Abstract
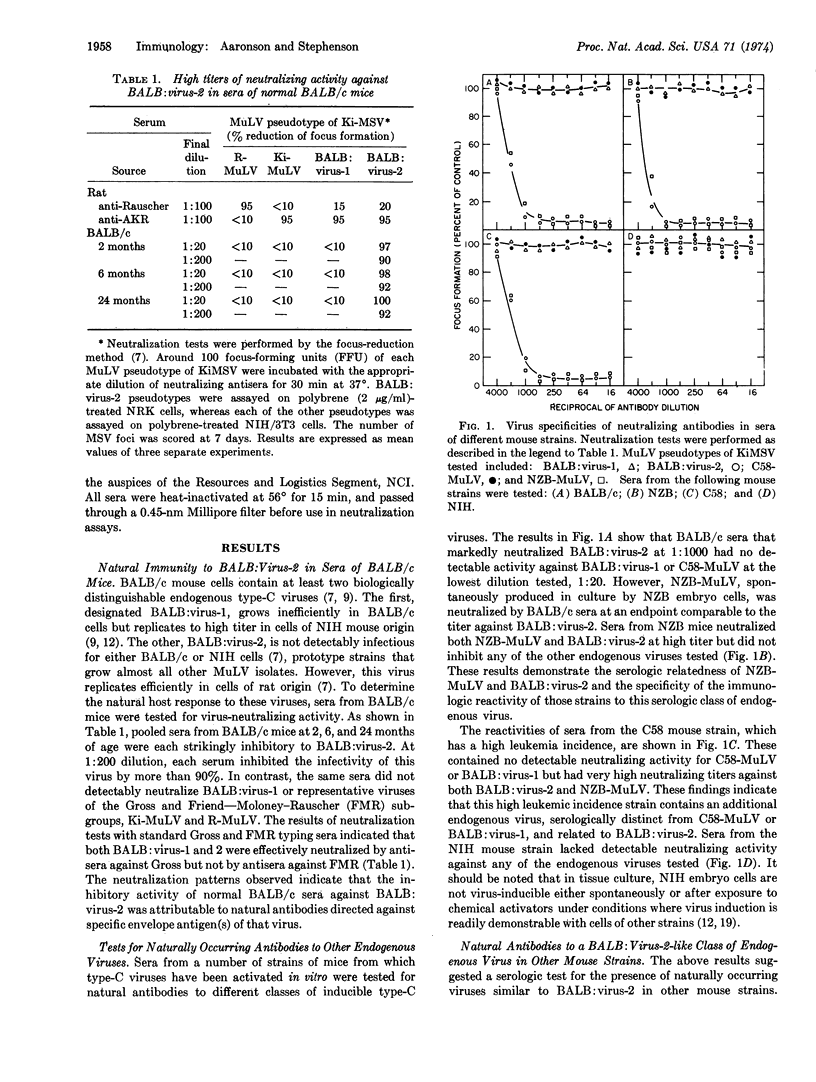
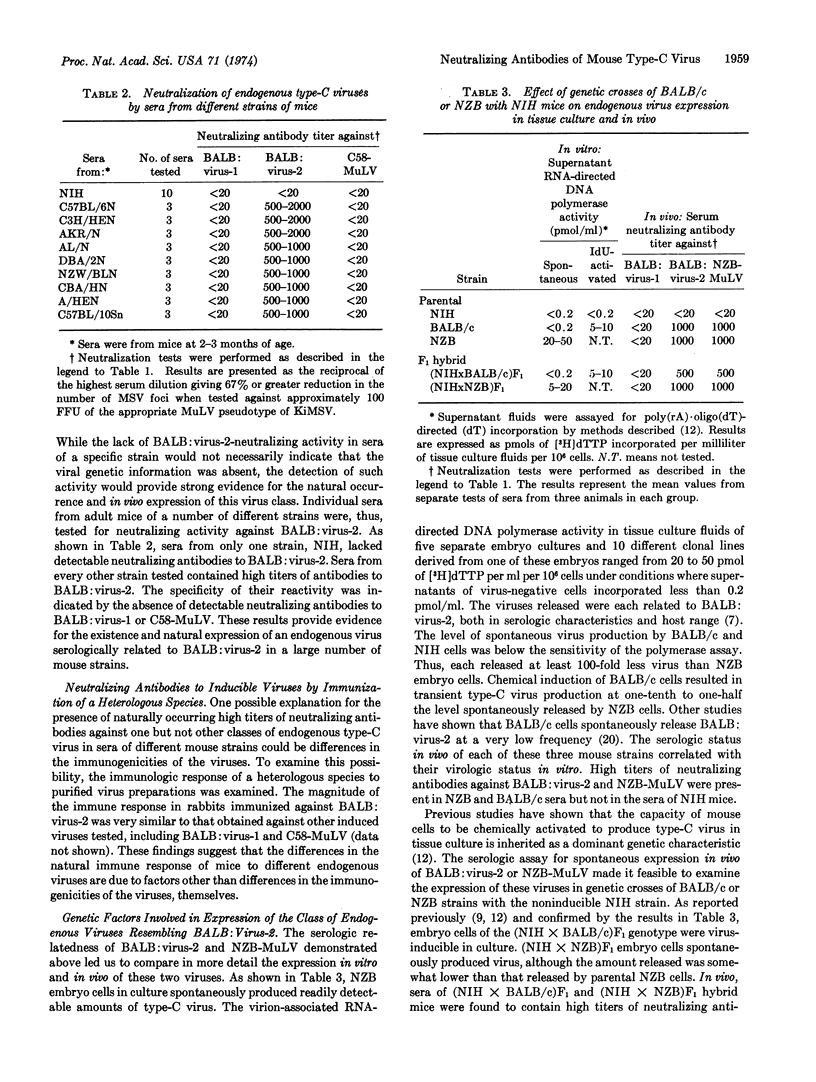
Mouse cells contain several biologically distinguishable endogenous type-C viruses. In the present studies, the immunologic response of different strains of mice to the natural expression of these viruses has been examined. High titers of neutralizing antibodies have been detected to a class of virus that lacks the ability to exogenously infect mouse cells. The expression of this virus, in vivo as well as in vitro, is shown to be vertically transmitted as a dominant genetic trait. The findings indicate the widespread occurrence of this endogenous virus and the ability of the mouse to immunologically respond to a class of type-C virus whose mode of transmission is similar to that of cellular genes.
Keywords: dominant genetic trait, RNA virus, cancer
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Chemical induction of focus-forming virus from nonproducer cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3069–3072. doi: 10.1073/pnas.68.12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Dunn C. Y. Endogenous C-type viruses of BALB-c cells: frequencies of spontaneous and chemical induction. J Virol. 1974 Jan;13(1):181–185. doi: 10.1128/jvi.13.1.181-185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Evidence that the AKR murine-leukemia-virus genome is complete in DNA of the high-virus AKR mouse and incomplete in the DNA of the "virus-negative" NIH mouse. Proc Natl Acad Sci U S A. 1974 Jan;71(1):167–171. doi: 10.1073/pnas.71.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS L. Pathogenic properties, and "vertical" transmission of the mouse leukemia agent. Proc Soc Exp Biol Med. 1951 Oct;78(1):342–348. doi: 10.3181/00379727-78-19068. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Aaronson S. A. In vivo inoculation of RNA C-type viruses inducing regression of experimental solid tumors. J Natl Cancer Inst. 1973 Dec;51(6):1935–1938. doi: 10.1093/jnci/51.6.1935. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Tennant R. W., Yuhas J. M., Clapp N. K., Batzing B. L., Snodgrass M. J. Autogenous immunity to endogenous RNA tumor virus antigens in mice with a low natural incidence of lymphoma. Cancer Res. 1972 Oct;32(10):2226–2234. [PubMed] [Google Scholar]
