was from Roche Diagnostics Corp. (Indianapolis, IN).
Cell culture
Primary human umbilical vein endothelial cells (HUVEC), EBM-2 basal medium and growth supplements were purchased from Lonza Walkersville, Inc. (Walkersville, MD). Endothelial cells were cultured in EBM-2 basal medium supplemented with the growth supplements and 5% fetal bovine serum (FBS). Mouse brain microvascular endothelial cells (bEND3) and wild-type CHO-K1 cells were obtained from American Type Culture Collection (Manassas, VA). bEND3 cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) containing 1% penicillin/streptomycin, supplemented with 10% heat-inactivated FBS. CHO cells were grown in F12K media containing 1% penicillin/streptomycin and heat-inactivated 10% FBS. All cell types were cultured at 37°C and 5% CO2 in a humidified incubator. For transfection, CHO-K1 cells seeded in a 6-well plate at 50% confluence were transfected with 1 µg of hEPCR or mEPCR cDNA using FuGENE HD transfection reagent as per the manufacturer’s instructions. Stable transfectants were selected by maintaining transfected cells in medium containing 500 µg/ml of Zeocin for hEPCR or 1mg/ml G418 for mEPCR.
Radiolabeling of ligands
Human FVIIa, mFVIIa, hAPC and mAPC were labeled with 125I using IODOGEN (Pierce)-coated polypropylene tubes and Na 125I (Perkin-Elmer Life Sciences, Wellesley, MA, USA) according to the manufacturer’s technical bulletin and as described previously (22).
Labeling of hFVIIa and mFVIIa with AF488
FVIIa was labeled with AF488 using AF488 microscale protein labeling kit as per manufacturer’s instructions. Briefly, 100 µg of FVIIa (at a concentration of 1 mg/ml) was incubated with 50-fold molar excess of AF488 tetrafluorophenyl ester for 15 min at room temperature to label primary amines of FVIIa with AF488 dye. Free AF488 molecules were removed from the conjugate by passing it through a Bio-Gel P-6 fine resin column or by dialysis. The degree of labeling, as determined by following the manufacturer’s instructional manual, was between 3 and 5.
Cell surface ligand binding
Binding of ligands in various cell model systems was carried out essentially as described in our earlier publications (2;12). Briefly, confluent monolayers were washed once with buffer A (10 mM HEPES, 0.15 M NaCl, 4 mM KCl, 11 mM glucose, pH 7.5) and then once with buffer B (Buffer A supplemented with 1 mg/ml BSA and 5 mM CaCl2). The cells were then incubated with buffer B or buffer B containing 1 mM Mg2+ (buffer B+) and chilled on ice before radiolabeled ligands were added. Varying concentrations of radiolabeled ligands were added to the cells and incubated for 3 h at 4°C on a rotator. After 3 h, the supernatant was removed, the cells were washed four times with ice-cold buffer B/B+ to remove the unbound radiolabeled protein, and the surface bound radiolabeled ligand was eluted by treating the cells with 0.1 M glycine (pH 2.3) for 5 min. The amount of surface binding was determined by counting the radioactivity in eluates. To measure EPCR specificity, EPCR blocking antibody (for hEPCR, JRK1494; mouse EPCR, mAb1560; 25 µg/ml) were added to cells in parallel wells 45 min before the addition of radiolabeled ligands. The difference in ligand binding between untreated cells and EPCR mAb treated cells was taken as EPCR-specific binding.
Surface plasmon resonance (SPR) binding studies
A BIACORE 3000 system (BIACORE, Uppsala, Sweden) was used for SPR binding studies. Soluble hEPCR or mEPCR were immobilized on a CM5 sensor chip using amine coupling chemistry according to the instructions provided by the manufacturer. Approximately 1000 RU of sEPCR (i.e., 1000 pg) was coupled to the chip by passing through 10 µg/ml EPCR at a rate of 5 µl/min. A flow cell blocked with ovalbumin was used as a reference. The chip was equilibrated by passing the running buffer (20 mM Tris, 150 mM NaCl, 5mM CaCl2 or 5 mM CaCl2 + 1 mM MgCl2, and 0.005% surfactant P-20) at a flow rate of 5 µl/min for 2 h. Ligand binding to sEPCR was analyzed by passing consecutively increasing concentrations of ligands (0 nM to 1 µM, in duplicates) in the running buffer containing 0.1% BSA over the sensor chip for 5 min (association time) followed by a 10 min dissociation period at a flow rate of 30 µl/min in parallel. Regeneration was performed with a 3-min pulse of 10 mM EDTA solution. Ligand binding to EPCR was determined by deducting the RU values noted in the reference flow cell from the RU values of flow cell coated with either hEPCR or mEPCR using BIAevaluation software 4.1. The experimental data was fitted for a Langmuir 1:1 binding model containing a mass transfer coefficient using the BIAevaluation software 4.1.
In vivo binding of FVIIa to EPCR
To compare cross-species differences in FVIIa binding to EPCR, wild-type C57BL/6J, EPCR-deficient (23) or EPCR-over expressing mice (21) were injected with AF488-labeled murine or hFVIIa (120 µg/kg body weight) via tail vein. After 30 min, mice were exsanguinated by flushing 15 mL saline (with 5 mM CaCl2 and 1 mM Mg2+) through the heart and draining the blood by severing the renal artery. Various organs were collected and fixed in Excel fixative (American Master*tech Scientific Inc., Lodi, CA) and processed for immunohistochemistry as described recently by our laboratory (24).
Mouse FVII and protein C activity and antigen assays
FVII clotting activity was measured as described earlier (25), except that pooled plasma from wild-type mice (C57BL/6) was used to construct a standard curve. Mouse FVII antigen level in plasma was measured in ELISA where rabbit anti-mFVII IgG was used as a capture antibody and biotinylated rabbit-anti mFVII IgG as the detecting antibody. Recombinant mFVIIa was used to generate the standard curve. AF488-FVIIa antigen levels in plasma was measured by capturing it with anti-AF488 antibody immobilized on 96-well plate and using antibodies against human FVII or mouse FVII as detecting antibodies in an ELISA. Mouse protein C antigen levels were measured as described earlier (21).
