Abstract
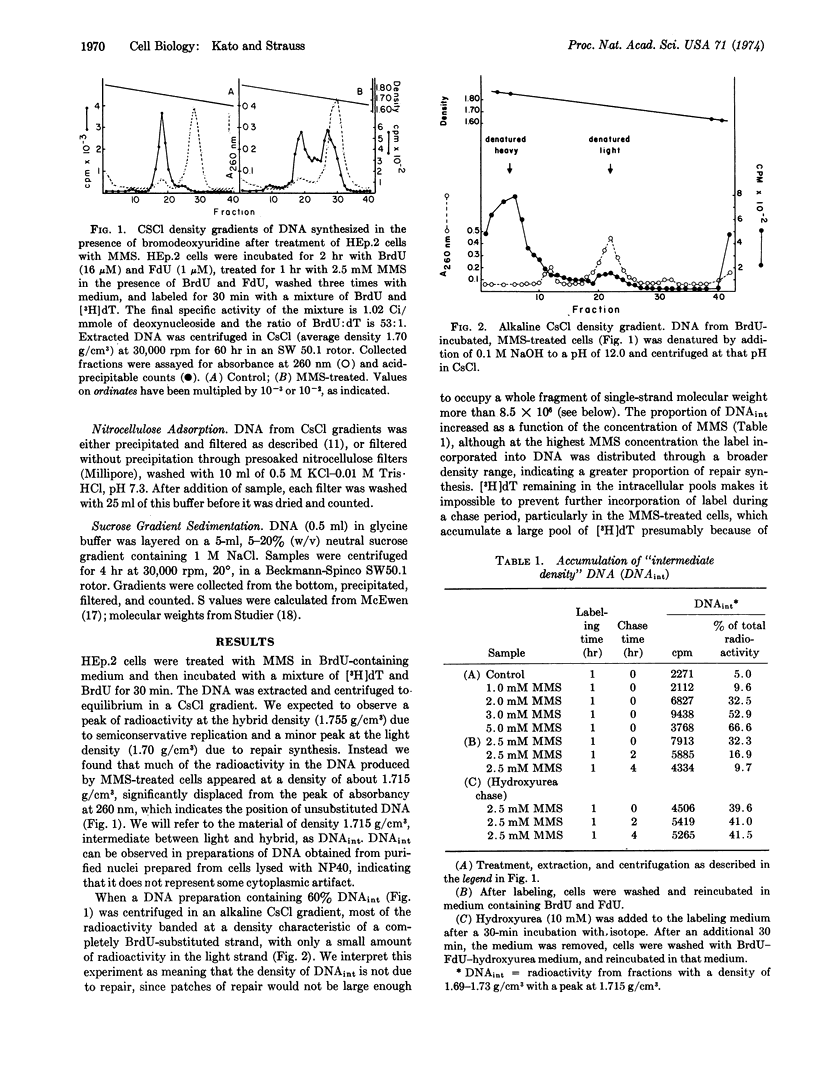
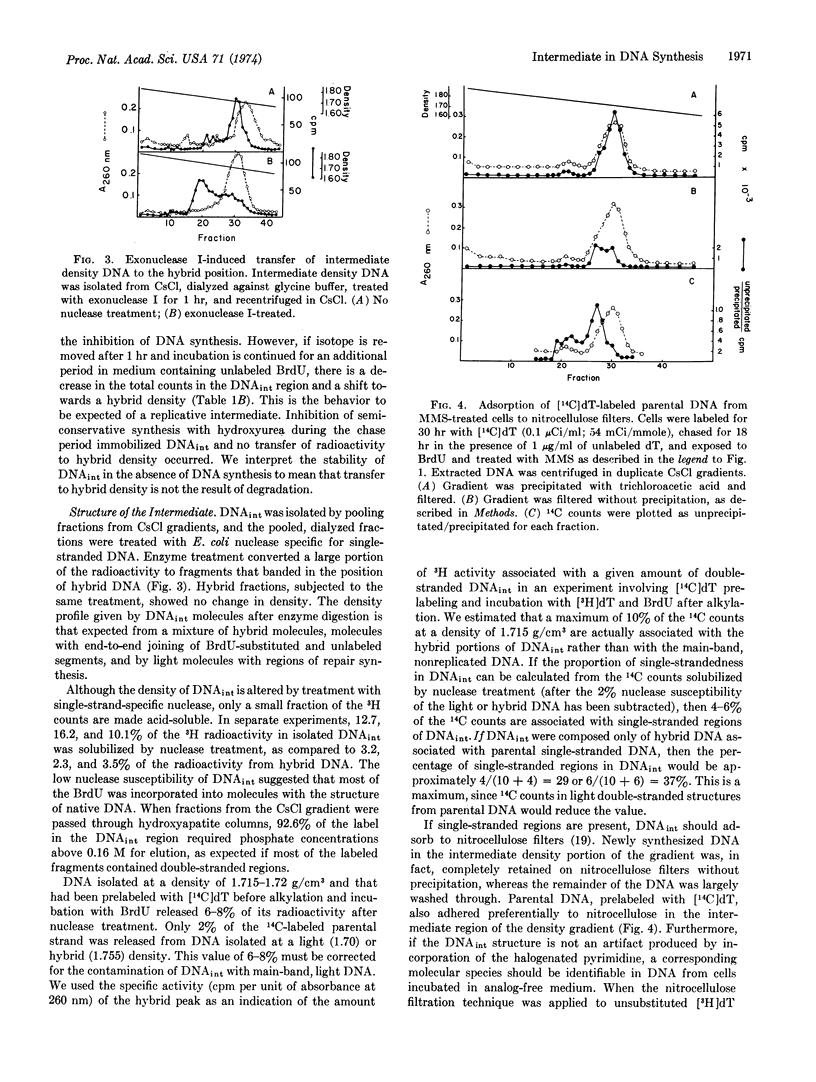
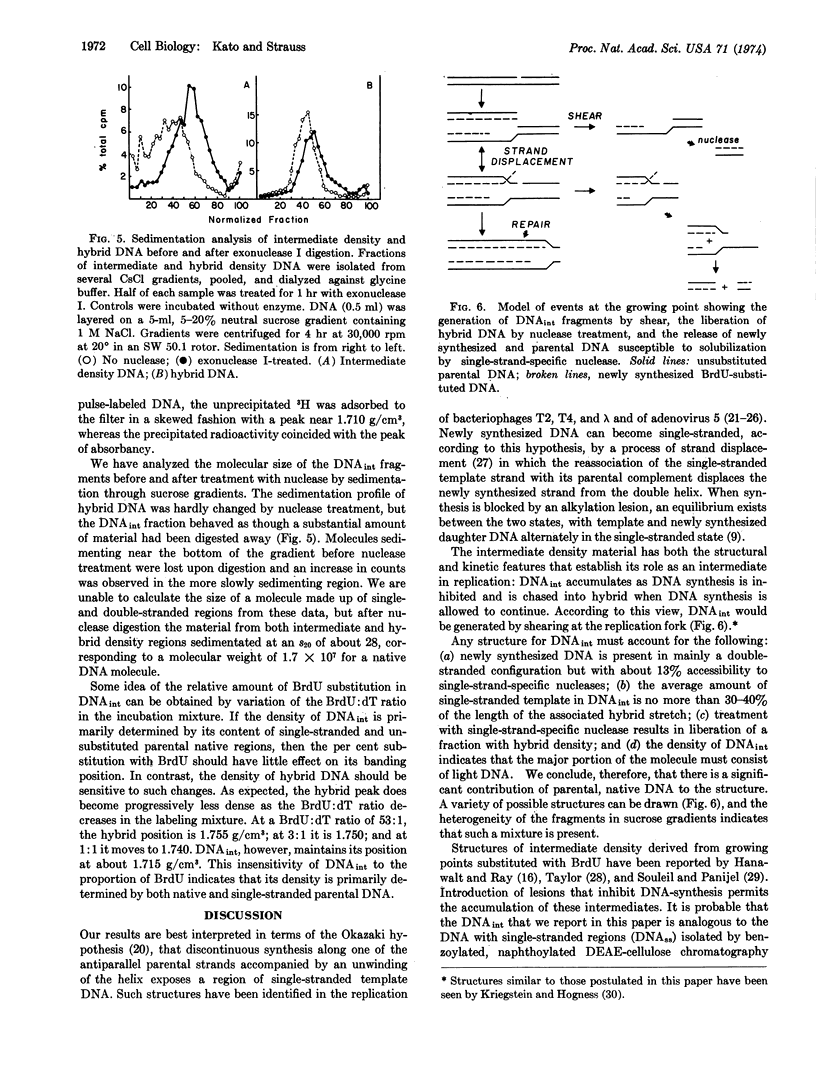
A portion of the DNA synthesized by HEp.2 cells after short incubation in the presence of BrdU bands in a CsCl gradient at a distinct peak of density 1.715-1.718 g/cm3. Inhibition of DNA synthesis by methyl methanesulfonate results in an increased proportion of this “intermediate density” DNA. Cells labeled and subsequently chased in nonradioactive BrdU yield only hybrid DNA. Treatment with single-strand-specific deoxyribonuclease converts a portion of the intermediate density material to molecules of hybrid density (1.755 g/cm3). The data suggest an intermediate in DNA replication that contains a single-stranded region in the parental strand and that accumulates when DNA synthesis is blocked by an alkylation-induced lesion.
