Abstract
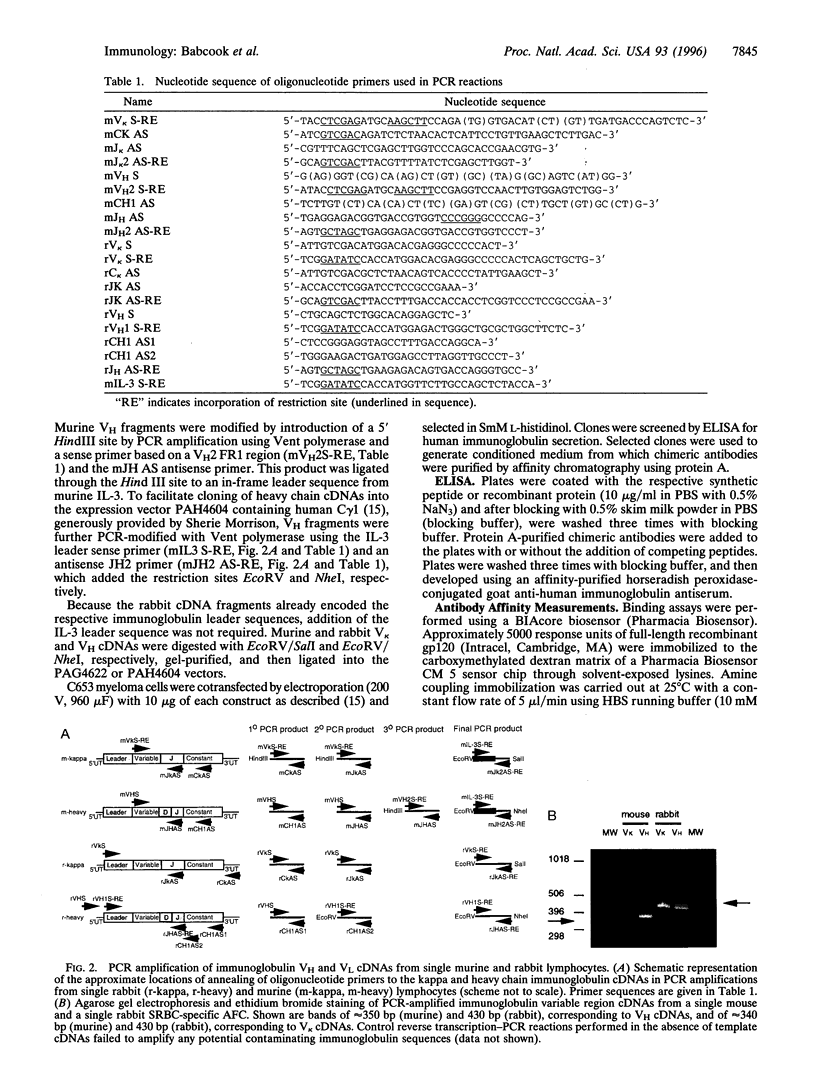
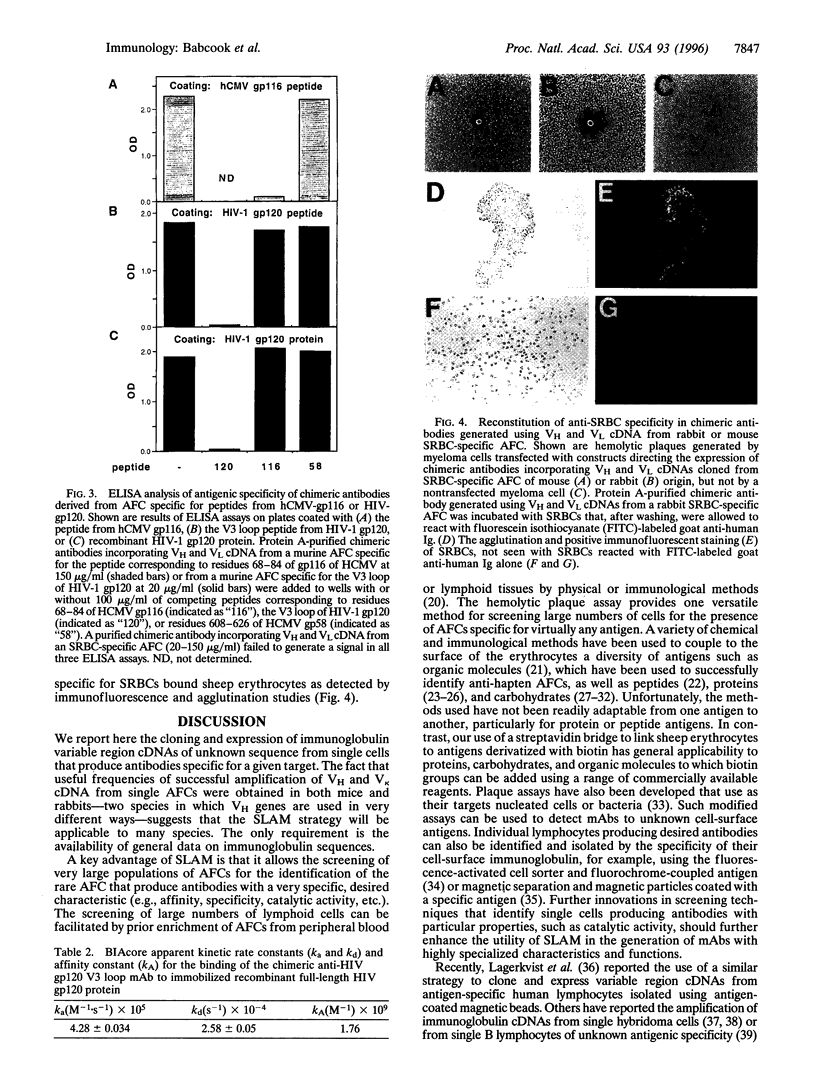
We report a novel approach to the generation of monoclonal antibodies based on the molecular cloning and expression of immunoglobulin variable region cDNAs generated from single rabbit or murine lymphocytes that were selected for the production of specific antibodies. Single cells secreting antibodies for a specific peptide either from gp116 of the human cytomegalovirus or from gp120 of HIV-1 or for sheep red blood cells were selected using antigen-specific hemolytic plaque assays. Sheep red blood cells were coated with specific peptides in a procedure applicable to any antigen that can be biotinylated. Heavy- and light-chain variable region cDNAs were rescued from single cells by reverse transcription-PCR and expressed in the context of human immunoglobulin constant regions. These chimeric murine and rabbit monoclonal antibodies replicated the target specificities of the original antibody-forming cells. The selected lymphocyte antibody method exploits the in vivo mechanisms that generate high-affinity antibodies. This method can use lymphocytes from peripheral blood, can exploit a variety of procedures that identify individual lymphocytes producing a particular antibody, and is applicable to the generation of monoclonal antibodies from many species, including humans.
Full text
PDF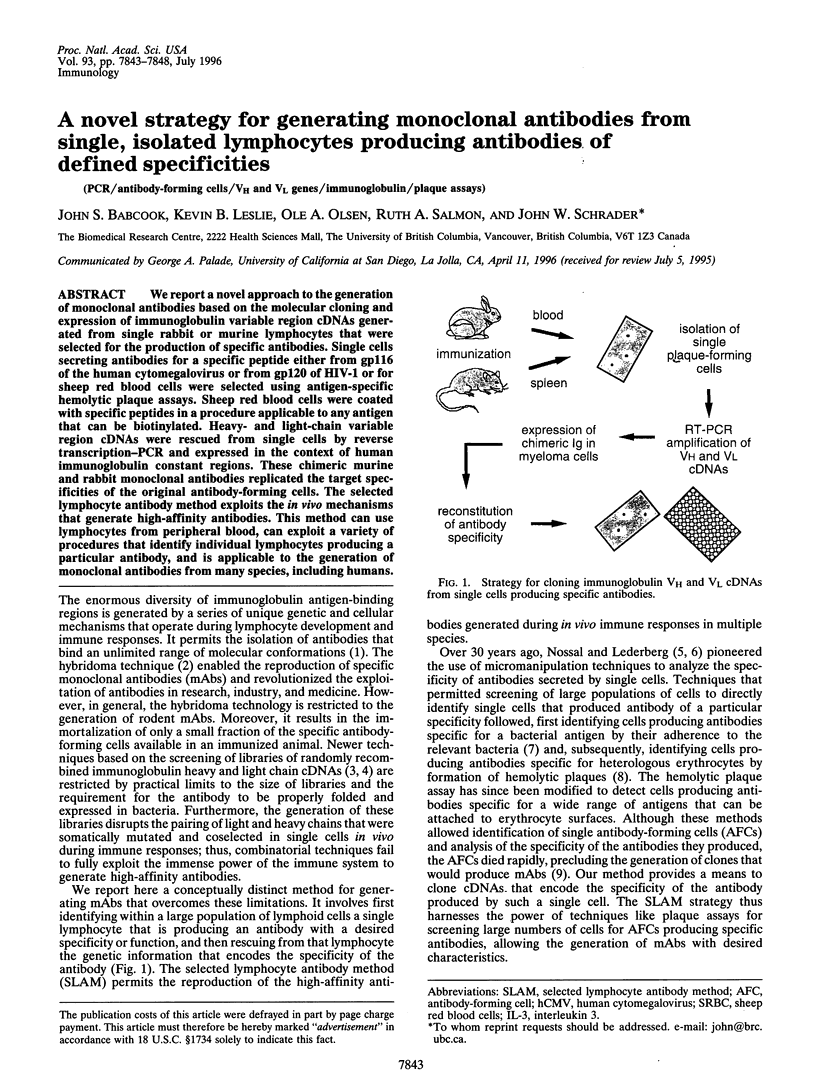



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Askenase P. W., Haynes J. D., Tauben D., De Bernardo R. Specific basophil hypersensitivity induced by skin testing and transferred using immune serum. Nature. 1975 Jul 3;256(5512):52–54. doi: 10.1038/256052a0. [DOI] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Barington T., Heilmann C. An improved haemolytic plaque assay for the detection of cells secreting antibody to bacterial antigens. J Immunol Methods. 1992 Jan 21;146(1):129–137. doi: 10.1016/0022-1759(92)90056-y. [DOI] [PubMed] [Google Scholar]
- Barington T., Heilmann C., Andersen V. Quantitation of antibody-secreting cells in the blood after vaccination with Haemophilus influenzae type b conjugate vaccine. Scand J Immunol. 1990 Apr;31(4):515–522. doi: 10.1111/j.1365-3083.1990.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Caruso A., Bonfanti C., Colombrita D., De Francesco M., De Rango C., Foresti I., Gargiulo F., Gonzales R., Gribaudo G., Landolfo S. Natural antibodies to IFN-gamma in man and their increase during viral infection. J Immunol. 1990 Jan 15;144(2):685–690. [PubMed] [Google Scholar]
