Abstract
Modern anti-retroviral therapy is highly effective at suppressing viral replication and restoring immune function in HIV-infected persons. However, such individuals show reduced physiological performance and increased frailty compared with age-matched uninfected persons. Contemporary anti-retroviral therapy is thought to be largely free from neuromuscular complications, whereas several anti-retroviral drugs previously in common usage have been associated with mitochondrial toxicity. It has recently been established that patients with prior exposure to such drugs exhibit irreversible cellular and molecular mitochondrial defects. However the functional significance of such damage remains unknown. Here we use phosphorus magnetic resonance spectroscopy (31P-MRS) to measure in vivo muscle mitochondrial oxidative function, in patients treated with contemporary anti-retroviral therapy, and compare with biopsy findings (cytochrome c oxidase (COX) histochemistry). We show that dynamic oxidative function (post-exertional ATP (adenosine triphosphate) resynthesis) was largely maintained in the face of mild to moderate COX defects (affecting up to ∼10% of fibers): τ½ ADP (half-life of adenosine diphosphate clearance), HIV-infected 22.1±9.9 s, HIV-uninfected 18.8±4.4 s, p = 0.09. In contrast, HIV-infected patients had a significant derangement of resting state ATP metabolism compared with controls: ADP/ATP ratio, HIV-infected 1.24±0.08×10−3, HIV-uninfected 1.16±0.05×10−3, p = 0.001. These observations are broadly reassuring in that they suggest that in vivo mitochondrial function in patients on contemporary anti-retroviral therapy is largely maintained at the whole organ level, despite histochemical (COX) defects within individual cells. Basal energy requirements may nevertheless be increased.
Introduction
Combination anti-retroviral therapy (cART) has transformed the prognosis for HIV-infected persons since the late 1990s. However, patients are at risk of mitochondrial toxicity, thought to be mediated very largely through exposure to certain nucleoside analog reverse transcriptase inhibitor (NRTI) anti-retrovirals. NRTIs were the first class of licensed anti-retroviral drug, and several of the older members of this class, zidovudine, stavudine, zalcitabine and didanosine, are known to inhibit the sole mitochondrial DNA (mtDNA) polymerase, pol γ, resulting in chain termination during mtDNA replication. During therapy, the molecular consequence of this inhibition is reduction in cellular mtDNA content (mtDNA depletion). A wealth of previous studies has demonstrated this phenomenon both in vitro, and in a variety of tissues in vivo [1]–[4]. These older NRTIs are no longer in common usage in industrialized countries owing to concerns over their toxicity profiles, although zidovudine and stavudine have been very extensively used in anti-retroviral therapy ‘roll-out’ programs in developing countries in recent years. Currently used NRTIs, such as tenofovir (a nucleotide RTI) and abacavir, have been shown to be essentially free from pol γ inhibition in vitro and to cause no significant mtDNA depletion in vivo [5], [6]. If a patient’s therapy is switched away from a pol γ inhibiting NRTI, the impairment of mtDNA replication is removed and mtDNA content returns to normal [7]. Therefore, although most patients are no longer exposed to pol γ inhibiting NRTIs, a significant cohort of long-term patients will have extensive prior exposure to such drugs. Although such patients do not have persistent mtDNA depletion, it has recently been established that they may have persistent histochemical mitochondrial defects evidenced by an increased proportion of COX (cytochrome c oxidase) deficient skeletal muscle fibers. These COX deficient fibers contain high levels of individual somatic (acquired) mtDNA mutations (principally large-scale deletion mutations) [8]. The relevance of this persistent cellular and molecular damage on mitochondrial function remains unknown. It is therefore unclear to what extent mitochondria may be functionally impaired in HIV-infected patients treated with contemporary cART.
