Abstract
We reviewed the Chinese and English literature for the efficacy and safety data of valsartan monotherapy or combination therapy in Chinese hypertensive patients. According to the data of ten randomized controlled trials, valsartan monotherapy was as efficacious as another angiotensin receptor blocker or other classes of antihypertensive drugs, excepting the slightly inferior diastolic blood pressure-lowering effect in comparison with calcium channel blockers. According to the data of six randomized controlled trials, valsartan combination, with hydrochlorothiazide, amlodipine, or nifedipine gastrointestinal therapeutic system, was more efficacious than monotherapy of valsartan, amlodipine, or nifedipine gastrointestinal therapeutic system. According to these trials, valsartan had an acceptable tolerability, regardless of whether it was used as monotherapy or in combination therapy. Nonetheless, several rare side effects have been reported, indicating that it should still be used with caution. This is of particular importance given that there are millions of hypertensive patients, worldwide, currently exposed to the drug.
Keywords: angiotensin receptor blocker, valsartan, hypertension, blood pressure, efficacy, side effect
Introduction
Since the first Chinese hypertension guidelines were published in 1999,1 angiotensin receptor blocker (ARB) has been among the five classes of antihypertensive drugs recommended for the initiation and maintenance of antihypertensive therapy. Subsequent Chinese hypertensive guidelines, published in 20052 and 2011,3 respectively, made similar recommendations for the choice of antihypertensive drugs. According to the 2012 Intercontinental Marketing Services report, valsartan, among several available agents in the class, is the most prescribed ARB for the management of hypertension in the People’s Republic of China.4 Valsartan is currently used as an agent of monotherapy or free-combination antihypertensive therapy and as a component of single-pill combination with hydrochlorothiazide or amlodipine as well.
In spite of its wide use in the People’s Republic of China, valsartan has never been studied in any hard-outcome study in this country, except for the 33 Chinese patients enrolled in the Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial.5 Nonetheless, several randomized controlled trials were conducted to study blood pressure-lowering efficacy and safety of valsartan monotherapy versus other antihypertensive drugs6–16 or combination therapy versus the component drugs.17–21 In addition, several case reports on rare side effects have been published in the Chinese literature.22–29
In the present review, we first summarized the results of the comparative therapeutic studies that investigated efficacy and safety of valsartan monotherapy or combination antihypertensive therapy in Chinese hypertensive patients. For side effects profile, we additionally reviewed case reports.
Selection of studies
We searched randomized controlled trials and side effect case reports involving valsartan via PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and VIP (http://www.cqvip.com/) for the English- and Chinese-language literature, respectively. For inclusion, a randomized controlled trial had to have been conducted in Chinese hypertensive patients and published in a peer-reviewed journal in the period from January 1, 1999 (from which time valsartan entered the Chinese market) to May 31, 2013; had a randomized parallel-group or cross-over design; compared valsartan monotherapy or combination therapy with placebo or other antihypertensive drugs; and assessed blood pressure at baseline and during follow-up. A case report must have been on a side effect attributable to the use of valsartan in the People’s Republic of China and published in a peer-reviewed journal before May 31, 2013. We excluded trials in Chinese patients with a disease other than hypertension, such as heart failure or albuminuria.
Efficacy of valsartan monotherapy in Chinese hypertensive patients
We identified eleven trials that compared valsartan monotherapy with angiotensin-converting enzyme (ACE) inhibitors (benazepril6 and enalapril7,8); another ARB (olmesartan9,10); calcium channel blockers ([CCBs] amlodipine,11–13 benidipine,14 and lacidipine15); or a diuretic (indapamide).16 Table 1 shows the characteristics of these trials and the randomized patients. These trials had a sample size of 42 subjects7 to 260 subjects,11 and follow-up time of 1 week15 to 48 weeks.14 All these trials individually had insufficient power to show superiority, equivalence, or noninferiority at a difference of 2–3 mmHg systolic or diastolic blood pressure. Nonetheless, the pooled analyses were able to provide sufficient power for all trials (n=1,232)6–16 as well as for the subgroup of trials that compared valsartan with CCBs (n=760),11–15 but not for the subgroups of trials that compared valsartan with ACE inhibitors (n=201)6–8 or another ARB (n=151).9,10
Table 1.
