Abstract
Background
Lead toxicity has been subjected to intensive research work, but some aspects of its mechanism needs to be elucidated.
Objectives
In the current study we aim to investigate the impact of lead toxicity on some different intermediates of apoptotic signaling pathway in experimental rats.
Design and methods
We measured caspase-8 and caspase-9 [by chemilumenescence], Bax and Bcl-2 [by ELISA] in Experimental rats, injected intraperitoneally with lead acetate for 7days at the dosage of 25, 50 and l00 mg/kg body weight and compared to control rats injected with deionized distilled water instead. instead.
Results
Lead acetate significantly increased the levels of caspase 8, caspase 9 and Bax in liver, kidney and brain of experimental animals especially those with high doses. Meanwhile, caspase 8 and Bax significantly increased in brain tissue at low dose of lead, while Bcl-2 significantly increased only with advanced toxicity. Furthermore, Bax/bcl2 ratio was significantly high in kidney (p<0.05), liver (p<0.01) and brain (p<0.01) at higher doses of lead toxicity. However, brain tissues showed significant Bax/Bcl2 ratio (p<0.05) at low lead dose. A significant positive correlation was noticed between the blood level of lead and enzymatic level of caspase 8, caspase 9 and Bax in different tissues.
Conclusion
: we concluded that lead might have toxic effect through intrinsic and extrinsic induction of apoptotic pathway with prominent effect on brain tissue even at low dose.
Keywords: Lead toxicity, Rats, Apoptosis, Bcl-2, Bax, Caspase 8 and Caspase 9
Introduction
Exposure to lead is unavoidable as it occurs through many routes including contaminated air, water, soil, food, and consumer products. The safe threshold for lead exposure has not been identified, as there is no accurate amount specified for lead toxicity. (1) Lead exists in different forms and is a constituent of a variety of organic compounds, which have the ability of direct penetration of the skin, respiratory tract, and it has a predominant effect on central nervous system. (2) Consequently, it constitutes a significant public health problem, despite efforts to reduce its level in the ecosystem. (3)
Neurological damage induced by lead toxicity is a well known condition that has been reported to be a base for several disorders like mental retardation; behavioral problems; nerve damage; Alzheimer’s disease; Parkinson’s disease; and possibly schizophrenia. (4) The ability of lead to pass through the blood-brain barrier is due in large part to its ability to substitute for calcium ions. At the molecular level, lead interferes with the regulatory action of calcium on cell functions and disrupts many intracellular biological activities. (5)
The mechanism of lead-induced toxicity is complex and needs more declaration. An important mechanism of lead toxicity is the induction of apoptosis. (6) The Bcl-2 (B cell Leukemia/Lymphoma) family comprises of approximately twenty proteins that collaborate to either maintain cell survival or initiate apoptosis. (7) It comprises several Bcl2-related genes of which some, promote apoptosis (Bax, Bad) and some others exert the opposite effect, inhibiting apoptosis (Bcl-2, Bcl-Xl, Bcl-w, NR-13, Mcl-1). (8) Flora et al. (2012)(9) found that, the combined exposure to lead and ethanol leads to increased oxidative stress and possible initiation of apoptosis in rats in a dose-dependent effects.
Experimental evidence has revealed that apoptosis can be regulated and abolished at several distinct points, especially the caspase activation process, in the apoptotic pathway. (10)
The main function of IAPs (Inhibitor of apoptosis proteins), is assumed to be the suppression of apoptosis via (baculovirus inhibitory repeat) BIR domain dependent interactions, inhibits the neuron-restricted calcium-binding protein, and inhibition of the processed caspases-3, -7 and -9. (11) Indeed, several experimental studies have reported of lead induced apoptosis in rat tissue including brain, (12) testis, (13) fibroblasts, (14) lung (15) and rat/mouse retinal rod cells, (16) but few of them focused on mechanism or dose dependent toxicity.
The current study was conducted to examine the effect of lead toxicity on apoptosis in different rat tissues [brain, liver and kidney], through estimation of caspase 9 (intrinsic pathway), caspase 8 (extrinsic pathway), pro-apoptotic (Bax) and anti-apoptotic (Bcl-2) molecules and to correlate these changes with different doses of lead toxicity.
