Abstract
Acute exacerbation of idiopathic pulmonary fibrosis (IPF) occurs in roughly 10% of patients annually, and is a leading cause of morbidity and mortality in this disease. While currently defined as idiopathic acute worsenings, acute exacerbations of IPF may in fact have a variety of causes, in particular infection and aspiration. Central to the pathobiology of clinically meaningful events is a diffuse injury to the IPF lung manifest histopathologically as diffuse alveolar damage, and biologically as accelerated alveolar epithelial cell injury or repair. Based on these recent observations, we propose a new paradigm for acute exacerbation of IPF that removes the idiopathic requirement and focuses on the pathophysiological mechanism involved.
Keywords: Idiopathic Pulmonary Fibrosis, Acute exacerbation, Interstitial Lung Disease, Definition, Diagnosis, Management
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease of unknown cause. The reported median survival for patients with IPF is approximately 3 years from the time of diagnosis, and there is no clearly effective therapy that improves survival [1]. IPF patients are known to experience episodes of acute respiratory worsening that result in substantial morbidity and mortality [1–3]. Although known causes of acute respiratory worsening such as pneumonia, heart failure and thromboembolism account for a proportion of these episodes, many remain idiopathic after clinical review. Idiopathic episodes of acute respiratory worsening have been termed acute exacerbations of IPF [2, 4]. This paper will review the current understanding of acute exacerbations of IPF (AE-IPF) and propose a new conceptual framework for thinking about acute exacerbation in IPF patients.
Definitions
Acute exacerbation of IPF was first defined by Kondo and colleagues in 1989 as an acute, clinically significant respiratory worsening of unidentifiable cause in a patient with underlying IPF [5]. Kondoh and colleagues further developed this concept [4]. Restricting acute exacerbation of IPF to idiopathic events distinguished IPF from other chronic lung diseases (e.g. asthma and chronic obstructive pulmonary disease) where definitions of acute exacerbation are not limited to idiopathic cases.
In 2007, the IPF Clinical Research Network (IPFnet) proposed diagnostic criteria for AE-IPF based on the criteria of Kondo, Kondoh, and others (Table 1) [2]. Acute exacerbation was defined by: subjective worsening of dyspnea within the prior 30 days; new bilateral opacities on high-resolution computed tomography (HRCT) of the chest; no evidence of infection by endotracheal or bronchoalveolar lavage analysis; and the exclusion of other alternative causes. Patients with idiopathic respiratory worsening who failed to meet all of the above criteria for whatever reason were defined as suspected acute exacerbation of IPF.
Table 1.
IPFnet diagnostic criteria for Acute Exacerbation of IPF
| Previous or concurrent diagnosis of idiopathic pulmonary fibrosis |
| Unexplained worsening or development of dyspnea within 30 days |
| High resolution computed tomography with new bilateral ground glass abnormality and/or consolidation superimposed on a background reticular or honeycomb pattern |
| No evidence of pulmonary infection by endotracheal aspirate or bronchoalveolar lavage |
| Exclusion of alternative causes, including left heart failure, pulmonary embolism, or identifiable cause of acute lung injury |
Ref [2]
These diagnostic criteria have succeeded in enhancing our understanding of a previously under-appreciated aspect of the natural history of IPF. Fundamental to their adoption, however, is the assumption that idiopathic acute respiratory worsening in IPF represents a distinct clinical entity that is important to distinguish from acute respiratory worsening of known cause. That is, AE-IPF is assumed to have a unique cause or pathobiology that has therapeutic or prognostic implications. In our opinion, data published over the last several years questions the validity of this assumption. As we will discuss below, we believe this has implications for how we should conceptualize acute exacerbation in IPF.
Incidence
Acute exacerbation of IPF occurs with an estimated incidence of 5–15% per year, with lower estimates typically arising from the placebo arms of clinical trials [6–9] and higher estimates from observational cohorts [3, 10, 11]. This variability is likely attributable to differences in patient characteristics (in particular severity of physiological impairment) between cohorts as well as logistical challenges inherent in identifying AEs-IPF in multicenter clinical trials.