Results
Plasma levels of FVII in EPCR transgenic mice
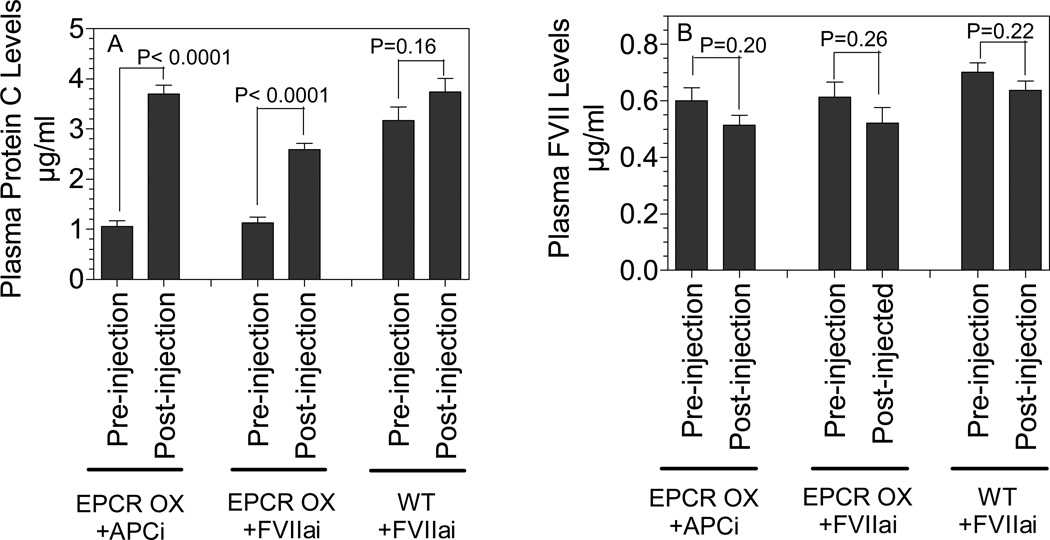
In earlier studies it had been found that the circulating PC antigen levels were slightly higher (~20%) in EPCR-deficient mice whereas they are markedly lower (~50%) in EPCR-over expressing mice, indicating mPC binds to EPCR and thus sequestered on the endothelium (21;23). Measurement of FVII plasma levels in a clotting activity assay in these mice showed a small (~10%) but statistically insignificant increase in circulating FVII levels in EPCR-deficient mice and a statistically significant decrease (~25%) in EPCR-over expressing mice (Table 1). Measurement of FVII clotting activity in a large number of plasma samples collected from EPCR transgenic mice in-house showed a similar trend, however the differences in circulating FVII levels between the wild-type and EPCR-over expressing mice were slightly diminished (Table 1). Measurement of FVII antigen levels in these plasma revealed a small but significant decrease in circulating FVII antigen levels in EPCR-over expressing mice compared to wild-type mice but no significant differences were found between the wild-type and EPCR-deficient mice. Although these data suggest that endogenous mFVII may associate to some extent with mEPCR on the vascular endothelium, it is difficult to reach a firm conclusion regarding the interaction of mFVII with mEPCR in vivo given that no significant differences in circulating FVII levels were observed between the wild-type and EPCR-deficient and only minor differences were observed between the wild-type and EPCR-over expressing mice. Li et al. (21) demonstrated that infusion of a high concentration of hPC to EPCR-over expressing mice increased plasma levels of mPC by 2-fold or more, indicating not only that a significant percentage of PC in EPCR-over expressing mice was bound to the vessel wall, but also that this bound ligand can be effectively displaced by infusion of a high concentration of binding competitor. Consistent with this earlier finding, circulating PC levels were substantially low in EPCR-over expressing mice compared to wild-type mice and injection of 400 µg of active site-inhibited hAPC (APCi) to EPCR-over expressing mice increased plasma levels of PC by about 3-fold (Fig. 1A), which predictably is near the plasma levels of PC in wild-type mice. Measurement of FVII antigen levels in these plasma samples showed no increase in circulating FVII antigen levels in EPCR-over expressing mice injected with APCi (Fig. 1B). Similar to hAPCi, injection of hFVIIai to EPCR-over expressing mice also led to a significant increase in the circulating PC antigen levels, indicating that hFVII effectively binds to mouse EPCR and displaces mPC associated with EPCR. Injection of hFVIIai to EPCR-over expressing mice did not increase plasma levels of mFVII. Overall these data indicate that there is no significant association of mFVII with EPCR in the mouse system, whereas exogenously administered hFVIIa readily binds to mEPCR. It is somewhat unexpected to find that endogenous mFVII does not significantly associate with EPCR on the vessel wall in mice given our recent immunohistochemical observation in wild-type mice that exogenously administered mFVIIa associated with the vessel wall (24). Based on these observations it is probable that this association with the vessel wall is independent of at least any significant interaction with EPCR.
Table 1.
Plasma levels of endogenous FVII in EPCR transgenic mice
| Genotype | Plasma FVII levels | |
|---|---|---|
| FVII clotting activity (U/ml)1 | FVII antigen levels (ng/ml) | |
| 2Experiment 1 (n=4) | ||
| Wild-type | 1.00 ± 0.09 | 717± 32 |
| EPCR-over expressor | 0.75 ± 0.04 (p=0.04) | 491± 47 (p<0.01) |
| EPCR-deficient | 1.10 ± 0.07 (p=0.41) | 638 ± 51 (p=0.24) |
| 3Experiment 2 (n= 10 to 36) | ||
| Wild-type | 1.00 ± 0.07 | 666 ± 11 |
| EPCR-over expressor | 0.85 ± 0.04 (p=0.06) | 611 ± 11 (p<0.01) |
| EPCR-deficient | 1.10 ± 0.06 (p=0.29) | 661 ± 13 (p=0.12) |
FVII clotting activity in wild-type mice was normalized to 1.0 U/ml. Wild-type mice includes littermates of EPCR-deficient and EPCR-over expressing mice and commercially obtained C57 BL/6 mice.
Plasma samples from Dr. Esmon’s laboratory
Plasma sample collected in-house
Figure 1. Ability of human APCi and FVIIai to displace endogenous ligands bound to EPCR in mice.
Wild-type or EPCR-over expressing mice were injected with human APCi or FVIIai (400 µg/mice) via tail vein. Plasma levels of mouse protein C (A) or FVII (B), pre- and post-injection (10 min after the injection), were determined in mouse specific protein C or FVII ELISA. The data shown are mean ± SEM (n = 3 to 8). P values indicate the statistical significance by t test with relative to plasma levels of protein C or FVII measured prior to injecting human APCi or FVIIai.