Material and Methods
Experimental Design
Forty young experimental male rats (6–8 months, 350–400 g body wt) were included in the current study. Animals were divided into four groups; (10 rats/group): group1, served as control group were injected with intraperitoneal deionized distilled water and lead toxicity was induced in second, third, and fourth groups by intraperitoneal injection of lead acetate (in deionized distilled water) of low dose (25 mg/kg/d), medium dose (50 mg/kg/d) and high dose (100 mg/kg/d) respectively, for seven days. All rats were fed with standard rodent chow, and caged in pairs and in an environment with 37 °C temperature, 60–65% relative humidity, 12 hours light and 12 hours dark cycles.
Data and Specimen Collection
At the end of the experimental treatment, the rats of each group were sacrificed. Blood samples were collected into heparinized tubes. Rat tissues (brain, liver, and kidney) were excised and washed with cold Phosphate buffer saline (PBS). The tissues were kept on ice all times.
Preparation of tissue homogenates
Rat tissues (1 gm) were minced and then homogenized in 1 ml lysis buffer [20 mM HEPES (pH 7.5), 150 mM NaCl, 1% NP-40, 0.1% SDS, 1 mM EDTA, and 1.0 mM DTT] with protease inhibitors (2 μg each of aprotinin, leupeptin, pepstatin A, and 0.5 mM phenylmethylsulfonyl fluoride), incubated on ice for 30 min, and then centrifuged at 10,000 xg at 4°C for 20 min. The resulting supernatant [cell lysates] were separated and stored at −70 °C until used for further analysis.
Lead analysis in whole blood
Blood Lead levels in rat were analyzed by employing flame atomic absorption spectrometry according to previously reported methods. (17) All laboratory glassware, polypropylene tubes, and disposable micropipette tips were immersed for several hours in 1:1 v/v concentrated HNO3/H2O, thoroughly rinsed in deionized water, and nitrogen gas dried before use, to avoid any possible contamination. Blood samples (200 ml) were added to 800 ml of Supra-pure HNO3, centrifuged at 15000 rpm for 15 min, and a 100 ml aliquot was taken from the clear solution and diluted (1:5 v/v) with deionized water. Calibration curves were constructed by adding known amounts of lead standard (E. Merck). Diluted blood samples were injected into the atomic absorption spectrophotometer (Perkin-Elmer Model 400, Shelton, CT, USA). Hollow cathode lamps of Pb were used at wavelength of 283.3 nm. The levels of blood lead (Pb) were expressed as part per million (ppm).
Measurement of Protein Concentration
The protein concentration in tissue lysates was measured by colorimetric method of Bradford (1976). (18)
Quantitative estimation of apoptotic markers in tissue lysates
Caspase 8 and caspase 9 were determined by Caspase-Glo ® 8 Assay and Caspase-Glo ® 9 Assay, Beckman, USA, respectively. The assay provides a luminogenic caspase substrate in buffer system optimized for caspase activity. The luminescence of each sample was measured in plate-reading luminometer by Ultra-Glo TMRecombinant Luciferase. One unit of caspase-8 is the amount of enzyme required to cleave 1pmol of substrate (Ac-LETD-pNA) per minute at 30°C. While one unit of caspase-9 is the amount of enzyme required to cleave 1pmol of substrate (Ac-LEHD-pNA) per minute at 30°C. Levels were expressed as Units/mg protein.
Bcl-2 protein and Bax protein levels were measured in tissue lysates by ELISA kits, Uscn Life Science Inc., the procedure was performed according to instructions of manufacturer. Levels were expressed as ng/mg tissue protein.
Statistical analysis
Data are expressed as mean values of estimated parameters ± SD. Comparison of different parameters between groups was done by t-test and ANOVA. Spearman correlation coefficient (r) was utilized to study the association between the different variables. Values of p lower than 0.05 were considered statistically significant. These analyses were performed using the Statistical Package for the Social Sciences (SPSS software, version 16.0, Chicago, Illinois) on a personal computer.