Risk Factors
Several risk factors for AE-IPF have been suggested including worse baseline lung function (in particular reduced forced vital capacity (FVC)), worse dyspnea, a history of coronary artery disease and the presence of pulmonary hypertension [3, 11–13]. These suggest that acute exacerbation of IPF is more common in patients with more advanced disease. Prednisone use has been associated with an increased risk of AE-IPF in retrospective cohorts, however it is difficult to exclude confounding by indication (i.e. that patients were getting prednisone because of the severity of their disease or because they were exacerbating) [3, 13]. Cigarette smoking may also increase acute exacerbation risk [3]. There appears to be a seasonal predilection to AE-IPF with increased frequency of exacerbations during the winter months [12–14]. Patients with a history of prior AE-IPF have greater than 4 times the risk of future AE-IPF events [15]. Other descriptive cohorts have identified subsets of patients with multiple AEs-IPF over time, but it is unclear whether this phenomenon arises from patient-specific or environmental factors.
Clinical presentation
Acute exacerbation of IPF can occur at any point during the course of IPF and may be the presenting manifestation of disease. When the latter occurs, characteristic radiographic and pathologic findings are essential tools in making the correct diagnosis. Patients with acute exacerbation typically present with symptoms of worsening dyspnea, cough and fever [1, 2]. By definition, AE-IPF is associated with new bilateral radiographic opacities on HRCT that can be peripheral, multifocal or diffuse patterns in distribution [16]. The pattern of radiographic involvement has prognostic implications (see below).
Surgical lung biopsy in patients with AE-IPF generally identifies acute and organizing diffuse alveolar damage (DAD), with a predominance of organizing pneumonia and extensive fibroblastic foci occasionally observed [17, 18]. Whether the latter cases represent sampling error is unknown. In most cases, dense, established fibrosis with or without microscopic honeycombing will also be present [19]. These findings, as well as exuberant fibroblastic foci, distinguish AE-IPF from isolated DAD.
Diagnostic Work-Up
There is no standardized work-up for patients presenting with possible AE-IPF, but testing often focuses on trying to identify a treatable underlying cause for respiratory worsening such as infection, pulmonary embolism, and congestive heart failure. Computed tomography-angiography is very useful in evaluating most of these possibilities and should be performed in all patients. The role of bronchoalveolar lavage to evaluate for infectious agents is less clear to many clinicians, since the sensitivity of most microbiological tests is poor; empiric therapy is often started regardless. There is also a risk of worsening hypoxemia with bronchoscopy in non-intubated patients with high baseline oxygen requirements; a sputum or nasopharyngeal swab can almost always be sent in these cases. In intubated patients, a bronchoalveolar lavage or an endotracheal aspirate can usually be obtained safely and easily. We believe the use of surgical lung biopsy in an established IPF patient with possible AE-IPF should be discouraged due to the high risk of peri-operative morbidity and mortality and the low likelihood of altered management or prognosis.
Etiology of acute exacerbation of IPF
The most commonly stated etiologic hypothesis for AE-IPF is that it is an acute, intrinsic acceleration of the IPF disease process. It is also possible that AE-IPF represents clinically occult secondary events such as infection, aspiration or heart failure. Recent studies have attempted to inform the etiology of AE-IPF by describing the biological phenotype of AE-IPF and looking more rigorously for potential causes. These studies suggest that AE-IPF is indeed an acceleration of the IPF disease process, but that it is likely caused by occult secondary insults such as infection, aspiration and mechanical stress.