Phosphorus magnetic resonance spectroscopy (31P-MRS) allows the dynamic measurement of in vivo skeletal muscle oxidative function through assessment of ATP (adenosine triphosphate) metabolites as well as acid handling. 31P-MRS has previously been employed in the longitudinal study of subjects with inherited mitochondrial disorders, both primary mtDNA defects, and secondary mtDNA defects consequent on nuclear gene disorders of mtDNA maintenance [9]–[11]. Limited data also suggests that 31P-MRS abnormalities in skeletal muscle may be demonstrated in the setting of acute exposure to pol γ inhibiting NRTIs: early in the HIV epidemic, in infected patients exposed to high-dose zidovudine therapy; and in uninfected volunteers treated with stavudine. Such measurements have not been performed in contemporary cART treated patients [12], [13].
We have therefore used 31P-MRS to determine whether patients on contemporary anti-retroviral therapy have abnormal in vivo mitochondrial oxidative function, and whether this correlates with biopsy COX defects.
Methods
Participants
Participants were adult HIV-1 infected patients, receiving ambulatory care at one of four specialist clinics (2 hospital-based, 2 community-based setting). Patients with current active hepatitis B or C co-infection were excluded. Participants were unselected with respect to the presence or absence of complications of HIV or anti-retroviral therapy. Patients with known inherited or non-HIV-associated neuromuscular disease were excluded. Demographic data, surrogate markers (CD4 T lymphocyte count, and HIV-1 RNA plasma viral load) and detailed lifetime anti-retroviral treatment history were obtained by case note review.
HIV-uninfected control subjects for 31P-MRS studies were age and sex matched to our cases, and we excluded persons with diabetes mellitus or abnormal glucose handling, thyroid disease, previous muscle injury, or diagnosed neuromuscular disease.
Research was approved by the Newcastle and North Tyneside Local Research Ethics Committee (ref. 06/Q0905/137). All subjects gave informed written consent for participation.
Phosphorus Magnetic Resonance Spectroscopy
MR studies were performed on calf muscle using a 3T Intera Achieva magnet (Philips). 31P-MRS measurements were obtained using a calf coil with a voxel within soleus muscle during: a 1 minute baseline resting period; a 3 minute period of calf flexion exercise at 25% of maximal voluntary contractile force; and a 6 minute recovery period [10], [14]. This exercise paradigm was specifically designed to keep metabolism within the aerobic phase. Analysis was performed in jMRUI v3.0 (Java Magnetic Resonance User Interface) using AMARES with appropriate prior knowledge parameters for skeletal muscle [15] and metabolite levels were calculated as previously described [14]. Phosphorylation potential was calculated from the concentration of ATP ([ATP], buffered at 8.2 mM), and the empirically calculated concentrations of adenosine diphosphate and inorganic phosphate ([ADP], [Pi]), as [ATP]/([ADP][Pi]) [16].
Skeletal Muscle Biopsies and Mitochondrial (COX) Histochemistry
Percutaneous lower limb skeletal muscle biopsies were performed under local anesthesia and snap-frozen in the liquid phase of isopentane, cooled in liquid nitrogen within 20 minutes of collection. Sequential COX-SDH (cytochrome c oxidase/succinate dehydrogenase) histochemistry was performed on 20 µm transverse cryo-sections. COX contains respiratory chain subunits encoded by the mitochondrial genome, and fibers stain brown (positive) in the presence of intact respiratory chain activity. SDH contains subunits encoded entirely by the nuclear genome and thus provides an effective counterstain (blue) as activity will be preserved in the presence of a cellular mtDNA defect. Proportional COX defect was determined by counting ≥500 fibers per biopsy.
Statistical Comparisons
Student’s paired t-test was used to compare MRS parameters between cases and controls. Correlation coefficients were calculated between COX and MRS data. All analyses were performed in SPSS 19.
Results
Patient Characteristics
23 HIV-infected subjects participated; 78% were male. Mean age was 57.6 years, with age range of 45–74 years. Mean duration of diagnosed HIV infection was 11.8 years. Mean current CD4 T lymphocyte count was 551 cells/µl; mean nadir CD4 count was 183 cells/µl. All subjects were currently receiving cART, of whom 96% had a fully suppressed HIV plasma viral load (<40 HIV-1 RNA copies/ml). In addition to their NRTIs, 70% of treated subjects were receiving a non-nucleoside reverse transcriptase inhibitor (NNRTI) and 35% a protease inhibitor (PI). With respect to the pol γ inhibiting NRTIs, 61% of patients had a prior history of zidovudine exposure, and 48% had dideoxynucleoside analog (stavudine, zalcitabine or didanosine) exposure (treatment details of individual patients are shown in Table 1 ).