Results
As shown in Table (1) blood lead levels of experimental rats of various groups expressed as ppm. There was a significant increase in blood lead concentrations with increasing intraperitoneal dose administration. In comparison to control group the level of blood lead was about 3.2 fold increase in (low lead dose; 25 mg/kg/d) group 2, 4.9 fold in (medium dose; 50 mg/kg/d) group 3 and 6.38 fold in (high dose;100 mg/kg/d) group 4, respectively. The rat liver lysate showed significant increase of caspase 8, caspase 9 and Bax with high lead toxicity (medium and high dose) as compared to controls. Bcl-2 showed no significant difference of Bcl-2 protein between studied groups (Table 2).
Table (1).
Lead concentrations (ppm), in blood in different treated groups of rats:
| Lead (ppm) | Controls | Group 1 | Group 2 | Group 3 |
|---|---|---|---|---|
| means ± SD | 0.15 ± 0.03 | 0.49 ± 0.07** | 0.69 ± 0.04** | 0.95 ± 0.06** |
| Median | 0.149 | 0.48 | 0.69 | 0.95 |
| Range | 0.12–0.18 | 0.42–0.55 | 0.56–0.73 | 0.98–1.19 |
p value was detected compared to control group
p < 0.01: is highly significant
Table (2).
Apoptotic makers in liver lysates.
| Variables | Controls | Group1 | Group 2 | Group 3 |
|---|---|---|---|---|
| Caspase 8 (units/mg protein) | 1.0±0.05 | 1.45± 0.04 | 1.4*2.9± | 10.8± 0.84** |
| Caspase 9 (units/mg protein) | 1.45± 0.49 | 1.55±0.63 | 7.5±0.56** | 8.7±2.8 ** |
| Bcl2 (ng/mg protein) | 45.5± 12.0 | 46.2± 5.3 | 45.4±6.8 | 63.3±4.1 |
| Bax (ng/mg protein) | 8.6±2.4 | 10.7±0..28 | 26.4±2.8** | 30.1±4.4** |
Values are means ± SD, p value was detected compared to control group
p ≤ 0.05: is significant,
p < 0.01: is highly significant
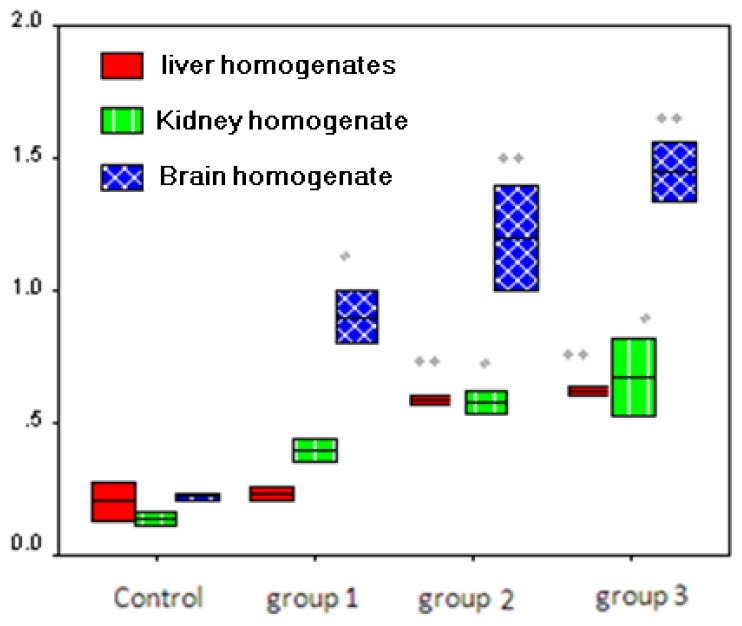
Moreover, the Kidney showed a significant higher level of Bcl-2 with advanced toxicity (high dose), beside the significant increase of caspase 8, caspase 9 and Bax with increasing dose of lead (Table 3). Furthermore, Bcl-2 increased significantly in brain in group 3 lead treated animals (100 mg/kg/d), meanwhile caspase 9, caspase 8 and Bax were significantly increased with higher doses of lead (50 mg/kg/d and 100 mg/kg/d), but both caspase 8 and Bax were higher even at low lead dose (25mg/kg/d) as shown in Table 4. By comparing Bax/bcl2 ratio in different rat tissues lysates, it was significantly higher in kidney (p < 0.05), liver (p < 0.01) and brain (p < 0.01) in group 2 and group 3 compared to control group. Moreover, brain tissues showed higher Bax/bcl2 ratio (p < 0.05) at low lead dose (25mg/kg/d) as shown in Figure (1). There were a significant positive correlation between the blood levels of lead with caspase 8 (p < 0.05), caspase 9 (p < 0.01) and Bax (p < 0.01) (Table 5).