FVIIa binding to vessel wall: EPCR dependency
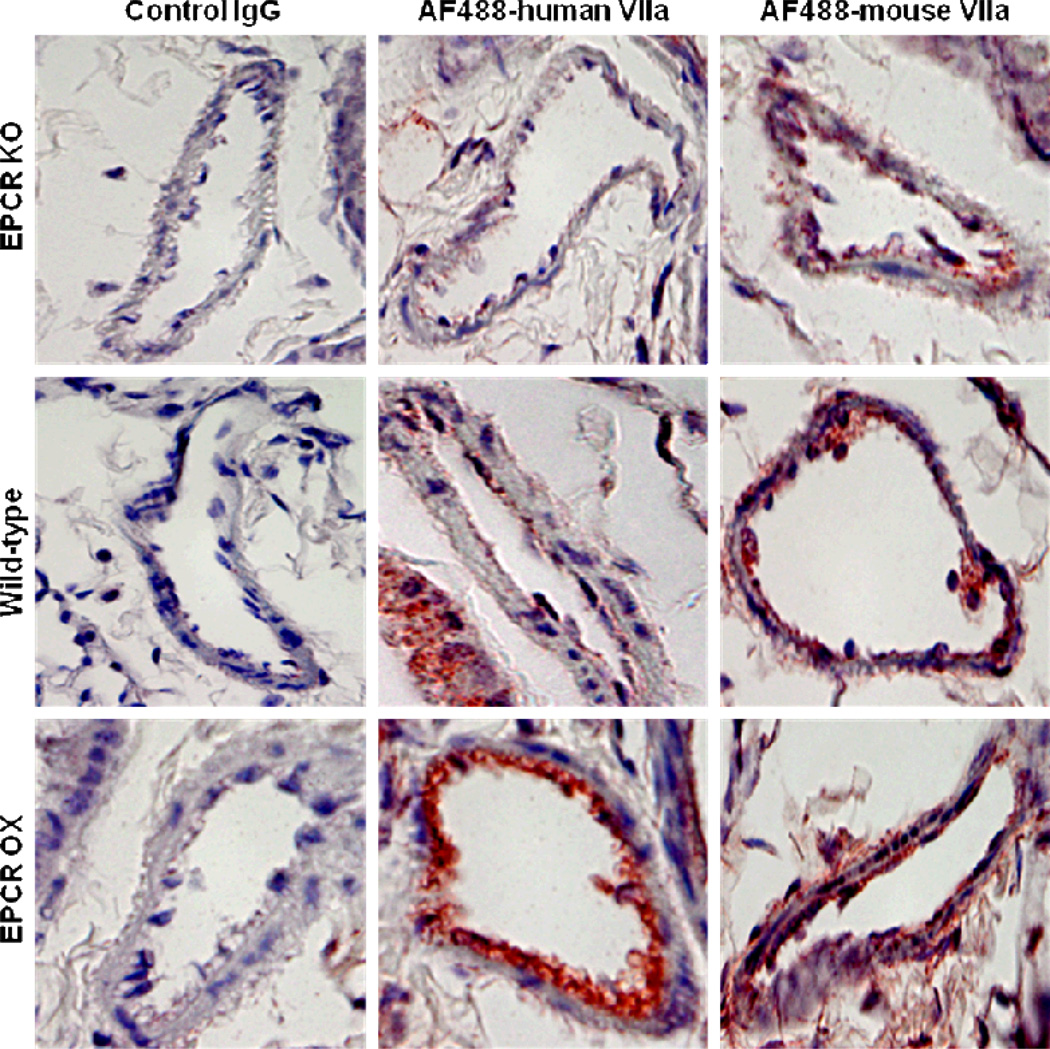
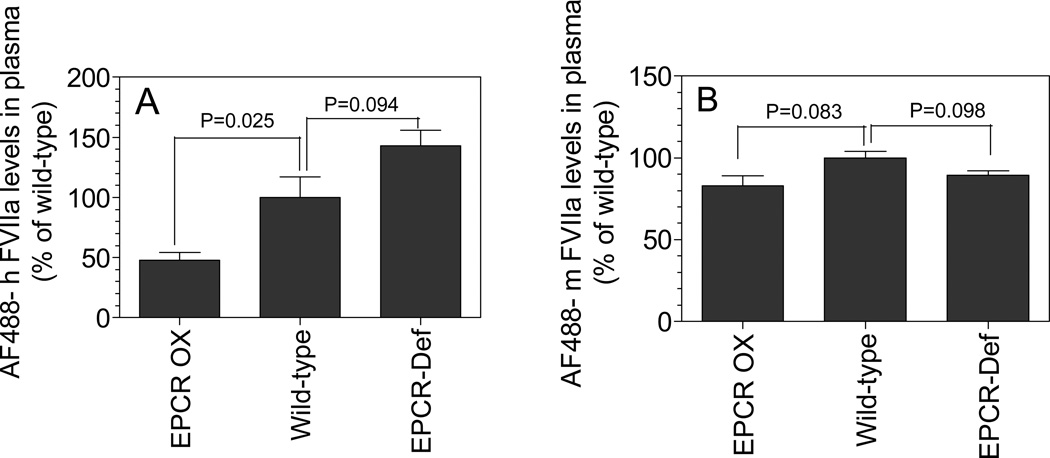
To properly determine whether FVIIa association with the vessel wall is the result of FVIIa binding to EPCR on the vessel wall, AF488-labeled mFVIIa or hFVIIa (120 µg/kg body weight) was injected via tail vein to wild type C57BL/6J, EPCR-deficient and EPCR-over expressing mice. Thirty minutes following the injection, mice were sacrificed by exsanguination followed by perfusion; lung and other tissues were collected and processed for immunohistochemistry. As shown in Fig. 2, hFVIIa associated with the vessel wall in EPCR-deficient mice is negligible compared to clearly visible association of hFVIIa with the vessel wall in wild-type mice. Very intense staining of hFVIIa was found at the endothelial layer of vessel walls in EPCR-over expressing mice. This distinct genotype-specific variation of hFVIIa staining on the vessel wall clearly indicates significant EPCR-specific binding of hFVIIa to the mouse endothelium. mFVIIa also associates with the mouse endothelium, but there are no discernable differences in vessel wall association among EPCR genotypes, indicating that mFVIIa interaction with the mouse endothelium is mostly or entirely EPCR independent. We observed similar results with other tissue sections (data not shown). Consistent with the immunohistochemical observation that hFVIIa injected into mice readily associates with EPCR, we observed a significant reduction in the recovery of injected AF488-hFVIIa in plasma of EPCR-over expressing mice compared to the wild-type (Fig. 3A). Although it is not statistically significant, mean plasma levels of AF488-hFVIIa were higher in EPCR-deficient mice compared to wild-type mice. No significant differences were observed in the recovery of AF488-mFVIIa in plasma among different genotypes (Fig. 3B).