Characteristics of controlled clinical trials that investigated the efficacy and safety of valsartan in Chinese hypertensive patients
| Author | Year | Design | Location (People’s Republic of China) | Subjects | Number of patients (Valsartan or valsartan combination/Control) | Men (%) | Age (SD) (years) | SBP/DBP (SD) (mmHg) at baseline
|
Antihypertensive medication (mg/day) | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Valsartan | Control | ||||||||||
| Valsartan monotherapya | |||||||||||
| vs ACEls | |||||||||||
| Zhang and Li6 | 2005 | Open | Northern | EH | 32/29 | 47.5 | 52 (9) | 152 (10)/103 (6) | 157 (10)/101 (6) | Valsartan 80–160 vs benazepril 10–20 | 8 weeks |
| Ko et al7 | 2005 | DB | Southern | EH/DM | 22/20 | 40.5 | 61 (11) | 144 (20)/79 (8) | 142 (13)/76(11) | Valsartan 80–160 vs enalapril 5–10 | 1 year |
| Li and Zhang8 | 2007 | Open | Northern | EH | 49/49 | 89.8 | NA | 185 (14)/115 (12) | 180 (16)/105 (15) | Valsartan 80–160 vs enalapril 10–20 | 8 weeks |
| vs ARBs | |||||||||||
| Zhang et al9 | 2008 | Open | Northern | EH | 30/34 | 54.7 | 54 (6) | 147 (10)/98 (10) | 148 (9)/97 (10) | Valsartan 80–160 vs olmesartan 20–40 | 8 weeks |
| Li et al10 | 2009 | Open | Southern | EH | 44/43 | 83.0 | 47 (7) | 154 (11)/95 (6) | 154 (10)/95 (5) | Valsartan 80 vs olmesartan 20 | 8 weeks |
| vs CCBs | |||||||||||
| Wang et al11 | 2002 | Open | Northern | EH | 130/130 | 100 | 46 (12) | 167 (9)/102 (8) | 168 (8)/101 (8) | Valsartan 80 vs amlodipine 5 | 8 weeks |
| Huang et al12 | 2007 | Open | Southern | EH/elderly/AF | 32/32 | 59.4 | 68 (6) | 162 (9)/83 (11) | 164 (8)/85(11) | Valsartan 80–160 vs amlodipine 5–10 | 12 weeks |
| Cai et al13 | 2011 | DB | Southern | EH/renal transplantation | 75/75 | 58.0 | 37 (2) | 147 (10)/87 (7) | 149 (11)/87 (9) | Valsartan 80 vs amlodipine 5b | 24 weeks |
| Peng et al (l)14 | 2010 | Open | Northern | EH/proteinuria (protein < 1 g/day) | 57/59 | 50.9 | 43 (9) | 149 (13)/97 (10) | 150 (16)/96 (9) | Valsartan 80 vs benidipine 8 | 48 weeks |
| Peng et al (2)15 | 2010 | Open | Northern | EH/proteinuria (protein 1–3 g/day) | 61/59 | 52.5 | 43 (8) | 150 (15)/95 (8) | 151 (17)/95 (7) | Valsartan 80 vs benidipine 8 | 48 weeks |
| Liu et al15 | 2008 | Open | Southern | EH | 25/25 | 68.0 | 57 (9) | 146 (15)/93 (13) | 145 (11)/94 (17) | Valsartan 80 vs lacidipine 4 | 1 week |
| vs diuretics | |||||||||||
| Yang et al16 | 2004 | Open | Southern | EH | 60/60 | 100 | NA | 148 (18)/97 (8) | 148 (18)/98 (6) | Valsartan 80 vs indapamide 1.5 | 8 weeks |
| Valsartan combinationa | |||||||||||
| Valsartan/HCTZ combination | |||||||||||