Biological phenotype of acute exacerbation of IPF
In 2009, Konishi and colleagues published a study of gene expression profiles in lungs from recently deceased patients with AE-IPF, non-exacerbated IPF, and non-diseased controls [20]. Compared to controls, AE-IPF and non-exacerbated IPF profiles were similar. Interestingly, no changes in inflammation-associated genes were identified. Rather, AE-IPF was primarily characterized by evidence of enhanced epithelial cell activity (injury and/or proliferation). Subsequently, our group in collaboration with investigators in Korea analyzed blood biomarker profiles in AE-IPF, stable IPF and acute lung injury (ALI) [21]. Consistent with the data of Konishi, biomarkers of type II alveolar epithelial cell activity were elevated in AE-IPF compared to stable IPF and ALI, and levels of inflammatory biomarkers were normal. Kakugawa and colleagues demonstrated elevated levels of heat shock 47 proteins (HSP47) in AE-IPF compared to stable IPF [22]. HSP47 is a collagen-specific molecular chaperone necessary for collagen synthesis and deposition that has been shown to correlate with survival in IPF [23]. Together, these results suggest that AE-IPF, once established, is characterized by an acceleration of the underlying IPF disease (i.e. an alveolar epithelial cell-driven driven fibroproliferative process).
Potential causes of acute exacerbation of IPF
Infection
Huie and colleagues investigated the potential role of infection in acute respiratory worsening in patients with fibrotic lung disease, 13 of whom had IPF [24]. In 8 of 27 overall cases, a potential infectious etiology was identified using standard culture-based techniques as well as polymerase chain reaction (PCR) or antigen-based testing for common respiratory viruses. Of these cases, three had bacterial pneumonia, one had Pneumocystis jirovecii pneumonia, and four had evidence of replicating virus (two herpes simplex virus, one cytomegalovirus, and one parainfluenza virus).
Building on these results, our group, again in collaboration with investigators in Korea and Japan, used genomics-based discovery techniques to define the role of viral infection as a potential cause of AE-IPF [25]. Bronchoalveolar lavage fluid from patients with AE-IPF was compared to stable IPF using a pan-viral microarray and, in a subset, deep sequencing. Four of 43 AE-IPF samples (9%) were positive for common respiratory viruses (two rhinovirus, one coronovirus, one parainfluenza virus) compared to none from the stable IPF group. Fifteen additional viruses were identified in AE-IPF samples (one herpes simplex virus, two Epstein-Barr virus, and twelve torque teno virus), while no additional viruses were detected in the stable IPF group. The clinical significance of these non-respiratory viruses was unclear. Respiratory viruses may have been undetectable in some additional samples due to a delay in time from the onset of symptoms to BAL collection. Collectively, these data suggest that a proportion of AE-IPF cases are caused by clinically occult viral infection.
Aspiration of gastric contents
Abnormal gastroesophageal reflux (GER) is near universal in IPF and is a known risk factor for aspiration [26–28]. Two small studies of patients with GER and IPF have suggested stabilization of disease and oxygenation with medical or surgical treatment of GER [29, 30]. Our group, in collaboration with investigators from Korea, measured pepsin levels (a biomarker of aspiration) in the BAL fluid of IPF patients with and without acute exacerbation [26]. In this cohort, BAL pepsin level was associated with acute exacerbation status, a finding driven by a subgroup of AE-IPF cases (33%) whose BAL pepsin levels were substantially elevated. A recent study comparing outcomes in patients on and off proton pump inhibitor (PPI) therapy for abnormal GER found PPI therapy to be associated with significantly reduced rates of AE-IPF. [31] These data suggest that aspiration of gastric contents, in particular acid, may be a cause of AE-IPF in a subgroup of patients.
Mechanical stress
There is increasing evidence that surgery can cause acute respiratory worsening in IPF, presumably through increased mechanical stress to the lungs. A large cohort of AE-IPF patients included 15 that occurred post-operatively (8 lung biopsies, 3 lung resections and 3 non-respiratory surgeries) [3]. Prolonged mechanical ventilation, high tidal volume and high concentration of supplemental oxygen during surgery have been proposed as potential causes [32, 33]. Other contributors to increased risk of AE-IPF after surgery may be the presence of typical histologic honeycombing [34], increased extent of radiographic fibrosis [35], elevated serum Krebs von den Lungen-6 (KL-6), greater extent of surgical resection (lobe vs. wedge resection) [36], high intra-operative fluid balance, and pre-operative elevations in C-reactive protein (CRP) [37]. There may also be an increased risk of AE-IPF post-BAL, particularly in IPF patients with poor lung function undergoing multiple BAL procedures [38, 39].