Table (3).
Apoptotic makers in kidney lysates.
| Variables | Controls | Group1 | Group 2 | Group 3 |
|---|---|---|---|---|
| Caspase 8 (units/mg protein) | 0.85± 0.03 | 1.5± 0.14 | 1.8± 0.02** | 2.1± 0.14** |
| Caspase 9 (units/mg protein) | 1.3± 0.28 | 1.5± 0.56 | 5.0± 1.27* | 6.95± 1.2** |
| Bcl2 (ng/mg protein) | 23.6±1.1 | 21.3±1.6 | 30.7±11.3 | 50.3±14.6* |
| Bax (ng/mg protein) | 3.2± 1.1 | 8.5±2.1 | 17.7±0.35** | 22.5±3.5** |
Values are means ± SD, p value was detected compared to control group
p ≤ 0.05: is significant,
p < 0.01: is highly significant
Table (4).
Apoptotic makers in brain lysates.
| Variables | Controls | Group1 | Group 2 | Group 3 |
|---|---|---|---|---|
| Caspase 8 (units/mg protein) | 1.1± 0.02 | 1.45± 0.04* | 1.8± 0.14** | 2.1± 0.15** |
| Caspase 9 (units/mg protein) | 1.0± 0. 5 | 4.75± 0.63 | 6.9± 0.42* | 12.2± 4.7** |
| Bcl2 (ng/mg protein) | 25.0± 1.4 | 24.0± 5.6 | 27.3±3.2 | 36.0±5.6* |
| Bax (ng/mg protein) | 5.5± 0.7 | 21.2± 1.7* | 36.5±10.6** | 52.5±3.5** |
Values are means ± SD, p value was detected compared to control group
p ≤ 0.05: is significant,
p < 0.01: is highly significant
Figure (1).
Bax/Bcl2 Ratio in Rat tissues Homogenates treated by Lead Acetate
Values are mean using one way ANOVA, p value was detected compared to control group. *p ≤ 0.05: is significant, **p < 0.01: is highly significant
Table (5).
Correlation coefficient (r) between lead and other apoptotic markers:
P ≤ 0.05: is significant,
p < 0.01: is highly significant
Discussion
Lead (Pb) is a prevalent occupational and environmental neurotoxin. (19) The wide use of lead had converted the lead poisoning into a ubiquitous worldwide environmental and medical challenge, and everyone has some measurable level of lead in blood. (20) Lead poisoning is one of the largest in terms of numbers of people exposed and the public health toll it takes. (21)
Lead is a potent neurotoxin that damages the nervous system and causes brain disorders. Although current lead usage has been minimized, lead exposure is still a risk because environmental lead is stable and no safe threshold for lead exposure has been established. The effects of lead are particularly damaging to the developing nervous system, causing potentially irreversible learning and behavior deficits. (22) The current study was conducted to explore the apoptotic effect of lead toxicity on tissues of experimental rats; when we analyzed the apoptotic effects of lead poisoning we have noticed a significant increase in enzymatic activity of caspase-8 and caspase-9 in hepatic, renal, and brain tissue lysates. This finding supports the apoptotic effect of lead on both intrinsic and extrinsic pathways. Agarwal et al. (2009)(23) found that stimulation of caspase cascade and simultaneous extracellular signal-regulated kinase (ERK) dephosphorylation are the most significant operative pathways directly associated with apoptotic signals triggered by lead acetate in adult rat hepatic stem cells. Moreover, Yedjouet al. (2010)(24) found that lead nitraterepresents an apoptosis-inducing agent in promyelocytic leukemia cells and its apoptotic mechanism functions, at least in part via, induction of phosphatidyl serine externalization and caspase-3 activation.