| Sun et al17 | 2007 | DB | Multiple* | EH | 419/423 | 58.2 | 52 (10) | 143 (12)/96 (5) | 144(12)/96 (5) | Valsartan 80/HCTZ 12.5 vs valsartan 80 | 8 weeks |
| Zhang et al18 | 2008 | DB | Northern | EH | 61/62 | 56.3 | 55 (8) | 151 (11)/99 (5) | 148 (12)/98 (5) | Valsartan 80/HCTZ 12.5 vs valsartan 80 | 6 weeks |
| Valsartan/amlodipine combination | |||||||||||
| Ke et al (1)19 | 2009 | DB | Multiple* | EH | 274/273/267 | 62.9 | 54 (9) | 142 (13)/95 (5) | 142 (13)/95 (5) 139 (12)/95 (5) |
Valsartan 80/amlodipine 5 vs valsartan 80 vs valsartan 160 | 8 weeks |
| Ke et al (2)19 | 2009 | DB | Multiple* | EH | 290/290 | 61.7 | 51 (10) | 141 (12)/73 (8) | 140 (11)/74 (8) | Valsartan 80/amlodipine 5 vs amlodipine 5 | 8 weeks |
| Wang et al20 | 2013 | Open | Multiple* | EH | 272/268 | 50.0 | 54 (9) | 147 (7)/87 (8) | 146 (7)/87 (8) | Valsartan 80/amlodipine 5 vs nifedipine GITS 30 | 12 weeks |
| Valsartan/nifedipine GITS combination | |||||||||||
| Ke et al (3)21 | 2012 | Open | Multiple* | EH/Asian | 177/182 | 52.4 | 56 (10) | 152 (8)/94 (7) | 152 (8)/94 (7) | Valsartan 80/nifedipine GITS 30 vs valsartan 160 | 12 weeks |
Notes: The valsartan monotherapy trials are listed in the order of comparison drug class, the drug, and the year of publication,6–16 and the valsartan combination therapy trials are listed in the order of the combination drug and the year of publication;17–21
at the end of 4-week monotherapy, 25 metoprolol per day was added if blood pressure was not controlled to a level below 130/80 mmHg.13
Multiple, multi-center studies across southern and northern People’s Republic of China.
Abbreviations: ACEls, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; DB, double-blind; DBP, diastolic blood pressure; DM, diabetes mellitus; EH, essential hypertension; GITS, gastrointestinal therapeutic system; HCTZ, hydrochlorothiazide; NA, not available; SBP, systolic blood pressure; SD, standard deviation; vs, versus.
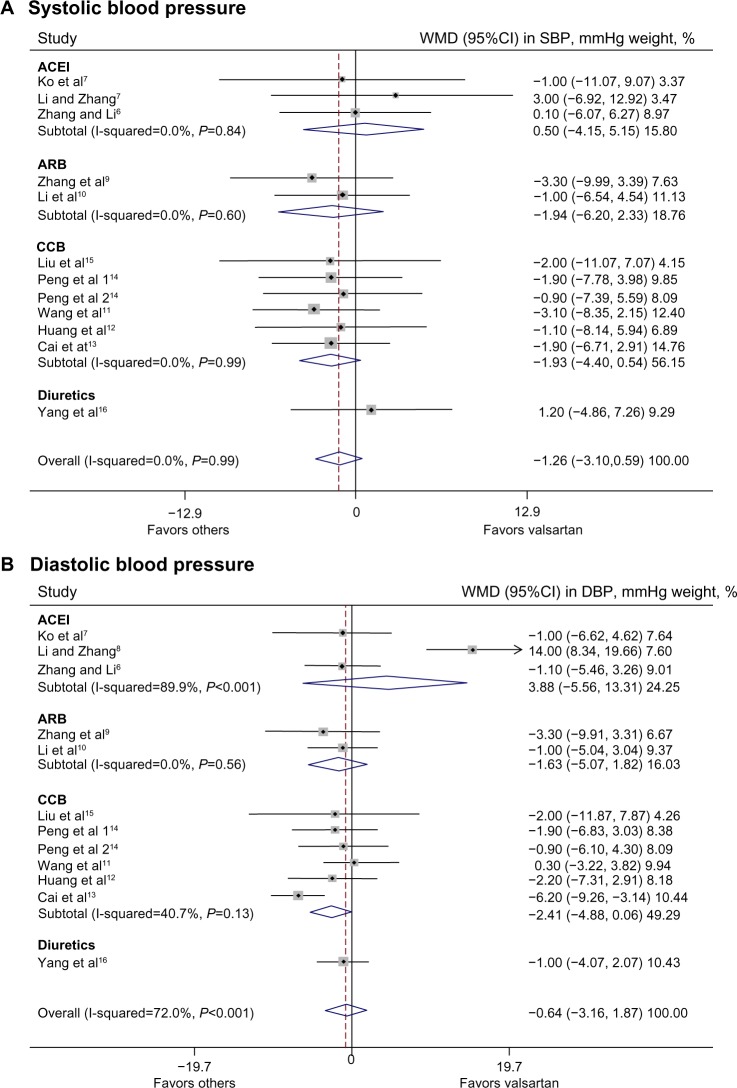
Overall, valsartan had similar blood pressure-lowering efficacy as the other classes of antihypertensive drugs or olmesartan, for systolic as well as diastolic blood pressure (P≥0.18) (Figure 1). There was significant heterogeneity across trials for diastolic blood pressure (P≤0.001) but not for systolic blood pressure (P=0.99). In drug-class-specific subgroup analyses, valsartan tended to be less efficacious than CCBs in reducing diastolic blood pressure (mean difference −2.41 mmHg; 95% confidence interval [CI]: −4.88 to 0.06 mmHg; P=0.056), with no significant heterogeneity across trials (P=0.13).11–15
Figure 1.