Ambient air pollution
Finally, there is recent evidence that ambient air pollution exposure, notably ozone (O3) and nitrogen dioxide (NO2), may cause AE-IPF in some patients with IPF. In a retrospective analysis of a large well-defined cohort of IPF patients, our group in collaboration with investigators in Korea found that the mean and maximum exposures to O3 and NO2 were associated with an increased risk of AE-IPF [15]. Additionally the frequency of exposure to pollution levels exceeding published air quality standards was associated with an increased risk of AE-IPF.
Management
Prevention
Prevention of AE-IPF may prove to be the most effective approach to management (Table 2); there are no data at this point on which to assess efficacy. Annual influenza vaccination (and pneumococcal vaccination where indicated) makes intuitive sense as a potential way to protect against respiratory infections that could cause acute exacerbation. Hand washing and other infection control policies are also prudent. Second, behavioural measures to reduce the risk of microaspiration (small meals, adequate time between meals and bed, elevation of the head of the bed) may make sense as general preventive measures in IPF patients. In those IPF patients with documented abnormal GER, it may also make sense to consider medical or surgical treatment of GER to reduce the risk of aspiration induced AE-IPF. In patients with IPF undergoing surgery, reducing the partial pressure of oxygen and tidal volumes used intra-operatively, when possible, may help reduce the risk of mechanical stress causing AE-IPF. Finally, there may be a role for air quality measures (choice of living environment, minimizing exposure to airborne irritants and pollutants like ambient ozone and nitrogen dioxide). Finally, therapies aimed at treating the underlying fibroproliferation in IPF may also reduce the risk of AE-IPF by controlling the pathologic response of the IPF lung to potential stressors [8].
Table 2.
Management considerations for acute exacerbation of IPF
| Intervention | Recommendation |
|---|---|
| Prevention of acute exacerbation of IPF | |
| Influenzae and pneumococcal vaccination | + |
| Hand washing, avoidance of sick contacts | + |
| Behavioral modifications for reflux and aspiration | + |
| Proton pump inhibitor therapy | +/− |
| Minimization of mechanical stress during surgery | + |
| Avoidance of airborne irritants and pollutants | +/− |
| Treatment of acute exacerbation of IPF | |
| Mechanical ventilation | +/− |
| Corticosteroids | + |
| Empiric antibiotics | + |
| Other therapies (tacrolimus, polymixin B hemoperfusion, cyclosporine A, thrombomodulin) | − |
| Pharmacological therapies for idiopathic pulmonary fibrosis | +/− |
| Lung transplantation | +/− |
+ = Would consider using in most patients as potential benefit seems to outweigh potential harm; +/− = Would consider using in selected cases as the balance of benefit and risk varies by clinical situation; − = Would not consider using in most patients as evidence is lacking to support a clinical benefit. Abbreviations: IPF = idiopathic pulmonary fibrosis.
Therapy
Once a patient with IPF develops an acute exacerbation, management is largely supportive (Table 2). Many patients with AE-IPF present in respiratory failure, and the decision to intubate and mechanically ventilate is a central one. A retrospective study of 24 IPF patients identified 8 admitted to the ICU during AE-IPF and found that overall, 22/24 died during hospital admission with none of the AE patients surviving to discharge [40]. In another retrospective study, 24/25 IPF patients admitted to the ICU for respiratory failure of unknown cause died in hospital despite thorough work-up and corticosteroid therapy [41]. In the largest cohort study of AE-IPF published to date, approximately 50% of patients required ICU admission and 80% of these patents died during the follow up period (not necessarily during the hospitalization) [3]. Based on these data, many IPF patients will decide against intubation. Ideally, this decision should be made with the patient and family well ahead of time and after a thoughtful and measured discussion.