- Cawley D., Chiang B. L., Naiki M., Ansari A. A., Gershwin M. E. Comparison of the requirements for cognate T cell help for IgG anti-double-stranded DNA antibody production in vitro: T helper-derived lymphokines replace T cell cloned lines for B cells from NZB.H-2bm12 but not B6.H-2bm12 mice. J Immunol. 1993 Mar 15;150(6):2467–2477. [PubMed] [Google Scholar]
- Chatenoud L., Baudrihaye M. F., Chkoff N., Kreis H., Goldstein G., Bach J. F. Restriction of the human in vivo immune response against the mouse monoclonal antibody OKT3. J Immunol. 1986 Aug 1;137(3):830–838. [PubMed] [Google Scholar]
- Chattopadhyay H., Chattopadhyay C., Natvig J. B., Wiger D., Mellbye O. J. Demonstration of anti-rubella antibody-secreting cells in rheumatoid arthritis patients. Scand J Immunol. 1979;10(1):47–54. doi: 10.1111/j.1365-3083.1979.tb01333.x. [DOI] [PubMed] [Google Scholar]
- Coloma M. J., Hastings A., Wims L. A., Morrison S. L. Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods. 1992 Jul 31;152(1):89–104. doi: 10.1016/0022-1759(92)90092-8. [DOI] [PubMed] [Google Scholar]
- Embleton M. J., Gorochov G., Jones P. T., Winter G. In-cell PCR from mRNA: amplifying and linking the rearranged immunoglobulin heavy and light chain V-genes within single cells. Nucleic Acids Res. 1992 Aug 11;20(15):3831–3837. doi: 10.1093/nar/20.15.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomsgaard A., Svenson M., Bendtzen K. Auto-antibodies to tumour necrosis factor alpha in healthy humans and patients with inflammatory diseases and gram-negative bacterial infections. Scand J Immunol. 1989 Aug;30(2):219–223. doi: 10.1111/j.1365-3083.1989.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Friedman M. L., Tunyaplin C., Zhai S. K., Knight K. L. Neonatal VH, D, and JH gene usage in rabbit B lineage cells. J Immunol. 1994 Jan 15;152(2):632–641. [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Gorny M. K., Xu J. Y., Karwowska S., Buchbinder A., Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993 Jan 15;150(2):635–643. [PubMed] [Google Scholar]