Figure 2. EPCR-specific binding of human FVIIa to mouse endothelium in vivo.
AF488-hFVIIa or AF488-mFVIIa (120 µg/kg) was injected to wild-type, EPCR-deficient or EPCR-over expressing mice via tail vein. Mice were killed 30 min after the injection and perfused with 15 ml of ice-cold saline (supplemented with 5 mM CaCl2 and 1 mM MgCl2). Various tissues were collected and fixed in Excel Plus fixative and processed for tissue sectioning and immunohistochemistry. Staining of tissue sections from lungs were shown here. AF488-FVIIa in the tissue was detected by probing the section with anti-AF488 antibody. The upper panel is EPCR-deficient, middle panel is wild-type and lower panel is EPCR-over expressing mice.
Figure 3. Recovery of exogenously administered hFVIIa in plasma inversely correlated with EPCR expression.
AF488-hFVIIa or AF488-mFVIIa (120µg/kg) was injected to wild-type, EPCR-deficient or EPCR-over expressing mice via tail vein. After 30 min, blood was collected from the mice via cardiac puncture. Amount of AF488-FVIIa levels in plasma were determined in ELISA that specifically detects human or murine AF488-FVIIa (n = 3 or more). AF488-FVIIa recovered in wild-type mouse was considered as 100% and statistical significances were calculated by t test against the wild-type mouse values.
APC and FVIIa binding to EPCR by SPR
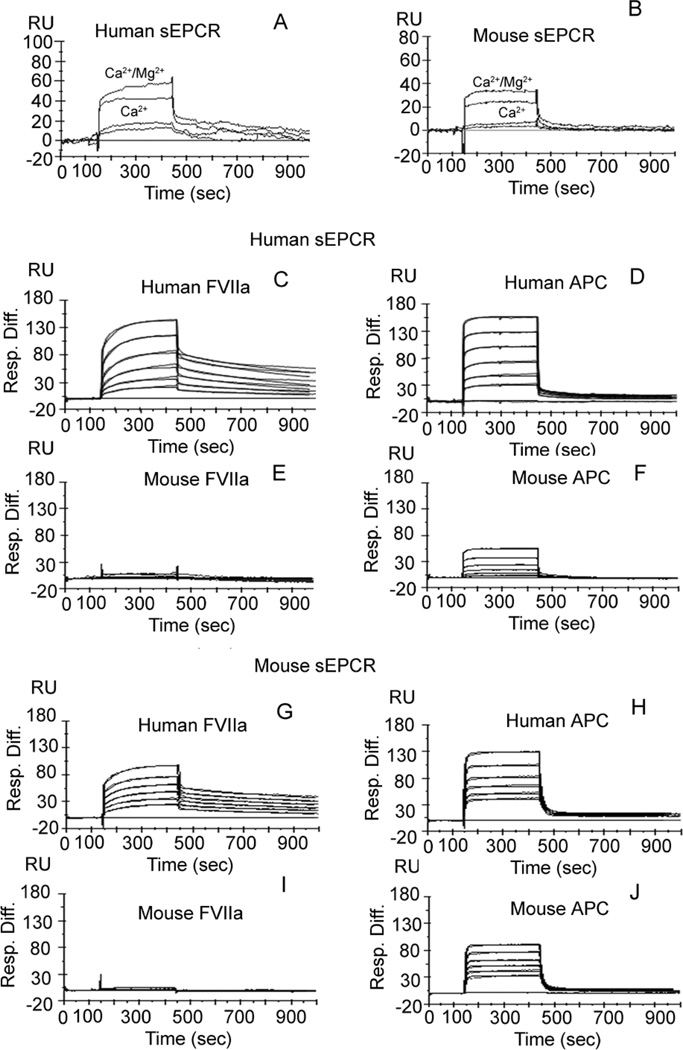
Given that the in vivo data described above clearly suggest that significant differences may exist between mFVIIa and hFVIIa regarding interaction with EPCR, further studies were performed in vitro to confirm and characterize these differences. SPR binding studies performed with soluble hEPCR or mEPCR immobilized on CM5 sensor chip show that hFVIIa binds to both hEPCR and mEPCR in the presence of Ca2+ while the presence of Mg2+ enhances this binding about 2 to 3-fold (Fig. 4A and 4B). Dose dependent binding studies in the presence of Ca2+/Mg2+ revealed that hFVIIa binds to hEPCR and mEPCR with a similar efficiency (compare Fig. 4C and 4G) and can be approximated to hAPC binding to hEPCR and mEPCR (Fig. 4D and 4H). In contrast to hFVIIa, mFVIIa binds very poorly as binding is barely detectable, particularly to mEPCR (Fig. 4J). Complex formation with soluble mTF (1:1 ratio) did not increase mFVIIa binding to EPCR (data not shown). mAPC binds to both hEPCR and mEPCR but with somewhat reduced affinity as compared to hAPC or hFVIIa (Fig. 4F and 4J). Given very high dissociation rates and the fact that we have not achieved saturation binding even at a 1 µM concentration of ligands in these binding studies, we have not estimated Kd values as these conditions may yield erroneous rate constants. Despite this limitation, these binding data clearly illustrate that, consistent with in vivo data, mFVIIa either does not bind or binds very poorly to mEPCR, whereas hFVIIa binds to mEPCR with a similar efficiency as hEPCR.
Figure 4. Interaction of human or murine FVIIa and APC with human or murine soluble EPCR.
Approximately 1000 RU of sEPCR (either human or mouse) was immobilized onto CM5 chip. hFVIIa (125 nM and 62.5 nM) were passed through a flow cell containing immobilized human sEPCR (A) or murine sEPCR (B) in the presence of Ca2+ (5 mM) or Ca2+ (5mM)/Mg2+ (1 mM). Varying concentrations (0, 62.5, 125, 250, 500, 1000 nM) of hFVIIa (C and G), hAPC, (D and H), mFVIIa (E and I) or mAPC (F and J) were passed through a flow cell containing immobilized human sEPCR (C to F) or mouse sEPCR (G to J) in the presence of Ca2+ (5 mM)/Mg2+ (1 mM). Blank and reference path corrected sensograms are presented along with the fitted curves. The experimental data were fitted with a Langmuir 1:1 binding model containing a mass transfer coefficient using BiaEvaluations software v 4.1 (Biacore).