SBP (A) and DBP (B)-lowering efficacy of valsartan monotherapy versus other classes of antihypertensive drug.
Notes: Squares indicate WMD in trials, with a size proportional to the number of patients. 95% CIs for individual trials are denoted by lines and those for the pooled mean differences by diamonds.
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure; WMD, weighted mean difference.
Since the follow-up times of these trials varied substantially, and valsartan may require a few weeks or even months to exert its full antihypertensive effect, we performed subgroup analysis in the three trials that had a follow-up time of at least 24 weeks.7,13,14 The results of this subgroup analysis were confirmatory: indeed, valsartan was similarly efficacious as enalapril in reducing systolic and diastolic blood pressure (P≥0.73), but tended to be less efficacious than CCBs in reducing diastolic (mean difference −3.52 mmHg; 95% CI: −7.01 to 0.01 mmHg; P=0.051) but not systolic blood pressure (P=0.32).
In addition, blood pressure-lowering efficacy of various classes of antihypertensive drugs, including valsartan, may be dependent on dietary sodium intake, which is known to be higher in northern than in southern People’s Republic of China. We therefore performed subgroup analysis in trials conducted in northern6,8,9,11,14 versus southern People’s Republic of China.7,10,12,13,15,16 The number of trials allowed comparison between northern and southern People’s Republic of China for the treatment effects of all trials6–16 and the trials of CCBs.11–15 Valsartan was similarly efficacious as CCBs or all the other antihypertensive drugs in northern and southern People’s Republic of China (P≥0.19), except that valsartan was significantly less efficacious in reducing diastolic blood pressure than CCBs (mean difference −4.86 mmHg; 95% CI: −7.53 to −2.19 mmHg; P<0.001) and all the other antihypertensive drugs (mean difference −2.50 mmHg; 95% CI: −4.59 to −0.40 mmHg; P=0.02) in southern People’s Republic of China. However, the treatment effects between northern and southern People’s Republic of China in reducing diastolic blood pressure differed significantly only in the trials of CCBs (P=0.02) but not all trials (P=0.60).
Efficacy of valsartan combination therapy in Chinese hypertensive patients
We identified six trials (Table 1) that compared valsartan single-pill (with hydrochlorothiazide17,18 or amlodipine19,20) or free (with nifedipine gastrointestinal therapeutic system [GITS]21) combination therapy with valsartan,17–19,21 amlodipine,19 or nifedipine GITS monotherapy.20 All trials had a two-group parallel comparison, except one that compared the single-pill combination of valsartan and amlodipine with two different dosage groups of valsartan (80 and 160 mg/day).19 These trials had a sample size of 123 subjects18 to 842 subjects17 and follow-up time of 6 weeks18 to 12 weeks.20,21 All but two18,21 of these trials individually had sufficient power to show superiority of valsartan combination against valsartan or amlodipine monotherapy at a difference of 2–3 mmHg systolic or diastolic blood pressure. Accordingly, all but the two inadequately powered18,21 trials showed significantly larger reductions in both systolic and diastolic blood pressure in patients on valsartan combination than those on monotherapy with valsartan or amlodipine.
Overall, valsartan combination, on average, showed reduced systolic and diastolic blood pressures 2–6 mmHg more than monotherapy (Figure 2). If the superiority in blood pressure-lowering efficacy was represented by the percentage of patients who achieved the blood pressure goal as defined in each trial, the improvement in the valsartan combination therapy group, compared with valsartan, amlodipine, or nifedipine GITS, was statistically significant in all trials (P<0.001), with an absolute percentage change of 10%17 to 25%.19
Figure 2.