Table 1. Characteristics of HIV-infected subjects.
| Subject | Age(yrs) | Gender | Duration of diagnosed HIV infection (mo) | CurrentCD4 count(cells/uL) | CurrentHIV VL(copies/mL) | Nadir CD4 count(cells/uL) | Duration of ART (mo) | Current HAART | Lifetime HAART | Biopsy COX defect (%) |
| 1 | 55 | M | 96 | 503 | <40 | 117 | 48 | TDF FTC ATV/r | TDF FTC ATV/r | 0.0% |
| 2 | 71 | M | 119 | 448 | <40 | UK | 119 | TDF FTC EFV | ddI AZT 3TC EFV TDF FTC | 3.0% |
| 3 | 74 | F | 201 | 825 | <40 | UK | 103 | TDF FTC EFV | AZT ddi d4T SQV TDF 3TC EFV | 0.4% |
| 4 | 45 | F | 71 | 537 | <40 | 10 | 70 | TDF FTC NVP | AZT 3TC EFV TDF FTC NVP | 0.1% |
| 5 | 55 | F | 59 | 406 | <40 | 112 | 22 | TDF FTC AZT DRV/r | TDF FTC LPV/r AZT DRV/r | 0.8% |
| 6 | 63 | M | 76 | 783 | <40 | 169 | 76 | ABC 3TC EFV | AZT ABC 3TC EFV | 0.0% |
| 7 | 63 | M | 215 | 361 | <40 | UK | 198 | ABC 3TC NVP | AZT ddI d4T 3TC ddC IDV NVP ABC | 2.2% |
| 8 | 62 | M | 43 | 180 | <40 | 56 | 42 | TDF FTC NVP | TDF FTC NVP | 0.2% |
| 9 | 49 | M | 193 | 762 | <40 | 120 | 193 | TDF FTC ATV/r | AZT ddC ddI 3TC d4T SQV NVP IDV NFV ABC TDFLPV/r FTC ATV/r | 1.3% |
| 10 | 48 | M | 158 | 872 | <40 | 10 | 151 | TDF ABC NVP | AZT ddI d4T 3TC RTV NVP IDV ddC ABC ATV/r TDF | 4.9% |
| 11 | 60 | F | 146 | 666 | <40 | 99 | 145 | ABC 3TC EFV | d4T ABC 3TC EFV | 0.2% |
| 12 | 51 | M | 141 | 494 | <40 | 151 | 140 | AZT 3TC NVP | AZT 3TC NVP | 1.4% |
| 13 | 66 | M | 57 | 403 | <40 | 287 | 12 | TDF FTC EFV | TDF FTC EFV | 11.2% |
| 14 | 63 | F | 182 | 865 | <40 | 300 | 154 | TDF FTC EFV | d4T 3TC NVP NFV EFV AZT TDF FTC | 1.2% |
| 15 | 60 | M | 101 | 419 | <40 | UK | 98 | TDF FTC NVP | AZT 3TC EFV NVP TDF FTC | 2.4% |
| 16 | 61 | M | 262 | 422 | <40 | UK | 160 | ABC NVP LPV/r | SQV AZT ddC 3TC d4T ddI IDV ABC NVP NFV LPV/r | 0.8% |
| 17 | 54 | M | 66 | 603 | <40 | 244 | 25 | TDF FTC DRV/r | TDF FTC EFV DRV/r | 3.4% |
| 18 | 51 | M | 237 | 559 | <40 | 327 | 165 | TDF FTC EFV | AZT ddI RTV NFV TDF FTC EFV | 1.5% |