- Green L. L., Hardy M. C., Maynard-Currie C. E., Tsuda H., Louie D. M., Mendez M. J., Abderrahim H., Noguchi M., Smith D. H., Zeng Y. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. 1994 May;7(1):13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- Griffiths A. D., Williams S. C., Hartley O., Tomlinson I. M., Waterhouse P., Crosby W. L., Kontermann R. E., Jones P. T., Low N. M., Allison T. J. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 1994 Jul 15;13(14):3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C., Henrichsen J., Pedersen F. K. Vaccination-induced circulation of human B cells secreting type-specific antibodies against pneumococcal polysaccharides. Scand J Immunol. 1987 Jan;25(1):61–67. doi: 10.1111/j.1365-3083.1987.tb01047.x. [DOI] [PubMed] [Google Scholar]
- Heilmann C., Pedersen F. K. Quantitation of blood lymphocytes secreting antibodies to pneumococcal polysaccharides after in vivo antigenic stimulation. Scand J Immunol. 1986 Feb;23(2):189–194. doi: 10.1111/j.1365-3083.1986.tb01957.x. [DOI] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Kapp J. A., Sorensen C. M., Pierce C. W. Antigen-specific suppressor T cell interactions. II. Characterization of two different types of suppressor T cell factors specific for L-glutamic acid50-L-tyrosine50 (GT) and L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). J Exp Med. 1983 Dec 1;158(6):1962–1978. doi: 10.1084/jem.158.6.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Fauci A. S. Activation of human B lymphocytes after immunization with pneumococcal polysaccharides. J Clin Invest. 1983 Apr;71(4):1032–1040. doi: 10.1172/JCI110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., Becker R. S. Molecular basis of the allelic inheritance of rabbit immunoglobulin VH allotypes: implications for the generation of antibody diversity. Cell. 1990 Mar 23;60(6):963–970. doi: 10.1016/0092-8674(90)90344-e. [DOI] [PubMed] [Google Scholar]
- Konecny J., Eckert M., Schöniger M., Hofacker G. L. Neutral adaptation of the genetic code to double-strand coding. J Mol Evol. 1993 May;36(5):407–416. doi: 10.1007/BF02406718. [DOI] [PubMed] [Google Scholar]
- Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. I. Direct evidence that lymphocytes with a high density of membrane-bound immunoglobulin are precursors of plasmacytes. Cell Immunol. 1974 Jun;12(3):396–406. doi: 10.1016/0008-8749(74)90096-3. [DOI] [PubMed] [Google Scholar]
- Küppers R., Zhao M., Hansmann M. L., Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993 Dec 15;12(13):4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerkvist A. C., Furebring C., Borrebaeck C. A. Single, antigen-specific B cells used to generate Fab fragments using CD40-mediated amplification or direct PCR cloning. Biotechniques. 1995 May;18(5):862–869. [PubMed] [Google Scholar]