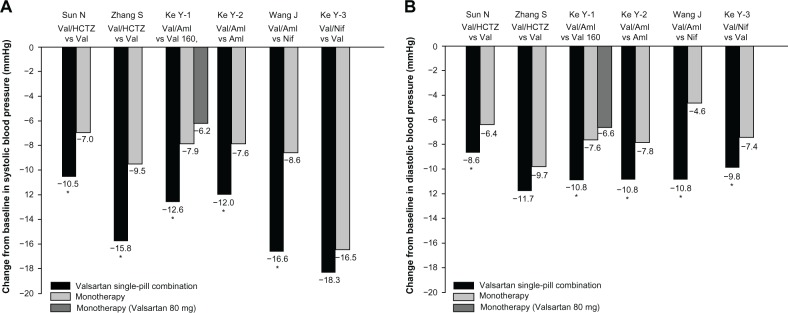
Systolic and diastolic blood pressure reductions in the combination therapy and monotherapy groups.
Note: *P<0.05 vs monotherapy.
Abbreviations: Aml, amlodipine; HCTZ, hydrochlorothiazide; Nif, nifedipine gastrointestinal therapeutic system; Val, valsartan; Val 80, Val 80 mg; Val 160, Val 160 mg; vs, versus.
In one trial that compared valsartan 80 mg/amlodipine 5 mg/day with amlodipine 5 mg/day, ambulatory blood pressure monitoring was performed in 82 of the 590 randomized subjects.19 In this particular sub-study, ambulatory blood pressure differences in favor of the valsartan/amlodipine combination (mean systolic/diastolic blood pressure difference −7.1/−6.6 mmHg, −7.2/−6.8 mmHg, and −6.3/−6.0 mmHg during the whole day, daytime and night-time, respectively) were much larger than those observed by clinic blood pressure measurement in the total study population (−4.4/−3.0 mmHg, mean systolic/diastolic blood pressure difference, respectively). These interesting observations warrant further investigation.
Side effects profile in randomized controlled clinical trials
In some,6–11,17–21 though not all,12–16 of the aforementioned randomized controlled trials, information on adverse events and serious adverse events was systematically collected and reported (Table 2). In the monotherapy trials,6–11 the incidence rate of adverse events with valsartan was lower than with ACE inhibitors (pooled odds ratio associated with ACE inhibition 3.51; 95% CI: 1.45–9.25; P=0.0035)6–8 and similar to the rate with another ARB (P=0.80)9,10 and amlodipine (P=0.99).11 There was no adverse event that was typically overrepresented in the valsartan group, regardless of the follow-up time.
Table 2.
Side effect profile in controlled clinical trials that compared valsartan with other antihypertensive drugs
| Author | Incidence rate of adverse events, (% number of events/subjects)a
|
Most frequently reported adverse events (number of patients)
|
||
|---|---|---|---|---|
| Valsartan | Other drugs | Valsartan | Other drugs | |
| Valsartan monotherapy | ||||
| vs ACEIs | ||||
| Zhang and Li6 | 12.5 (4/32) | 15.6 (5/29) | Weakness (2) Dizziness (1) Dry mouth (1) |
Cough (3) Dizziness (1) Tinnitus (1) |
| Ko et al7 | 13.6 (3/22) | 45.0 (9/20) | Numbness (1) Joint pain (1) |
Cough (7) Palpitations (1) Minor stroke (1) |
| Li and Zhang8 | 4.1 (2/49) | 20.4 (10/49) | Headache (1) Dry mouth (1) |
Cough (5) Headache (3) Tinnitus (1) |
| vs ARBs | ||||
| Zhang et al9 | 6.7 (2/30) | 8.8 (3/34) | Dizziness (1) Weakness (1) |
Dizziness (2) Weakness (1) |
| Li et al10 | 2.3 (1/44) | 4.7 (2/43) | Headache (1) | Cough (1) Headache (1) |
| vs CCBs | ||||
| Wang et al11 | 1.5 (2/130) | 1.5 (2/130) | Cough (1) Dizziness (1) |
Edema (1) Headache (1) |
| Combination | Monotherapy | Combination | Monotherapy | |
|
| ||||
| Valsartan combination therapy | ||||
| Valsartan/hydrochlorothiazide | ||||
| Sun et al17 | 8.9 (38/429) | 5.1 (22/435) | Hyperuricemia (8) Dizziness (7) Hypokalemia (4) |
Dizziness (8) Headache (3) Hypokalemia and abnormal liver function (2) |
| Zhang et al18,b | 21.0 (13/62) | 15.6 (10/64) | Headache Dizziness Chest distress |
Headache Dizziness Chest distress |
| Valsartan/amlodipine | ||||
| Ke et al (1)19,c | 4.4 (12/274) | 4.4 (12/274)/4.9 (13/268) | Edema (4) Dizziness (3) |
Edema (2) Dizziness (1)/Dizziness (4) Edema (1) |
| Ke et al (2)19,c | 10.7 (31/291) | 9.0 (26/290) | Abnormal liver function (7) Dyslipidemia (6) Dizziness (3) |
Abnormal liver function (4) Dizziness (3) Dyslipidemia (2) |
| Wang et al20 | 5.7 (16/282) | 15.6 (44/282) | Headache (3) Edema (2) Dizziness (1) |
Headache (13) Palpitations (11) Edema (7) |
| Valsartan/nifedipine GITS | ||||
| Ke et al (3)21 | 4.5 (8/177) | 4.4 (8/182) | Peripheral edema (1) Flushing (1) Palpitation (1) |
Dizziness (2) Headache (1) |
Notes:
The incidence rate was reported for withdrawals in the trial of Li W et al10 and for drug-related adverse events in all the combination therapy trials;17–21
the number of patients was not reported;18
there were two control groups with two different dosages of valsartan monotherapy (80 and 160 mg/day).
Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers; GITS, gastrointestinal therapeutic system; vs, versus.
In the combination therapy trials,17–21 the incidence rate of drug-related adverse events was higher with valsartan/hydrochlorothiazide combination than with valsartan monotherapy (pooled odds ratio associated with the combination 1.71; 95% CI: 1.05–2.82; P=0.029)17,18 and lower with valsartan/amlodipine combination (5.7%) than with nifedipine GITS (15.6%; odds ratio associated with nifedipine GITS 3.07; 95% CI: 1.65–5.99; P<0.001).20 However, the incidence rate of the drug-related adverse events was similar between valsartan/amlodipine combination and valsartan or amlodipine monotherapy (P≥0.59)19 and between valsartan/nifedipine GITS combination and valsartan monotherapy (P=0.99).21 The adverse events reported in the combination groups to a large extent reflected a component of the combination other than valsartan, such as hyperuricemia and hypokalemia associated with hydrochlorothiazide,17 palpitations and flushing associated with nifedipine GITS,21 and peripheral edema associated with amlodipine19,20 and nifedipine GITS.21
In addition, one randomized study specifically investigated the hematologic effect of valsartan (n=30) versus benazepril (n=30).22 In this study, valsartan significantly (P<0.001) decreased serum concentrations of erythropoietin (mean ± standard deviation from 14.2±3.2 to 12.1±2.9 U/L) and hemoglobin from baseline (from 144.3±13.8 to 135.2±14.8 g/L), whereas these hematologic measurements did not change with benazepril (P>0.05). This observation also warrants further investigation.
Side effects profile in clinical practice
Because of the limited number of patients in a randomized controlled trial, rare side effects are usually difficult to detect; however, in clinical practice, with millions of users of a drug, rare side effects can be discovered. We reviewed case reports that described side effects probably or possibly related to the use of valsartan, and identified eight publications (Table 3).23–30 There was one case in each of these eight reports. Of these eight cases, seven had a single clinical manifestation (angioedema, cough, drug eruption, hematuria, hypotension, muscle pain, or urticaria), and one had multiple clinical manifestations (urticaria, vertigo, muscle pain, and upper respiratory tract infection). Angioedema, drug eruption, and urticaria can be similarly attributable to hypersensitivity to valsartan.
Table 3.
Side effect profile of valsartan in case or case series reports in the therapeutic management of hypertension in the People’s Republic of China
| Author | Year | Side effectsa | Number of patients | Age (years) | Sex |
|---|---|---|---|---|---|
| Huang et al23 | 2004 | Hypotension | 1 | 62 | Male |
| Li et al24 | 2004 | Angioedema | 1 | 65 | Male |
| Li et al25 | 2006 | Muscle pain | 1 | 69 | Female |
| Zhang26 | 2008 | Cough | 1 | 80 | Male |
| Jiao27 | 2008 | Urticaria, vertigo, muscle pain, and upper respiratory tract infection | 1 | 63 | Female |
| Xu28 | 2009 | Hematuria | 1 | 60 | Female |
| Hua and Zhou29 | 2011 | Urticaria | 1 | 62 | Male |
| Zhuang30 | 2012 | Drug eruption | 1 | 50 | Male |
Notes:
the side effects in all reports disappeared after the drug was discontinued.