| 19 | 62 | M | 143 | 329 | <40 | 163 | 55 | TDF FTC DRV/r | AZT 3TC NVP FOS-APV RTV TDF FTC DRV/r | 1.2% |
| 20 | 50 | F | 120 | 1358 | <40 | 541 | 0 | nil | nil | 0.0% |
| 21 | 53 | M | UK | 804 | <40 | 301 | 48 | TDF FTC EFV | TDF FTC EFV | NA |
| 22 | 56 | M | 240 | 401 | 97 | 150 | 224 | TDF FTC ETR DRV/r | AZT ddC SQV 3TC IDV d4T NVP ddI ABC LPV/r TDFATV/r FOS-APV/r DRV/r MVC FTC | 2.2% |
| 23 | 45 | M | 165 | 592 | <40 | 305 | 146 | RAL ABC ATV/r | d4T 3TC NVP ddI IDV ABC ATV/r RAL | 9.8% |
| 24 | 57 | M | 145 | 435 | <40 | 379 | 21 | TDF FTC EFV | TDF FTC EFV | 0.4% |
VL, plasma HIV-1 RNA viral load; (c)ART, (combination) anti-retroviral therapy; AZT, zidovudine; d4T, stavudine; ddI, didanosine; ddC, zalcitabine; 3TC, lamivudine; ABC, abacavir; TDF, tenofovir; FTC, emtricitabine; EFV, efavirenz; NVP, nevirapine; ATV, atazanavir; DRV, darunavir; LPV, lopinavir; SQV, saquinavir; NFV, nelfinavir; IDV, indinavir; FOS-APV, fosamprenavir; RTV, ritonavir at therapeutic dose;/r, ritonavir at pharmacokinetic boosting dose; MVC, maraviroc; RAL, raltegravir; UK, unknown; NA, not available; COX, cytochrome c oxidase. COX data (but not MRS data) from 5 subjects has been previously described [8].
Measures of Muscle ATP and Acid Metabolism by 31P-MRS
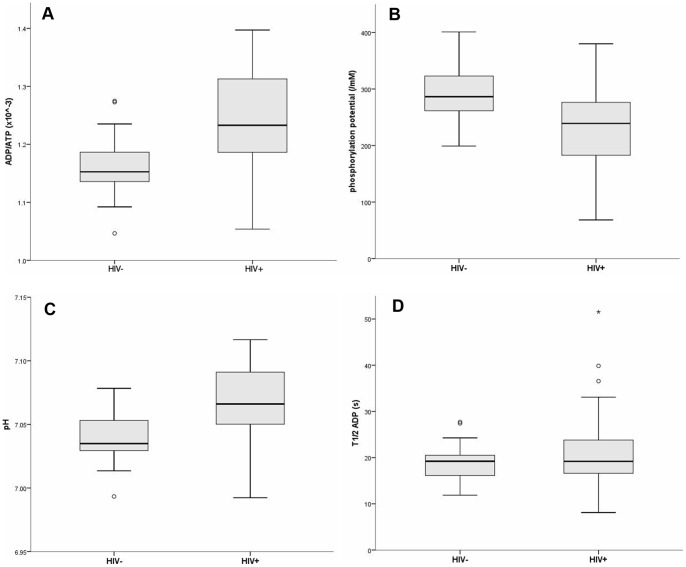
In the resting state, baseline ATP metabolites and pH values were significantly higher in cART-treated HIV-infected subjects compared to age and gender-matched controls (mean ±SD): ADP/ATP ratio, HIV-infected 1.24±0.08×10−3, HIV-uninfected 1.16±0.05×10−3, p = 0.001; phosphocreatine/ATP (PCr/ATP) ratio, HIV-infected 5.04±1.89, HIV-uninfected 3.75±0.26, p = 0.004; pH, HIV-infected 7.07±0.03, HIV-uninfected 7.04±0.02, p = 0.002. Correspondingly, calculated basal phosphorylation potential was significantly lower in HIV-infected subjects compared with controls: HIV-infected 227±86 mM−1, HIV-uninfected 292±53 mM−1, p = 0.003 ( Figure 1a–c ). (Further details of calculated 31P-MRS parameters are shown in the Table S1.).