- Laman J. D., Schellekens M. M., Abacioglu Y. H., Lewis G. K., Tersmette M., Fouchier R. A., Langedijk J. P., Claassen E., Boersma W. J. Variant-specific monoclonal and group-specific polyclonal human immunodeficiency virus type 1 neutralizing antibodies raised with synthetic peptides from the gp120 third variable domain. J Virol. 1992 Mar;66(3):1823–1831. doi: 10.1128/jvi.66.3.1823-1831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A. H., Creadon G., Wysocki L. J. Sequencing heavy- and light-chain variable genes of single B-hybridoma cells by total enzymatic amplification. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7610–7614. doi: 10.1073/pnas.89.16.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg N., Taylor L. D., Harding F. A., Trounstine M., Higgins K. M., Schramm S. R., Kuo C. C., Mashayekh R., Wymore K., McCabe J. G. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994 Apr 28;368(6474):856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- MAKELA O., NOSSAL G. J. Autoradiographic studies on the immune response. II. DNA synthesis amongst single antibody-producing cells. J Exp Med. 1962 Jan 1;115:231–244. doi: 10.1084/jem.115.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKELA O., NOSSAL G. J. Bacterial adherence: a method for detecting antibody production by single cells. J Immunol. 1961 Oct;87:447–456. [PubMed] [Google Scholar]
- Manyonda I. T., Soltys A. J., Hay F. C. A critical evaluation of the magnetic cell sorter and its use in the positive and negative selection of CD45RO+ cells. J Immunol Methods. 1992 Apr 27;149(1):1–10. doi: 10.1016/s0022-1759(12)80042-1. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J., Lalor P. A. Molecular characterization of single memory B cells. Nature. 1991 Apr 11;350(6318):502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- Merchant B., Hraba T. Lymphoid cells producing antibody against simple haptens: detection and enumeration. Science. 1966 Jun 3;152(3727):1378–1379. doi: 10.1126/science.152.3727.1378. [DOI] [PubMed] [Google Scholar]
- Meyer H., Sundqvist V. A., Pereira L., Mach M. Glycoprotein gp116 of human cytomegalovirus contains epitopes for strain-common and strain-specific antibodies. J Gen Virol. 1992 Sep;73(Pt 9):2375–2383. doi: 10.1099/0022-1317-73-9-2375. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Warner N. L. The immune response of normal, irradiated and thymectomized mice to fowl immunoglobulin G as detected by a hemolytic plaque technique. Int Arch Allergy Appl Immunol. 1971;40(1):59–71. doi: 10.1159/000230395. [DOI] [PubMed] [Google Scholar]