Most patients with AE-IPF receive corticosteroids, in keeping with the 2011 international evidence-based guideline’s weak positive recommendation [1]. There have been no randomized trials investigating the use of corticosteroids in AE-IPF. The rationale for corticosteroid use in AE-IPF is two-fold. First, a minority of cases of AE-IPF have demonstrated organizing pneumonia on surgical lung biopsy suggesting a corticosteroid-responsive element. Second, there are some data supporting the use of late steroids in acute lung injury (although this is a contentious topic in the acute lung injury community), and acute lung injury demonstrates diffuse alveolar damage on surgical lung biopsy similar to AE-IPF. It is unknown what dose and duration of corticosteroids to use. In AE-IPF patients with respiratory failure, our practice is to treat with high-dose intravenous corticosteroids (e.g. 500 mg solumedrol daily) for 3–5 days with tapering to a lower dose or discontinuation based on clinical response. In less severe cases, oral prednisone 60 mg daily is used for 10–14 days with tapering as clinically indicated. Careful attention must be given to the individual risks of corticosteroid treatment in AE-IPF patients.
The majority of patients presenting with AE-IPF receive empiric antibiotics targeting respiratory pathogens though there is no data to support their use [42]. This is based on the clinical reasoning that underlying infection can be easily missed on microbiological testing, and that a treatment course of antimicrobial therapy is low risk. Our practice is to treat patients empirically for common community-acquired organisms.
Several small studies have reported varying efficacy of novel therapies in AE-IPF but none have been rigorously tested in large randomized trials. These experimental therapies include combined tacrolimus and corticosteroids [43], polymyxin B-immobilized fiber column perfusion treatment [44–48], cyclosporine-A [49, 50] and recombinant thrombomodulin.[51].
Prognosis
Short-term survival post AE-IPF is poor, with a median survival of around 2 months post event [3]. Several markers of worse prognosis have been identified in AE-IPF including lower baseline FVC and DLCO, elevated lactate dehydrogenase and CRP, and higher body mass index [3, 12, 52]. Circulating fibrocytes and elevated Interleukin-17 levels may also prove prognostic [45, 53]. Not surprisingly, worsening gas-exchange despite treatment is a poor prognostic marker in AE-IPF [12]. The radiographic pattern of involvement at the time of AE-IPF is also prognostic, with higher mortality in the presence of diffuse ground-glass abnormality compared to a multifocal or peripheral distribution [16]. A composite HRCT score including extent of ground-glass opacification, consolidation, traction bronchiectasis, and honeycombing demonstrated that higher scores predicted mortality risk after AE-IPF [54]. Such prognostic information may be useful for appropriate triage and decision-making regarding level of and continuation of care.
At this time, the paucity of data on outcomes after lung transplantation precludes a recommendation for or against lung transplantation in patients experiencing AE-IPF, though individual outcomes likely depend on a combination of patient factors and center-specific experience.
Proposed conceptual framework
Defining AE-IPF has catalyzed the recognition and characterization of a previously under-recognized and under-appreciated aspect of the natural history of IPF. We believe the current definition, however, has led to an organizational framework for acute respiratory worsening in IPF that emphasizes and prioritizes idiopathic events over those of known cause – a framework that emerging data suggest may be conceptually flawed.
Data reviewed earlier in this article suggest that many so-called acute exacerbations of IPF are in fact not idiopathic. Instead, they appear to be caused by a variety of secondary events including infection and aspiration. Is support of this concept, epidemiological data show that AE-IPF and acute respiratory worsening of known cause share similar clinical characteristics and outcomes [3, 13]. Taken together, the above data suggest that AE-IPF as currently defined may be an artificial construct - a clinical distinction without biological or clinical evidence to support it. This hypothesis could of course be wrong, but we believe the current balance of evidence argues in its favor.
This does not mean that the concept of acceleration of the underlying disease in AE-IPF is incorrect. Indeed, microarray and biomarker data reviewed earlier suggest that the global signal from AE-IPF cases is indeed one of accelerated disease. We speculate that in patients with AE-IPF, unrelated clinical events (e.g. infection, microaspiration), that in non-diseased lungs would perhaps cause no clinically significant sequellae, lead to diffuse alveolar injury and acceleration of the fibroproliferative process. Like a dry forest going up in flames after one stray lightening strike, the IPF lung may be primed for sudden decline due to its inability to undergo normal alveolar repair, and it may require only minor stressors to tip it towards a rapid decline.