FVIIa and APC binding to EPCR on cell surfaces
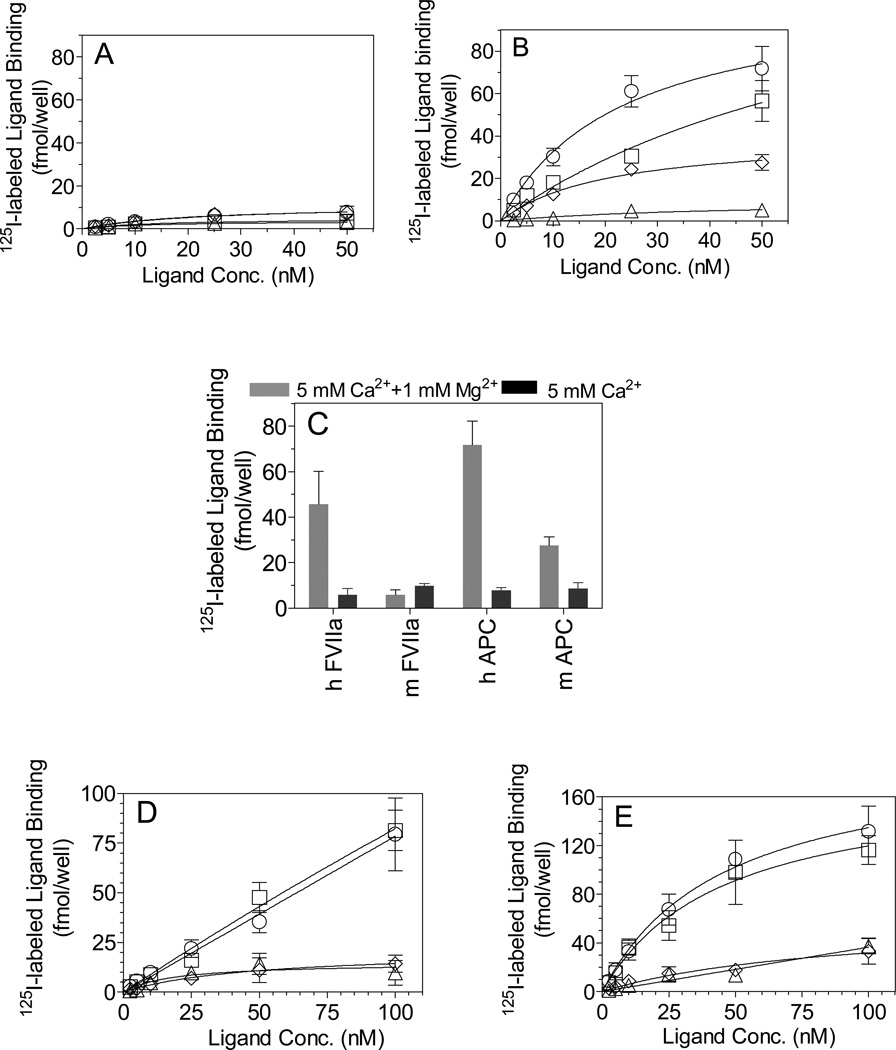
Next, we examined the binding of hFVIIa and mFVIIa to EPCR on cells expressing EPCR, i.e., mouse or human endothelial cells, and CHO cells stably transfected to express either murine or human EPCR. Since Mg2+ was shown to influence binding in SPR studies, we have also evaluated Mg2+ dependency in these studies. As shown in Fig. 5A, in the presence of Ca2+ alone, little human or murine FVIIa or APC bound to mouse endothelial cells. However, inclusion of Mg2+ along with Ca2+ increases the binding of hFVIIa and hAPC markedly (approx. 10-fold increase) to EPCR on mouse endothelial cells (Fig. 5B and 5C). In the presence of Mg2+, mAPC binding to mEPCR was also increased, but to a lesser extent (~3-fold increase). In contrast, no detectable increase in the binding of mFVIIa to mEPCR was noted even in the presence of Mg2+ (Fig. 5B and 5C). In the presence of Ca2+/Mg2+, both hAPC and hFVIIa bound to EPCR on mouse endothelial cells in a dose-dependent manner. The Kd values for hAPC and hFVIIa are 23 nM and 80nM, respectively. mAPC also showed a dose-dependant binding to mEPCR on endothelial cells, but the amount of binding is only about 30% of human APC at 50 nM concentration. We obtained essentially similar binding data with CHO cells stably transfected to express mEPCR (data not shown). Binding studies performed with HUVEC show that all the ligands bound specifically to hEPCR, although human ligands bound significantly more compared to the mouse ligands (Fig. 5D). The binding curves obtained in the presence of Ca2+ alone for hFVIIa and hAPC or mFVIIa and mAPC to hEPCR are superimposable (Fig. 5D). Non-saturable binding of hFVIIa and hAPC to hEPCR shown in Fig. 5D stems from the use of limited dose-range of hFVIIa and hAPC (≤ 100 nM) we employed in the present study along with mFVIIa and mAPC, which were available only in limited quantities. Inclusion of Mg2+ in the binding buffer increased the binding of hFVIIa and hAPC by about 2–3 fold and both ligands bound to hEPCR with a similar affinity having a Kd of ~ 40 nM. These data indicate that Mg2+ increases the affinity of the human ligands for hEPCR. A small increase in the binding of mFVIIa and mAPC to human EPCR was also noted upon inclusion of Mg2+, but this enhancing effect is minimal (compare Fig. 5D and 5E). Binding studies performed with CHO cells stably transfected with hEPCR gave similar results (data not shown).
Figure 5. Human or murine FVIIa and APC binding to EPCR on mouse or human endothelial cells.
Confluent monolayer’s of Bend.3 (mouse endothelial cells) (A – C) or HUVEC (D and E) were incubated with varying concentrations of 125I-labeled hFVIIa (□), hAPC (○), mFVIIa (Δ) or mAPC (◊) or a fixed concentration (50 nM, C) for 3 h at 4°C in a buffer containing Ca2+ (5 mM) (A, C and D) or Ca2+ (5 mM) and Mg2+ (1 mM) (B, C and E) ± EPCR blocking mAb. EPCR-specific ligand binding was determined as described in the methods. The data shown are mean ± SEM (n = 3 or more independent experiments).
Comparison of the number of specific binding sites for the various ligands and an EPCR antibody on the mouse and human endothelial cells showed that the number of binding sites for human FVIIa and human APC on both mouse and human endothelial cells was very similar to that of EPCR mAb (Table 2). However, the number of binding sites for mouse ligands, particularly for mouse FVIIa, was substantially lower to that of EPCR mAb (Table 2). These data indicate that a fraction of EPCR sites on endothelial cells are unable to interact with mouse ligands, including mAPC, suggesting a possible heterogeneity in EPCR expressed at the cell surface.