In the current study, Bax protein levels were significantly increased in liver, kidney and brain. Its increasing levels were significantly higher with higher doses of lead toxicity. While Bcl-2 levels were only increased significantly in advanced lead toxicity in both kidney and brain. The increased levels of Bcl-2 may be as compensated balanced due to over activation of Bax especially in advanced lead toxicity. These data may be indicated as the primary pathway of apoptotic stimulation by lead is through stimulation of apoptotic marker; Bax ; rather than through its inhibitory effect on anti-apoptotic molecules as Bcl-2. In this respect, the ratio of antiapoptotic molecules to proapoptotic molecules might be more important than absolute amounts of each of them. In the present study, comparing Bax/bcl2 ratio in different tissues in lead treated animals, showed significantly high level in both kidney and liver. However, brain tissues showed significant elevated Bax/bcl2 ratio at high as well as low lead doses.
Our findings support those of Sharifi et al. (2010)(25) who suggested that neurotoxicity of chronic lead exposure in hippocampus in vivo may be due to facilitation of apoptosis. They found high expression of Bax protein and no significant change in bcl-2 expression by western blot analysis in rat hippocampus and accordingly increased the Bax/Bcl-2 ratio compared to control group. By contrast, Rong et al. (2008)(26) reported that, lead acetate induces apoptosis in Bcl-2culture cells, which may be related to activation of NF-kappa B and p53 gene and depression of Bcl-2 gene.
We recorded in this study that, liver and kidney tissues were almost not affected from lead toxicity at low levels. While, brain showed significant apoptotic changes, as caspase-8 activity, BAX and Bax/bcl2 ratio were significantly increased. That might indicate higher hazardous effects of lead toxicity especially on neural tissues even at low doses.
These findings support the hypothesis that lead-related apoptosis evokes an intrinsic and extrinsic pathway of pro-apoptotic signaling within the rat liver, kidney and brain. Furthermore, our results partially explained the prominent neurotoxic effect of lead even at low level of toxicity.
Acknowledgment
This study was supported by Sabic Research grants grant [SRD-011-21], Qassim University, Saudi Arabia.
References
- 1.Rossi E. The Clinical biochemist. Vol. 29. Reviews/Australian Association of Clinical Biochemists; 2008. Low level environmental lead exposure—a continuing challenge; pp. 63–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, Schwartz BS, Hu H, Woolf A. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115:463–71. doi: 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rojas-Castañeda JC, Vigueras-Villaseñor RM, Rojas P, Chávez-Saldaña M, Gutiérrez-Pérez O, Montes S, Ríos C. Alterations induced by chronic lead exposure on the cells of circadian pacemaker of developing rats. Int J Exp Pathol. 2011;4:243–50. doi: 10.1111/j.1365-2613.2011.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Han D, Li Y, Zheng L, Gu C, Piao Z, Au W, Xu Z, Huo X. Lead Affects Apoptosis and Related Gene XIAP and Smac Expression in the Hippocampus of Developing Rats. Neurochem Res. 2010;35:473–479. doi: 10.1007/s11064-009-0083-9. [DOI] [PubMed] [Google Scholar]
- 5.Sanders T, Liu Y, Buchner V, Tchounwou P. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev Environ Health. 2009;24:15–45. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulido MD, Parrish AR. Metal-induced apoptosis: mechanisms. Mutat Res. 2003;533:227–241. doi: 10.1016/j.mrfmmm.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flora SJ, Gautam P, Dwivedi N. Dose-dependent effects of ethanol on lead-induced oxidative stress in rats. J Environ Pathol Toxicol Oncol. 2012;31:61–73. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i1.70. [DOI] [PubMed] [Google Scholar]