Conclusion
Valsartan monotherapy was as efficacious as any another ARB or other classes of antihypertensive drugs, except in the case of the slightly inferior diastolic blood pressure-lowering effect in comparison with CCBs. However, valsartan combination therapy, either with amlodipine, hydrochlorothiazide, or nifedipine GITS was more efficacious than monotherapy of amlodipine or valsartan. Valsartan had acceptable tolerability, regardless of whether it was used as monotherapy or in combination therapy. Nonetheless, several rare side effects have been reported, indicating that valsartan should still be used with caution. This point is of particular importance given the millions of hypertensive patients currently exposed to the drug. In addition, all trials included in the present review were conducted exclusively or predominantly in ethnic Han Chinese. More research is required in ethnic minority Chinese populations, especially those with different lifestyle.
Acknowledgments
The authors were financially supported by grants from the National Natural Science Foundation of China (30871360, 30871081, 81170245, and 81270373); the Ministry of Science and Technology (a grant for China–European Union collaborations [1012]); the Ministry of Education (NCET-09-0544), Beijing, People’s Republic of China; the Shanghai Commissions of Science and Technology (11QH1402000) and Education (the “Dawn” project 08SG20); the Shanghai Bureau of Health (XBR2011004 and 20101051); and Shanghai Jiaotong University School of Medicine (a grant of Distinguished Young Investigators to Yan Li).
Footnotes
Disclosure
Dr Wang reports receiving lecture and consulting fees from Boehringer-Ingelheim, MSD, Novartis, Omron, Pfizer, Servier, and Takeda. The authors report no other conflicts of interest in this work.
References
- 1.Writing Group of Chinese Guidelines for the Management of Hypertension Preventive and therapeutic instruction of Chinese hypertension. Chinese Journal of Hypertension. 2000;8(2):103–112. Chinese. [Google Scholar]
- 2.Writing Group of Chinese Guidelines for the Management of Hypertension 2004 update: Chinese guideline for prevention and treatment of patients with hypertension (practical edition) Zhonghua Xin Xue Guan Bing Za Zhi. 2004;32(12):1060–1064. Chinese. [Google Scholar]
- 3.Liu LS, Writing Group of 2010 Chinese Guidelines for the Management of Hypertension 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(7):579–615. Chinese. [PubMed] [Google Scholar]
- 4.Intercontinental Marketing Services (IMSHealth) [Accessed December 11, 2013]. Available from http://www.imshealth.com/portal/site/imshealth.
- 5.Zhu J-R, Fujita T, Shimamoto K, et al. Implication of clinical studies for the Asian population: focus on Chinese/Asian population. Int J Clin Pract. 2006;60(Suppl 150):14–16. [Google Scholar]
- 6.Zhang W, Li J. Effects of valsartan on renal function in hypertensive patients. South China Journal of Cardiology. 2005;11(1):42–44. Chinese. [Google Scholar]
- 7.Ko GT, Tsang CC, Chan HC. Stabilization and regression of albuminuria in Chinese patients with type 2 diabetes: a one-year randomized study of valsartan versus enalapril. Adv Ther. 2005;22(2):155–162. doi: 10.1007/BF02849886. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Zhang Y. Effects of valsartan and enalapril on hypertension and renal function in primary hypertensive patients. Hebei Medical Journal. 2007;29(11):1211–1212. Chinese. [Google Scholar]
- 9.Zhang J, Chen S, Zhang S, Tao Z. Efficacy and safety of olmesartan medoxomil in the treatment of essential hypertension. Chinese Pharmacist. 2008;11(11):1351–1352. Chinese. [Google Scholar]
- 10.Li W, Chen X, Guan S, Xu Q, Wang B, Ke Q. Comparison of olmesartan and valsartan in the treatment of middle aged primary hypertensive patients. Clin Med Chin. 2009;25(7):704–705. Chinese. [Google Scholar]
- 11.Wang Y, Li Y, Sun N. Effect of valsartan in the treatment of essential hypertension in male patients with erectile dysfunction. Clin Med Chin. 2002;18(10):939–940. Chinese. [Google Scholar]
- 12.Huang M, Liu J, He X, et al. Effects of valsartan on endothelial function in essential hypertension patients with atrial fibrillation. Chinese Journal of Hypertension. 2007;15(2):151–154. Chinese. [Google Scholar]