Table 2.
Number of EPCR-specific binding sites for the various ligands and EPCR mAb on mouse and human endothelial cells.
| Ligand | Number of binding sites (103/cell)1 | |
|---|---|---|
| Mouse endothelial cells | Human endothelial cells | |
| Mouse EPCR mAb (1560) | 990 ± 86 | - |
| Mouse FVIIa | 59 ± 52* | 169 ± 63* |
| Mouse APC | 243 ± 44* | 360 ± 114* |
| Human FVIIa | 879 ± 101 | 1048 ± 254 |
| Human APC | 654 ± 110 | 1181 ± 214 |
| Human EPCR mAb (JRK1500) | - | 1006 ± 156 |
Number of EPCR-specific binding sites shown in the table was estimated by the analysis of the binding curves in the presence of Ca2+ and Mg2+ ions using hyperbola curve-fitting program (Graph Pad Prism 4).
indicates that the values were significantly lower (P < 0.03) from the number of EPCR mAb binding sites. Two independent experiments performed with a single concentration of radioligands in a concentration close to a saturating concentration (100 nM) ± EPCR blocking mAb also gave a similar data.
Discussion
Recent studies, performed mostly in silico and in cell model systems, show that FVIIa acts as a true-ligand for EPCR (2–4) and suggest that FVIIa binding to EPCR may play an important role in hemostasis (2;4;26) and inflammation (6). The observation that intravenously injected FVIIa readily associates with murine vascular endothelium supports the possibility that FVIIa binds to EPCR on vascular endothelium in vivo (24). Nonetheless, at present, there is no direct evidence supporting FVIIa association with EPCR in vivo. Given that the murine model system has become a preferred animal model system for studies elucidating mechanisms involved in hemostasis and thrombosis as well as the role of blood coagulation in inflammation, it is important to know the characteristics of mFVIIa interaction with mEPCR, particularly in comparison to hFVIIa binding to hEPCR, before using the murine model to investigate the importance of FVIIa binding to EPCR in human pathophysiology. The data presented in this manuscript show that mFVIIa binds very poorly, if at all, to either mEPCR or hEPCR. Interestingly, hFVIIa binds to both hEPCR and mEPCR with a similar affinity as that of hAPC. A comparison of the binding of human or murine ligands to hEPCR and mEPCR in the presence of Ca2+ ± Mg2+ showed that Mg2+ differentially influences the interaction of human and murine ligands with EPCR. Overall, these data may provide insights into the potential contribution of various binding region residues in the interaction of FVIIa with EPCR.
Earlier studies have shown that endogenous protein C levels were significantly higher in EPCR-deficient mice (23) and substantially lower in EPCR-over expressing mice (21). Moreover, infusion of a high concentration of hAPC increased mouse plasma PC via displacement by 10 to 20% in wild-type mice and by more than 2-fold in EPCR-over expressing mice (21), establishing that PC associates with EPCR on the vascular endothelium in mice. Plasma FVII levels in EPCR-over expressing mice were 15 to 20% lower compared to wild-type mice, suggesting that endogenous FVII associates to some extent with EPCR. However, infusion of high concentrations of hAPCi failed to increase the plasma levels of FVII while they increased plasma levels of PC by 2–3-fold, indicating the lack of EPCR-dependent vascular compartmentalization of FVII. Thus, at present, the reason for decreased plasma levels of FVII in EPCR-over expressing mice is unclear. It is conceivable that EPCR overexpression in mice could alter overall metabolism. Alternatively, poor interaction of mouse FVII with EPCR may be sufficient to increase catabolism of FVII slightly in mice over expressing EPCR.
As observed with infusion of hAPCi, infusion of a high concentration of FVIIai also increased plasma PC levels in EPCR-over expressing mice by more than 2-fold. Moreover, infusion of therapeutic concentrations of hFVIIa (120 µg/kg) to mice showed clear EPCR-dependent association of hFVIIa with the vascular endothelium. Consistent with the observation that hFVIIa binds to EPCR, the recovery of injected hFVIIa in plasma was lower in EPCR-over expressing mice in comparison to wild-type mice. These data suggest that it is very likely that hFVIIa administered for instance to hemophilic patients could readily associate with EPCR on the endothelium. Although we have observed mFVIIa association with the endothelium as reported earlier (24), this association appears to be EPCR-independent. It is unlikely that the observed association of mFVIIa to the mouse endothelium reflects mFVIIa binding to TF on the endothelium as we have found no evidence for the presence of TF on the endothelium. It is possible that mFVIIa may specifically bind to mouse endothelium at sites other than EPCR. Limited competition studies in vitro showed that 50-fold molar excess of unlabeled mFVIIa but not hFVIIa or EPCR blocking Mab reduced the binding of 125I-mFVIIa to mouse endothelial cells by about 40%, indicating that mFVIIa may specifically associate with mouse endothelium but this binding site is not EPCR (data not shown). Extensive studies may be required to identify mFVIIa binding site on the endothelium.
In vitro binding studies performed using mouse and human ligands and EPCR confirm the in vivo observations, i.e., mFVIIa does not bind or binds very poorly to murine EPCR whereas hFVIIa binds to mEPCR with a similar affinity as that which is observed in the human system. Our data on mFVIIa binding to mEPCR in SPR is similar to that of Puy et al. (9), but differs from that of Disse et al. (8), who showed mFVIIa binding to mEPCR. Disse et al. (8) also reported that mFVIIa binding to mEPCR is increased in the presence of soluble TF. It is possible that mFVIIa binding to TF may stabilize the weak interaction of mFVIIa with EPCR. However, we were unable to detect any significant amount of mFVIIa binding to EPCR even when we passed mFVIIa-soluble mTF complexes over the immobilized sEPCR in SPR binding studies. The reason for the discrepancy between our present data and the earlier finding (8) remains unclear.
As expected from our earlier data (2), hFVIIa and hAPC bind to EPCR on human endothelial cells in a very similar fashion. Both hFVIIa and hAPC also bind to EPCR on murine endothelial cells. Under similar experimental conditions, mFVIIa binding to mEPCR is negligible. Although these data firmly suggest that FVIIa interaction with EPCR may not play a similarly important role in hemostasis or inflammation within the murine system, use of hFVIIa in the murine model system may simulate the human system for studying the importance of the interaction between FVIIa and EPCR. Here it may be pertinent to note that although hFVIIa binds poorly to mTF, hFVIIa at 10 nM or higher is sufficient to saturate mTF (27). Further, use of transgenic mice expressing human TF (28) would remove any significant limitation in using hFVIIa in mouse models.