- Huebner R. J., Kelloff G. J., Sarma P. S., Lane W. T., Turner H. C., Gilden R. V., Oroszlan S., Meier H., Myers D. D., Peters R. L. Group-specific antigen expression during embryogenesis of the genome of the C-type RNA tumor virus: implications for ontogenesis and oncogenesis. Proc Natl Acad Sci U S A. 1970 Sep;67(1):366–376. doi: 10.1073/pnas.67.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Yurconic M., Jr, Hanna M. G., Jr Autogenous immunity to endogenous RNA tumor virus. Radioimmune precipitation assay of mouse serum antibody levels. J Exp Med. 1973 Jul 1;138(1):194–208. doi: 10.1084/jem.138.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Pincus T. Demonstration of biological activity of a murine leukemia virus of New Zealand black mice. Science. 1970 Oct 16;170(3955):326–327. doi: 10.1126/science.170.3955.326. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Mellors R. C., Aoki T., Huebner R. J. Further implication of murine leukemia-like virs in the disorders of NZB mice. J Exp Med. 1969 May 1;129(5):1045–1062. doi: 10.1084/jem.129.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C., Shirai T., Aoki T., Huebner R. J., Krawczynski K. Wild-type Gross leukemia virus and the pathogenesis of the glomerulonephritis of New Zealand mice. J Exp Med. 1971 Jan 1;133(1):113–132. doi: 10.1084/jem.133.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. The antibody response of mice to murine leukemia virus in spontaneous infection: absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):134–138. doi: 10.1073/pnas.69.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lymphocytic choriomeningitis: production of antibody by "tolerant" infected mice. Science. 1967 Dec 1;158(3805):1193–1195. doi: 10.1126/science.158.3805.1193. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Studies of genetic transmission of murine leukemia virus by AKR mice. II. Crosses with Fv-1 b strains of mice. J Exp Med. 1972 Nov 1;136(5):1286–1301. doi: 10.1084/jem.136.