Keywords: alkylating agents, replication, single-stranded DNA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H., Jr, Irvin J. L. Changes in the physical state of DNA during replication in regenerating liver of the rat. Proc Natl Acad Sci U S A. 1970 Jan;65(1):152–159. doi: 10.1073/pnas.65.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle M., McMahon M., Strauss B. Failure of alkylated HEp.2 cells to replicate newly synthesized DNA. Mutat Res. 1971 Aug;12(4):427–440. doi: 10.1016/0027-5107(71)90093-5. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Turner G. N., Pagano J. S., Hancock R. Sites of replication of chromosomal DNA in a eukaryotic cell. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2300–2305. doi: 10.1073/pnas.69.8.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. Studies on the nature of replicating DNA of HeLa cells. Biochim Biophys Acta. 1969 Jan 21;174(1):253–263. doi: 10.1016/0005-2787(69)90249-4. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C., RAY D. S. ISOLATION OF THE GROWING POINT IN THE BACTERIAL CHROMOSOME. Proc Natl Acad Sci U S A. 1964 Jul;52:125–132. doi: 10.1073/pnas.52.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Structure of branch points in replicating DNA: presence of single-stranded connections in lambda DNA branch points. J Mol Biol. 1971 Mar 14;56(2):319–325. doi: 10.1016/0022-2836(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune Y., Richardson C. C. Strand displacement during deoxyribonucleic acid synthesis at single strand breaks. J Biol Chem. 1971 Apr 25;246(8):2692–2701. [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo, II. Evidence for the second intermediate. Proc Natl Acad Sci U S A. 1968 Jun;60(2):691–698. doi: 10.1073/pnas.60.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo. I. Isolation of the first intermediate of DNA replication in bacteria as single-stranded DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):329–336. doi: 10.1073/pnas.60.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Schaefer A. State of newly synthesized HeLa DNA. Nature. 1969 Mar 29;221(5187):1215–1217. doi: 10.1038/2211215a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M., Smith B. A., Crathorn A. R. Quantitative aspects of the repair of alkylated DNA in cultured mammalian cells. II. Non-semiconservative DNA synthesis ('repair synthesis') in HeLa and Chinese hamster cells following treatment with alkylating agents. Chem Biol Interact. 1971 Feb;3(1):49–68. doi: 10.1016/0009-2797(71)90025-1. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sato S., Ariake S., Saito M., Sugimura T. Properties of nascent DNA of Ehrlich ascites tumor cells obtained by nitrocellulose column chromatography. Biochem Biophys Res Commun. 1972 Oct 6;49(1):270–277. doi: 10.1016/0006-291x(72)90040-x. [DOI] [PubMed] [Google Scholar]
- Sato S., Tanaka M., Sugimura T. Short chain intermediate in DNA replication of Ehrlich ascites tumor cells. Biochim Biophys Acta. 1970 May 21;209(1):43–48. doi: 10.1016/0005-2787(70)90659-3. [DOI] [PubMed] [Google Scholar]
- Schandl E. K., Taylor J. H. Oligodeoxyribonucleotides from pulse-labeled mammalian cells. Biochim Biophys Acta. 1971 Feb 11;228(3):595–609. doi: 10.1016/0005-2787(71)90724-6. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Scudiero D., Strauss B. Accumulation of single-stranded regions in DNA and the block to replication in a human cell line alkylated with methyl methane sulfonate. J Mol Biol. 1974 Feb 15;83(1):17–34. doi: 10.1016/0022-2836(74)90421-5. [DOI] [PubMed] [Google Scholar]
- Souleil C., Panijel J. Effect of antigen dose on the presence of a particular BrdU-labelled DNA fraction in lymph node cells. Exp Cell Res. 1973 Apr;78(2):462–466. doi: 10.1016/0014-4827(73)90093-1. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C., Ellens D. J., Jansz H. S. Linear intermediates in the replication of adenovirus DNA. Nat New Biol. 1972 Sep 13;239(89):47–49. [PubMed] [Google Scholar]
- Taylor J. H. Replication of DNA in mammalian chromosomes: isolation of replicating segments. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1083–1087. doi: 10.1073/pnas.70.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Regions of single-stranded DNA in the growing points of replicating bacteriophage T7 chromosomes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2682–2686. doi: 10.1073/pnas.69.9.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]