Interestingly, both mFVIIa and mAPC bind poorly to hEPCR, indicating that species-specific differences in EPCR also determine the degree of ligand binding. Species-specific differences in the ligand and EPCR also appear to play a role in how effectively Mg2+ modulates these interactions. In the absence of Mg2+ (i.e., in the presence of Ca2+ alone), all ligands bind very poorly to mEPCR. Mg2+ increases the binding of hFVIIa, hAPC and mAPC to mEPCR dramatically, particularly the binding of hFVIIa and hAPC. In contrast to the above, a considerable amount of hFVIIa and hAPC bind to hEPCR in the presence of Ca2+ alone. Although Mg2+ increases the affinity of hFVIIa and hAPC binding to hEPCR, the Mg2+ effect is not as remarkable as that observed for mEPCR. Mg2+ had only a minimal effect on the binding of mFVIIa or mAPC to hEPCR.
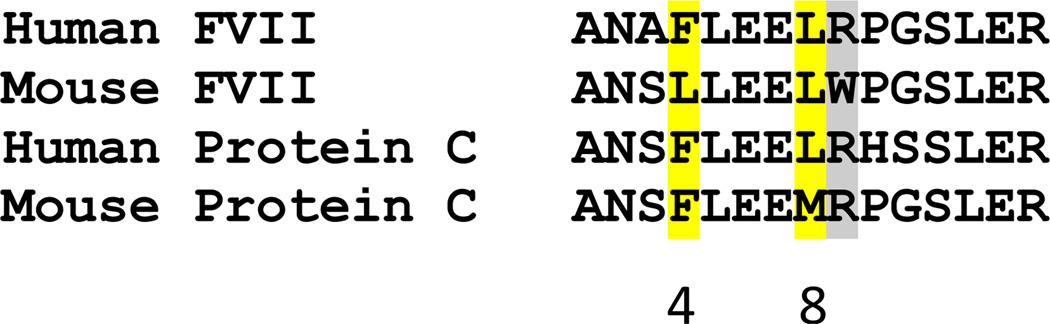
The Gla domain of FVIIa and APC plays a critical role in their binding to EPCR (2;3;29). In the absence of Mg2+, seven molecules of Ca2+ bind to the Gla domain of hFVIIa and when Mg2+ is added along with Ca2+, three of the Ca2+ are replaced by Mg2+ molecules (15). Mg2+ binding at these sites induces conformational changes in the Ω loop of the FVIIa Gla domain involving the three hydrophobic residues (Phe4, Leu5 and Leu8), as well as Gla6 and Gla7. This conformational change in FVIIa results in additional contacts between the Gla domain of FVIIa with the carboxyl-terminal module of TF (15). Based on the present observation that Mg2+ greatly enhances the binding of FVIIa to EPCR, it is likely that Mg2+-induced conformational changes in the Ω loop of the FVIIa Gla domain results in additional contacts between the Gla domain and EPCR thereby stabilizing their interaction. Although Leu8, Leu5 and Gla6 and 7 are conserved in mFVIIa (Fig. 6), it does not appreciably bind EPCR indicating that Phe4 plays a crucial role in the binding. The substitution of Phe4 with Leu4 in mFVIIa may fail to form the ordered Ω loop upon Ca2+/Mg2+ binding to the Gla domain as the side chain phenyl group of Phe4 is involved in the Ω loop formation (15).
Figure 6. Amino acid sequence comparison of the EPCR binding region of the Gla domain of human and murine FVII and protein C.
In addition to the subtle sequence differences in human and murine ligands in the Gla region involved in EPCR binding, other sequence differences between human and murine EPCR might have also played a role in the differential binding of ligands to human and murine EPCR as observed in this study. A comparison of three-dimensional models of human, bovine and murine EPCR showed that EPCR from different species adopts a similar conformation except one notable difference in mEPCR (19). Several areas in and around the ligand binding groove were neutral in hEPCR and bEPCR, whereas a very negative region was observed in mEPCR, in part due to the presence of specific residues in the unique 106-loop (19). The electronegative 106-loop in mouse EPCR has the potential to interact with metal ions (19). Thus, it is possible that Mg2+ could bind to mEPCR in this loop and neutralize the negative charge, which facilitates ligand binding.
In summary, our data indicate quantitatively that the reactivity of EPCR with its ligands differs between human and mouse systems. As such, care should be taken in the design of experiments in the mouse model system in investigating the importance of the FVIIa–EPCR interaction, as mouse FVII may not appreciably bind to mEPCR. However, the observation that human FVIIa binds effectively to mouse EPCR and along with the availability of mice expressing human TF (28), may substantiate the use of hFVIIa in the mouse model system in order to investigate the importance of EPCR in FVIIa transport and therapeutical action.
What is known about this topic?
EPCR, a cellular receptor for protein C/APC, also acts as receptor for FVII/FVIIa.
FVIIa binding to EPCR may facilitate FVIIa transport from blood to extravasculature and provide endothelial barrier protection.
Conflicting data exist in recent literature as to whether or not murine FVIIa can interact with murine EPCR.
What does this paper add?
Endogenous plasma FVII or exogenously administered murine FVIIa does not significantly associate with murine EPCR in vivo.
Human FVIIa readily and significantly binds to murine EPCR in vivo.
Infusion of high concentrations of human FVIIa can replace endogenous protein C bound to EPCR and increase plasma concentrations of protein C
Very little binding of human FVIIa or APC to murine EPCR in the presence of calcium ions alone. Mg2+ ions have a profound effect on the ligand binding to EPCR, particularly to murine EPCR.
Highlights limitations in using murine models for studying importance of FVIIa-EPCR interaction, which could be overcome partly by using human FVIIa as the ligand.
Acknowledgments
This work has been partly supported by Novo Nordisk Inc. as a part of the Investigator Initiated Grant and NHLBI grants (HL058869 and HL107483). The authors are thankful to Lars C. Petersen, Novo Nordisk, Denmark, for providing mouse FVIIa and soluble TF, and Pierre Neuenschwander, University of Texas Health Science Center, Tyler, TX for help in performing SPR studies.
The study was partly supported by a research grant from Novo Nordisk.