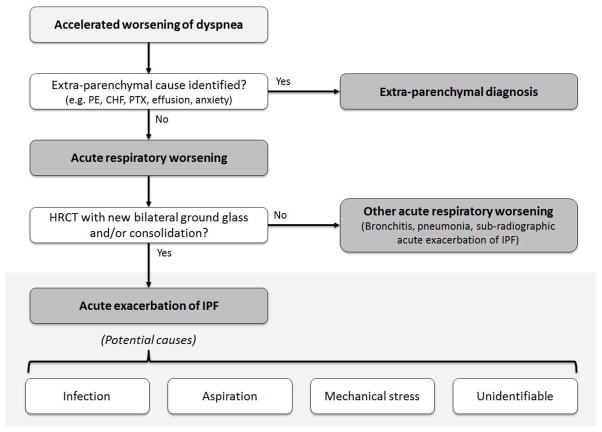
In conclusion, we propose that clinicians and researchers consider a new conceptual framework for acute exacerbation in IPF that de-emphasizes etiology (i.e. removes the idiopathic requirement) and emphasizes the presence of diffuse injury signifying clinically significant disease worsening (Figure). In this conceptual model, the hypothesis is that all acute respiratory worsenings of IPF have a secondary cause (some are clinically identifiable, some are not), and that some acute respiratory worsenings lead to acceleration of the underlying disease resulting in diffuse alveolar injury – i.e. exacerbation. We encourage researchers to think creatively about AE-IPF and consider alternative conceptual frameworks such as the one presented here. More research into AE-IPF is sorely needed to further improve our understanding of these clinically meaningful events.
Figure 1. Proposed conceptual framework for acute exacerbation of idiopathic pulmonary fibrosis (IPF).
Patients with IPF and accelerated worsening of their dyspnea can have parenchymal or extra-parenchymal explanations for their symptoms. We propose that those with parenchymal causes be categorized as acute respiratory worsening. Cases of acute respiratory worsening that demonstrate evidence of diffuse alveolar injury on high-resolution computed tomography (HRCT) scanning are subsequently categorized as acute exacerbation of IPF. In some cases of acute exacerbation of IPF, a cause will be identified (e.g. infection). Cases of acute respiratory worsening that do not have HRCT evidence for diffuse alveolar injury are categorized as other acute respiratory worsening, possible explanations for which are bronchitis, lobar pneumonia, and sub-radiographic acute exacerbation of IPF. Abbreviations: PE = pulmonary embolism; CHF = congestive heart failure; PTX = pneumothorax; HRCT = high resolution computed tomography; IPF = idiopathic pulmonary fibrosis.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Kerri Johannson declares that she has no conflicts of interest. Harold R. Collard is a consultant for Biogen, FibroGen, Genoa, Gilead, InterMune, MedImmune, Mesoblast, and Promedior. His institution receives money as a result. His institution also receives funding through grants he has from Boehringer-Ingelheim, Genentech, and NIH/NGLBI.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
References
- **1.Raghu G, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. These are the most recent internationally accepted consensus guidelines on the diagnosis and management of IPF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard HR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–43. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **3.Song JW, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–63. doi: 10.1183/09031936.00159709. This manuscript represents the largest descriptive cohort to date describing clinical features and outcomes in acute exacerbations of IPF. It also demonstrates that acute exacerbation may occur after surgery. [DOI] [PubMed] [Google Scholar]
- 4.Kondoh Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103(6):1808–12. doi: 10.1378/chest.103.6.1808. [DOI] [PubMed] [Google Scholar]
- 5.Kondo ASS. Intractable Diseases Research Foundation Publication No. 27 . Interstitial pneumonia of unknown etiology. Tokyo, Japan: University of Tokyo Press; 1989. Acute exacerbation in idiopathic interstitial pneumonia (IIP) [Google Scholar]
- 6.King TE, Jr, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(1):92–9. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 7.Noble PW, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 8.Richeldi L, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 9.Zisman DA, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–8. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mura M, et al. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: a 3-year prospective study. Eur Respir J. 2012;40(1):101–9. doi: 10.1183/09031936.00106011. [DOI] [PubMed] [Google Scholar]
- 11.Judge EP, et al. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J. 2012;40(1):93–100. doi: 10.1183/09031936.00115511. [DOI] [PubMed] [Google Scholar]