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. A., Fitch F. W., Stuart F. P., Köhler H., Cosenza H. Specific suppression of immune responses. Science. 1973 Sep 21;181(4105):1133–1141. doi: 10.1126/science.181.4105.1133. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W., Kawakami T., Kohne D., Okabe H., Gilden R., Hatanaka M. Primate and murine type-C viral nucleic acid association kinetics: analysis of model systems and natural tissues. J Virol. 1974 Feb;13(2):363–369. doi: 10.1128/jvi.13.2.363-369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. A genetic locus for inducibility of C-type in BALB-c cells: the effect of a nonlinked regulatory gene on detection of virus after chemical activation. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2798–2801. doi: 10.1073/pnas.69.10.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Antigenic properties of murine sarcoma virus-transformed BALB-3T3 nonproducer cells. J Exp Med. 1972 Mar 1;135(3):503–515. doi: 10.1084/jem.135.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Genetic factors influencing C-type RNA virus induction. J Exp Med. 1972 Jul 1;136(1):175–184. doi: 10.1084/jem.136.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Segregation of loci for C-type virus induction in strains of mice with high and low incidence of leukemia. Science. 1973 May 25;180(4088):865–866. doi: 10.1126/science.180.4088.865. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Greenberger J. S., Aaronson S. A. Oncogenicity of an endogenous C-type virus chemically activated from mouse cells in culture. J Virol. 1974 Jan;13(1):237–240. doi: 10.1128/jvi.13.1.237-240.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Arnstein P., Parks W. P., Lennette E. H., Huebner R. J. A type-C virus in human rhabdomyosarcoma cells after inoculation into NIH Swiss mice treated with antithymocyte serum. Proc Natl Acad Sci U S A. 1973 Mar;70(3):859–862. doi: 10.1073/pnas.70.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]