Footnotes
Disclosure and conflict of interests
U. Hedner is a consultant to Novo Nordisk A/S, Zurich, Switzerland.
References
- 1.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S, Pendurthi UR, Steinoe A, Esmon CT, Rao LV. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J Biol Chem. 2007;282:11849–11857. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston RJ, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlback B, et al. Multifunctional specificity of the protein C/activated protein C Gla domain. J Biol Chem. 2006;281:28850–28857. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Sagaseta J, Montes R, Puy C, Diez N, Fukudome K, Hermida J. Binding of factor VIIa to the endothelial cell protein C receptor reduces its coagulant activity. J Thromb Haemost. 2007;5:1817–1824. doi: 10.1111/j.1538-7836.2007.02648.x. [DOI] [PubMed] [Google Scholar]
- 5.Nayak RC, Sen P, Ghosh S, Gopalakrishnan R, Esmon CT, Pendurthi UR, et al. Endothelial cell protein C receptor cellular localization and trafficking. Blood. 2009;114:1974–1986. doi: 10.1182/blood-2009-03-208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen P, Gopalakrishan R, Kothari H, Keshava S, Clark C, Esmon CT, et al. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011;117:3199–3208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuepbach RA, Riewald M. Coagulation factor Xa cleaves protease-activated receptor-1 and mediates signaling dependent on binding to the endothelial protein C receptor. J Thromb Haemost. 2010;8:379–388. doi: 10.1111/j.1538-7836.2009.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disse J, Petersen HH, Larsen KS, Persson E, Esmon N, Esmon CT, et al. The endothelial protein c receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors. J Biol Chem. 2011;286:5756–5767. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puy C, Hermida J, Montes R. Factor X and factor VII binding to endothelial protein C receptor differs between species. J Thromb Haemost. 2011;9:1255–1257. doi: 10.1111/j.1538-7836.2011.04295.x. [DOI] [PubMed] [Google Scholar]
- 10.Sen P, Nayak R, Clark CA, Gopalakrishan R, Esmon CT, Pendurthi UR, et al. Factor X binding to endothelial cell protein C receptor: A comparison with factor VIIa and activated protein C. Blood. 2011;118:2635–2636. doi: 10.1182/blood-2011-05-354571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne R, Amphlett GW, Castellino FJ. Metal ion specificity of the conversion of bovine factors IX, IX alpha, and IXa alpha to bovine factor IXa beta. J Biol Chem. 1980;255:1430–1435. [PubMed] [Google Scholar]
- 12.Sen P, Sahoo S, Pendurthi UR, Rao LV. Zinc modulates the interaction of protein C and activated protein C with endothelial cell protein C receptor. J Biol Chem. 2010;285:20410–20420. doi: 10.1074/jbc.M110.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Church WR, Boulanger LL, Messier TL, Mann KG. Evidence for a common metal ion-dependent transition in the 4-carboxyglutamic acid domains of several vitamin K-dependent proteins. J Biol Chem. 1989;264:17882–17887. [PubMed] [Google Scholar]
- 14.Butenas S, Lawson JH, Kalafatis M, Mann K. Cooperative interaction of divalent metal ions, substrate, and tissue factor with factor VIIa. Biochem. 1994;33:3449–3456. doi: 10.1021/bi00177a039. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj SP, Schmidt AE, Agah S, Bajaj MS, Padmanabhan K. High resolution structures of p-aminobenzamidine- and benzamidine-VIIa/soluble tissue factor: unpredicted conformation of the 192–193 peptide bond and mapping of Ca2+, Mg2+, Na+, and Zn2+ sites in factor VIIa. J Biol Chem. 2006;281:24873–24888. doi: 10.1074/jbc.M509971200. [DOI] [PubMed] [Google Scholar]
- 16.Vermeer C. Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990;266:625–636. doi: 10.1042/bj2660625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman PA, Przysiecki CT. Vitamin K-dependent carboxylation. Int J Biochem. 1987;19:1–7. doi: 10.1016/0020-711x(87)90116-9. [DOI] [PubMed] [Google Scholar]
- 18.Freedman SJ, Blostein MD, Baleja JD, Jacobs M, Furie BC, Furie B. Identification of the phospholipid binding site in the vitamin K-dependent blood coagulation protein factor IX. J Biol Chem. 1996;271:16227–16236. doi: 10.1074/jbc.271.27.16227. [DOI] [PubMed] [Google Scholar]
- 19.Villoutreix BO, Blom AM, Dahlback B. Structural prediction and analysis of endothelial cell protein C/activated protein C receptor. Protein Eng. 1999;12:833–840. doi: 10.1093/protein/12.10.833. [DOI] [PubMed] [Google Scholar]
- 20.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Zheng X, Gu J, Hunter J, Ferrell GL, Lupu F, et al. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3:1351–1359. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 22.Rao LVM, Williams T, Rapaport SI. Studies of the activation of factor VII bound to tissue factor. Blood. 1996;87:3738–3748. [PubMed] [Google Scholar]
- 23.Li W, Zheng X, Gu JM, Ferrell GL, Brady M, Esmon NL, et al. Extraembryonic expression of EPCR is essential for embryonic viability. Blood. 2005;106:2716–2722. doi: 10.1182/blood-2005-01-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopalakrishnan R, Hedner U, Ghosh S, Nayak R, Allen TC, Pendurthi UR, et al. Bio-distribution of pharmacologically administered rFVIIa. J Thromb Haemost. 2010;8:301–310. doi: 10.1111/j.1538-7836.2009.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao LVM, Bajaj SP, Rapaport SI. Activation of human factor VII during clotting in vitro. Blood. 1985;65:218–226. [PubMed] [Google Scholar]
- 26.Puy C, Lopez-Sagaseta J, Hermida J, Montes R. The endothelial cells downregulate the generation of factor VIIa through EPCR binding. Br J Haematol. 2010;149:111–117. doi: 10.1111/j.1365-2141.2009.08060.x. [DOI] [PubMed] [Google Scholar]
- 27.Petersen LC, Norby PL, Branner S, Sorensen BB, Elm T, Stennicke HR, et al. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: a human-murine species compatibility study. Thromb Res. 2005;116:75–85. doi: 10.1016/j.thromres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, et al. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–1700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 29.Regan LM, Mollica JS, Rezaie AR, Esmon CT. The interaction between the endothelial cell protein C receptor and protein C is dictated by the gamma-carboxyglutamic acid domain of protein C. J Biol Chem. 1997;272:26279–26284. doi: 10.1074/jbc.272.42.26279. [DOI] [PubMed] [Google Scholar]