- 13.Cai J, Huang Z, Yang G, et al. Comparing antihypertensive effect and plasma ciclosporin concentration between amlodipine and valsartan regimens in hypertensive renal transplant patients receiving ciclosporin therapy. Am J Cardiovasc Drugs. 2011;11(6):401–409. doi: 10.2165/11593800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Peng T, Hu Z, Guo L, et al. Renal protective effects of benidipine and valsartan in primary hypertension patients with proteinuria. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38(1):20–22. Chinese. [PubMed] [Google Scholar]
- 15.Liu L, Zhao SP, Zhou HN, Xu DY, Li JX. Effect of valsartan on postprandial plasma inflammatory factors in patients with essential hypertension. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33(9):809–813. Chinese. [PubMed] [Google Scholar]
- 16.Yang T, Ma Q, Zeng X, et al. Effects of valsartan or indapamide on sexual activity in middle-aged hypertensive men. Chinese Journal of Hypertension. 2004;12(4):297–299. Chinese. [Google Scholar]
- 17.Sun NL, Wang HY, Zhu JR, Valsartan/HCTZ Clinical Investigation Group Therapeutic efficacy of valsartan and valsartan/HCTZ in mild to moderate hypertensive patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(8):715–718. Chinese. [PubMed] [Google Scholar]
- 18.Zhang S, Yu B, Li L, Du Z, Guan Z. Randomized, double-blinded trial evaluation of valsartan/hydrochlorothiazide combination therapy in mild to moderate essential hypertension in north-east China. J Int Med Res. 2008;36(4):630–637. doi: 10.1177/147323000803600403. [DOI] [PubMed] [Google Scholar]
- 19.Ke YN, Huang J, Zhu JR, Valsartan/Amlodipine Single Pill Combination Study Group Efficacy and safety of the single pill combination of valsartan 80 mg plus amlodipine 5 mg in mild to moderate essential hypertensive patients without adequate blood pressure control by monotherapy. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37(9):794–799. Chinese. [PubMed] [Google Scholar]
- 20.Wang JG, Zeng WF, He YS, et al. EXAM Investigators Valsartan/amlodipine compared to nifedipine GITS in patients with hypertension inadequately controlled by monotherapy. Adv Ther. 2013;30(8):771–783. doi: 10.1007/s12325-013-0048-x. [DOI] [PubMed] [Google Scholar]
- 21.Ke YN, Dong YG, Ma SP, Yuan H, Ihm SH, Baek SH, ADVISE study group Improved blood pressure control with nifedipine GITS/valsartan combination versus high-dose valsartan monotherapy in mild-to-moderate hypertensive patients from Asia: results from the ADVISE study, a randomized trial. Cardiovasc Ther. 2012;30(6):326–332. doi: 10.1111/1755-5922.12003. [DOI] [PubMed] [Google Scholar]
- 22.Guo LL, Li M, Wang AH. Effects of benazepril and valsartan on erythropoietin levels in patients with essential hypertension. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(10):1761–1763. Chinese. [PubMed] [Google Scholar]
- 23.Huang G, Chen K, Fang Y, Li W. A case of first dose hypotension induced by usual dose of valsartan. Journal of Gannan Medical College. 2005;25(1):99. Chinese. [Google Scholar]
- 24.Li B, Wei M, Zhang X, Liu X, Sheng S, Qiu C. A case of angioedema induced by valsartan. Herald of Medicine. 2004;23(10):782. Chinese. [Google Scholar]
- 25.Li J, Xiong A, Zhang Z. A case of muscle pain induced by valsartan capsules. World Clin Drugs. 2006;27(2):85. Chinese. [Google Scholar]
- 26.Zhang Z. A misdiagnosed case of cough induced by valsartan. Chinese Journal of Healthcare Medicine. 2008;10(5):328. Chinese. [Google Scholar]
- 27.Jiao W. A case of urticaria, vertigo, infection of upper respiratory tract and muscle pain induced by long term use of valsartan. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2008;17(24):3843. Chinese. [Google Scholar]
- 28.Xu Q. A case of hematuria induced by valsartan capsules. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2009;17(9):806. Chinese. [Google Scholar]
- 29.Hua W, Zhou X. A case of urticaria induced by valsartan. Sichuan Med J. 2011;32(10):1662. Chinese. [Google Scholar]
- 30.Zhuang L. A case of drug eruption caused by valsartan capsules. Clinical Journal of Medical Officers. 2012;40(3):510. Chinese. [Google Scholar]