- NOSSAL G. J. Antibody production by single cells. III. The histology of antibody production. Br J Exp Pathol. 1959 Aug;40:301–311. [PMC free article] [PubMed] [Google Scholar]
- NOSSAL G. J., LEDERBERG J. Antibody production by single cells. Nature. 1958 May 17;181(4620):1419–1420. doi: 10.1038/1811419a0. [DOI] [PubMed] [Google Scholar]
- Ohlin M., Sundqvist V. A., Mach M., Wahren B., Borrebaeck C. A. Fine specificity of the human immune response to the major neutralization epitopes expressed on cytomegalovirus gp58/116 (gB), as determined with human monoclonal antibodies. J Virol. 1993 Feb;67(2):703–710. doi: 10.1128/jvi.67.2.703-710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi R., Güssow D. H., Jones P. T., Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 May;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Hayward J. A. The lymphocyte surface. I. Relation between Fc receptors, C'3 receptors and surface immunoglobulin. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):47–63. doi: 10.1098/rspb.1974.0060. [DOI] [PubMed] [Google Scholar]
- Razin E., Leslie K. B., Schrader J. W. Connective tissue mast cells in contact with fibroblasts express IL-3 mRNA. Analysis of single cells by polymerase chain reaction. J Immunol. 1991 Feb 1;146(3):981–987. [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Nossal G. J. Effector cell blockade. A new mechanism of immune hyporeactivity induced by multivalent antigens. J Exp Med. 1974 Jun 1;139(6):1582–1598. doi: 10.1084/jem.139.6.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. D., Benkovic S. J. Transition-state stabilization as a measure of the efficiency of antibody catalysis. Nature. 1995 Jun 1;375(6530):388–391. doi: 10.1038/375388a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kamimura J., Ayabe T., Kashiwagi H. Demonstration of neutralizing autoantibodies against IL-1 alpha in sera from patients with rheumatoid arthritis. J Immunol. 1990 Oct 1;145(7):2140–2146. [PubMed] [Google Scholar]
- Thomson P. D., Harris N. S. Detection of plaque-forming cells in the peripheral blood of actively immunized humans. J Immunol. 1977 Apr;118(4):1480–1482. [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]