Figure 1. Phosphorus magnetic resonance spectroscopy.
Resting state metabolic parameters differed significantly between HIV-infected subjects (HIV+) and HIV-uninfected controls (HIV−): ADP/ATP (adenosine diphosphate/ATP) ratio (a), phosphorylation potential (b), and pH (c) (n = 23 each; ADP/ATP, p = 0.001; phosphorylation potential, p = 0.003; pH, p = 0.002). In contrast, the rate of ATP re-synthesis (estimated as τ½ ADP) following exertion was not significantly impaired in HIV-infected subjects compared with controls (p = 0.09) (d).
In terms of dynamic oxidative function, mean post-exercise ATP metabolite recovery rates did not differ significantly between HIV-infected subjects and controls. For example, τ½ ADP: HIV-infected 22.1±9.9 s, HIV-uninfected 18.8±4.4 s, p = 0.09 ( Figure 1d ). None of the clinical variables (age, duration of diagnosed HIV infection, CD4 T lymphocyte count, or anti-retroviral treatment history) correlated significantly with any of the baseline or post-exercise 31P-MRS parameters in HIV-infected subjects.
Mitochondrial (COX) Histochemistry and Correlation with ATP Metabolism
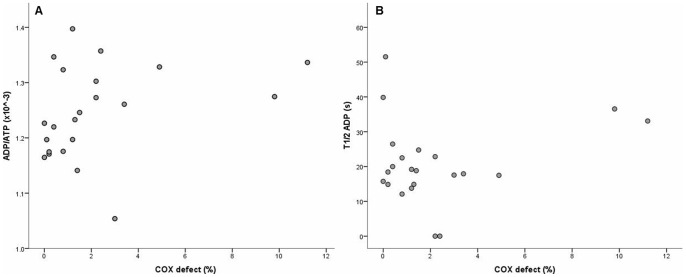
A wide range of COX defects were observed across the subject group (0 to >10% of muscle fibers affected per biopsy). Interestingly, we observed significant COX defects both in patients with prior exposure to pol γ inhibiting NRTIs, and in some patients without such exposure (COX defects for individual patients are shown in Table 1 ). Resting state ADP/ATP ratio showed a moderate correlation with biopsy proportional COX defect (Kendall’s τ = 0.34, p = 0.034) ( Figure 2a ). There was no correlation between dynamic ATP metabolism, for example as estimated by τ½ ADP, and biopsy COX defect ( Figure 2b ).
Figure 2. Relationship of phosphorus magnetic resonance spectroscopy and muscle histochemistry.
Resting state ADP/ATP ratio showed moderate correlation with the percentage frequency of COX deficient muscle fibers in treated HIV-infected subjects (Kendall’s τ = 0.34, p = 0.034) (a), whereas the rate of ATP re-synthesis following exertion (estimated as τ½ ADP) did not (Kendall’s τ = 0.04) (b).
Discussion
In our study, most anti-retroviral treated HIV-infected subjects demonstrated dynamic in vivo tissue mitochondrial function comparable with uninfected control subjects, whereas it is generally impaired in inherited mitochondrial disorders. Our subject group included patients with very long durations of HIV infection and extensive anti-retroviral drug treatment histories, including past exposure to pol γ inhibiting NRTIs. Interestingly, in the present study we observed COX defects both in subjects with prior exposure to pol γ inhibiting NRTIs, and in some subjects without such exposure. This observation contrasts with our previous work in younger HIV-infected patients (all aged ≤50 years), where COX defects appeared to be attributable almost entirely to exposure to pol γ inhibiting NRTIs [8]. In the present study, the heterogeneous COX defects are most likely to reflect the significantly older subject age range (45–74 years). In this age group it is expected to see some COX defects due to normal aging [17], although it is also possible that there are other unmeasured HIV or treatment-associated factors driving COX defects in some of these patients. As with NRTI-associated COX defects, these COX deficient fibers also contain high levels of individual somatic mtDNA mutations [18]. What is therefore the likely explanation of our finding of largely normal in vivo muscle mitochondrial function? COX-deficient fibers contain high levels of mutant mtDNA, whereas COX positive fibers contain almost exclusively wild-type mtDNA. Therefore function at the whole tissue level is presumably compensated by the larger number of fibers with normal COX function. In contrast, in the historical context when patients were actively treated with a pol γ inhibiting NRTI, the molecular defect was one of mtDNA depletion, and the impairment of oxidative function would be expected to affect all fibers [13]. We therefore conclude that although somatic mtDNA mutations and associated cellular COX defects are frequently present in contemporary cART treated patients, there is some ability to compensate for in vivo exertional oxidative function in the muscle as a whole.