- 10.Rami BG, Chin LS, Lazio BE, Singh SK. Okadaic-acid-induced apoptosis in malignant glioma cells. Neurosurg Focus. 2003;14(2):e4. doi: 10.3171/foc.2003.14.2.5. [DOI] [PubMed] [Google Scholar]
- 11.Niu Y, Zhang R, Cheng Y, Sun X, Tian J. Effect of lead acetate on the apoptosis and the expression of and bax genes in rat brain cells. 2002;36(1):30–3. [PubMed] [Google Scholar]
- 12.Sharifi AM, Baniasadi S, Jorjani M, Rahimi F, Bakhshayesh M. Investigation of acute lead poisoning on apoptosis in rat hippocampus in vivo. Neurosci Lett. 2002;329:45–48. doi: 10.1016/s0304-3940(02)00576-1. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari N, Sinha N, Narayan R, Saxena DK. Lead induced cell death in testis of young rats. J Appl Toxicol. 2001;21:275–277. doi: 10.1002/jat.754. [DOI] [PubMed] [Google Scholar]
- 14.Iavicoli I, Carelli G, Sgambato A, Masci O, Ardito R, Cittadini A, Castellino N. Lead inhibits growth and induces apoptosis in normal rat fibroblasts. Altern Lab Anim. 2001;29:461–469. [PubMed] [Google Scholar]
- 15.Shabani A, Rabbani A. Lead nitrate induced apoptosis in alveolar macrophages from rat lung. Toxicology. 2000;149:109–114. doi: 10.1016/s0300-483x(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 16.He L, Perkins GA, Poblenz AT, Harris JB, Hung M, Ellisman MH, Fox DA. Bcl-xL overexpression blocks bax mediated mitochondrial contact site formation and apoptosis in rod photoreceptors of lead-exposed mice. Proc Natl Acad Sci USA. 2003;100:1022–1027. doi: 10.1073/pnas.0333594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villeda-Hernandez J, Barroso-Moguel R, Mendez-Armenta M, Nava-Ruız C, Huerta-Romero R. Enhanced brain regional lipid peroxidation in developing rats exposed to low level lead acetate. Brain Research Bulletin. 2001;55:247–251. doi: 10.1016/s0361-9230(01)00512-3. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MA. rapid and sensitive method for the quantitation of microgram quantities of prtein utilizing the principle of protein – dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH. Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. Int J Dev Neurosci. 2003;21:1–12. doi: 10.1016/s0736-5748(02)00137-5. [DOI] [PubMed] [Google Scholar]
- 20.Payne M. Lead in drinking water. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2008;179(3):253–4. doi: 10.1503/cmaj.071483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environmental health perspectives. 2007;115(3):455–62. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiang J, Díaz E. Lead and developmental neurotoxicity of the central nervous system. Current Neurobiology. 2011;2(1):35–42. [Google Scholar]
- 23.Agarwal S, Roy S, Ray A, Mazumder S, Bhattacharya S. Arsenic trioxide and lead acetate induce apoptosis in adult rat hepatic stem cells. Cell Biol Toxicol. 2009;25(4):403–13. doi: 10.1007/s10565-008-9094-6. [DOI] [PubMed] [Google Scholar]
- 24.Yedjou CG, Milner JN, Howard CB, Tchounwou PB. Basic apoptotic mechanisms of lead toxicity in human leukemia (HL-60) cells. Int J Environ Res Public Health. 2010;7(5):2008–17. doi: 10.3390/ijerph7052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharifi AM, Mousavi SH, Jorjani M. Effect of chronic lead exposure on pro-apoptotic Bax and anti-apoptotic Bcl-2 protein expression in rat hippocampus in vivo. Cellular and Molecular Neurobiology. 2010;30:769–774. doi: 10.1007/s10571-010-9504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rong J, Chang W, Lv L, Chen J. Study on the roles of nuclear factor-kappaB, p53 and Bcl-2 gene in lead acetate in lead acetate induced apoptosis in PC12 cells. Wei Sheng Yan Jiu. 2008;37(3):262–3. [PubMed] [Google Scholar]