- 12.Simon-Blancal V, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration. 2012;83(1):28–35. doi: 10.1159/000329891. [DOI] [PubMed] [Google Scholar]
- 13.Collard HR, et al. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res. 2013;14:73. doi: 10.1186/1465-9921-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson AL, et al. Seasonal variation: mortality from pulmonary fibrosis is greatest in the winter. Chest. 2009;136(1):16–22. doi: 10.1378/chest.08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannson KA, VE, Lee K, Balmes JR, Ji W, Kaplan GG, Kim DS, Collard HR. Acute Exacerbation Of Idiopathic Pulmonary Fibrosis Is Associated With Short-Term Air Pollution Exposure. American Journal of Respiratory and Critical Care Medicine. 2013:187. doi: 10.1183/09031936.00122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akira M, et al. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(4):372–8. doi: 10.1164/rccm.200709-1365OC. [DOI] [PubMed] [Google Scholar]
- 17.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128(5):3310–5. doi: 10.1378/chest.128.5.3310. [DOI] [PubMed] [Google Scholar]
- 18.Churg A, et al. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol. 2007;31(2):277–84. doi: 10.1097/01.pas.0000213341.70852.9d. [DOI] [PubMed] [Google Scholar]
- 19.Churg A, Wright JL, Tazelaar HD. Acute exacerbations of fibrotic interstitial lung disease. Histopathology. 2011;58(4):525–30. doi: 10.1111/j.1365-2559.2010.03650.x. [DOI] [PubMed] [Google Scholar]
- 20.Konishi K, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180(2):167–75. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Collard HR, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L3–7. doi: 10.1152/ajplung.90637.2008. This manuscript investigatoes the plasma biomarker profile for acute exacerbation of IPF demonstrating that it is predominantly suggesting of increased alveolar epithelial cell injury and/or activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakugawa T, et al. Serum heat shock protein 47 levels are elevated in acute exacerbation of idiopathic pulmonary fibrosis. Cell Stress Chaperones. 2013 doi: 10.1007/s12192-013-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahloon RA, et al. Patients with Idiopathic Pulmonary Fibrosis with Antibodies to Heat Shock Protein 70 Have Poor Prognoses. American Journal of Respiratory and Critical Care Medicine. 2012;187(7):768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huie TJ, et al. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: aetiology and outcomes. Respirology. 2010;15(6):909–17. doi: 10.1111/j.1440-1843.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- *25.Wootton SC, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(12):1698–702. doi: 10.1164/rccm.201010-1752OC. This manuscript demonstrates that viral infection is present in a minority of acute exacerbation of IPF cases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Lee JS, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39(2):352–8. doi: 10.1183/09031936.00050911. This manuscript demonstrates that elevated pepsin levels are present in the bronchoalveolar lavage of patients with acute exacerbation of IPF suggesting that aspiration is responsible for a subgroup of cases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobin RW, et al. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158(6):1804–8. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 28.Raghu G, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27(1):136–42. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 29.Raghu G, et al. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129(3):794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 30.Linden PA, et al. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131(2):438–46. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- *31.Lee JS, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. The Lancet Respiratory Medicine. 2013;1(5):369–376. doi: 10.1016/S2213-2600(13)70105-X. This manuscript demonstrates that anti-acid therapy may reduce the incidence of acute exacerbation of IPF in a clinical trial cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondoh Y, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med. 2006;100(10):1753–9. doi: 10.1016/j.rmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto S, et al. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med. 2011;50(2):77–85. doi: 10.2169/internalmedicine.50.3390. [DOI] [PubMed] [Google Scholar]
- 34.Sugiura H, et al. Acute exacerbation of usual interstitial pneumonia after resection of lung cancer. Ann Thorac Surg. 2012;93(3):937–43. doi: 10.1016/j.athoracsur.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today. 2011;41(7):914–21. doi: 10.1007/s00595-010-4384-z. [DOI] [PubMed] [Google Scholar]
- 36.Yano M, et al. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg. 2012;14(2):146–50. doi: 10.1093/icvts/ivr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuno Y, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg. 2012;41(6):e161–5. doi: 10.1093/ejcts/ezs147. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto K, et al. Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir Med. 2012;106(3):436–42. doi: 10.1016/j.rmed.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Ghatol A, Ruhl AP, Danoff SK. Exacerbations in idiopathic pulmonary fibrosis triggered by pulmonary and nonpulmonary surgery: a case series and comprehensive review of the literature. Lung. 2012;190(4):373–80. doi: 10.1007/s00408-012-9389-5. [DOI] [PubMed] [Google Scholar]
- 40.Rangappa P, Moran JL. Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Crit Care Resusc. 2009;11(2):102–9. [PubMed] [Google Scholar]
- 41.Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11(2):117–22. doi: 10.1155/2004/379723. [DOI] [PubMed] [Google Scholar]
- 42.Collard HR, et al. Current diagnosis and management of idiopathic pulmonary fibrosis: a survey of academic physicians. Respir Med. 2007;101(9):2011–6. doi: 10.1016/j.rmed.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horita N, et al. Tacrolimus and steroid treatment for acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2011;50(3):189–95. doi: 10.2169/internalmedicine.50.4327. [DOI] [PubMed] [Google Scholar]
- 44.Abe S, et al. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med. 2012;51(12):1487–91. doi: 10.2169/internalmedicine.51.6965. [DOI] [PubMed] [Google Scholar]
- 45.Tachibana K, et al. Polymyxin-B hemoperfusion for acute exacerbation of idiopathic pulmonary fibrosis: serum IL-7 as a prognostic marker. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):113–22. [PubMed] [Google Scholar]
- 46.Noma S, et al. Two cases of acute exacerbation of interstitial pneumonia treated with polymyxin B-immobilized fiber column hemoperfusion treatment. Intern Med. 2007;46(17):1447–54. doi: 10.2169/internalmedicine.46.0117. [DOI] [PubMed] [Google Scholar]
- 47.Seo Y, et al. Beneficial effect of polymyxin B-immobilized fiber column (PMX) hemoperfusion treatment on acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2006;45(18):1033–8. doi: 10.2169/internalmedicine.45.6018. [DOI] [PubMed] [Google Scholar]
- 48.Oishi K, et al. Association between cytokine removal by polymyxin B hemoperfusion and improved pulmonary oxygenation in patients with acute exacerbation of idiopathic pulmonary fibrosis. Cytokine. 2013;61(1):84–9. doi: 10.1016/j.cyto.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 49.Inase N, et al. Cyclosporin A followed by the treatment of acute exacerbation of idiopathic pulmonary fibrosis with corticosteroid. Intern Med. 2003;42(7):565–70. doi: 10.2169/internalmedicine.42.565. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto S, et al. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49(2):109–15. doi: 10.2169/internalmedicine.49.2359. [DOI] [PubMed] [Google Scholar]
- 51.Kondoh Y, et al. B102. INTERSTITIAL LUNG DISEASE: NOVEL MANAGEMENT AND OUTCOME STRATEGIES. American Thoracic Society; Recombinant Thrombomodulin In Acute Exacerbation Of Idiopathic Pulmonary Fibrosis; pp. A3811–A3811. [Google Scholar]
- 52.Kondoh Y, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(2):103–10. [PubMed] [Google Scholar]
- 53.Moeller A, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 54.Fujimoto K, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol. 2012;22(1):83–92. doi: 10.1007/s00330-011-2211-6. [DOI] [PubMed] [Google Scholar]