The decreased basal phosphorylation potential, as we have observed in our patients, implies an increased rate of ATP synthesis at rest [19]. Cytosolic ATP concentration is tightly buffered, and results from the balance of the ATP hydrolysis required for the maintenance of cellular integrity and the synthesis of ATP from oxidative phosphorylation [16]. The rate of ATP synthesis is strongly dependent on the phosphorylation potential in resting muscle, with a decreased phosphorylation potential, as we have observed in our patients, implying an increased rate of ATP synthesis [19]. This notion suggests that there is a requirement for an increased basal rate of intracellular ATP hydrolysis in HIV-infected subjects, and an increased basal rate of ATP synthesis is therefore required to maintain ATP homeostasis. Although this might imply increased basal energy expenditure in these patients compared with healthy subjects, given that dynamic in vivo mitochondrial function is unimpaired, the physiological significance of this observation remains uncertain. Further work should therefore examine correlates of this finding, such as fatigue [20].
In conclusion, in a cohort of predominantly older HIV-infected patients with longstanding cART, we observed frequent histochemical COX defects both in patients with and without prior exposure to pol γ inhibiting NRTIs. It is, however, broadly reassuring that in vivo whole tissue mitochondrial function in most contemporary anti-retroviral treated patients appears to be largely maintained, despite the presence of this frequent mitochondrial damage within individual cells.
Supporting Information
Phosphorus magnetic resonance data. Calculated 31P-MRS parameters, in resting state and during recovery from sub-maximal exercise.
(DOCX)
Funding Statement
This work received financial support from: Medical Research Council (MRC, UK) (BP)(www.mrc.ac.uk); Newcastle National Institute for Health Research (NIHR) Biomedical Research Centre in Ageing and Age-Related Diseases (BP, PFC) (http://www.ncl.ac.uk/biomedicine/research/brc); Medical Research Council (MRC, UK) Centre for Translational Muscle Disease (PFC); Wellcome Trust (084980/Z/08/Z & 096919Z/11/Z, PFC) (www.wellcome.ac.uk); Medical Research Council (MRC, UK) (G1100160, KGH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Arnaudo E, Dalakas M, Shanske S, Moraes CT, DiMauro S, et al. (1991) Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 337: 508–510. [DOI] [PubMed] [Google Scholar]
- 2. Cote HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, et al. (2002) Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 346: 811–820. [DOI] [PubMed] [Google Scholar]
- 3. Cherry CL, Gahan ME, McArthur JC, Lewin SR, Hoy JF, et al. (2002) Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. J Acquir Immune Defic Syndr 30: 271–277. [DOI] [PubMed] [Google Scholar]
- 4. Hoschele D (2006) Cell culture models for the investigation of NRTI-induced mitochondrial toxicity. Relevance for the prediction of clinical toxicity. Toxicol In Vitro 20: 535–546. [DOI] [PubMed] [Google Scholar]
- 5. Lim SE, Copeland WC (2001) Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. J Biol Chem 276: 23616–23623. [DOI] [PubMed] [Google Scholar]
- 6. Birkus G, Hitchcock MJ, Cihlar T (2002) Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 46: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McComsey GA, Paulsen DM, Lonergan JT, Hessenthaler SM, Hoppel CL, et al. (2005) Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. Aids 19: 15–23. [DOI] [PubMed] [Google Scholar]
- 8. Payne BA, Wilson IJ, Hateley CA, Horvath R, Santibanez-Koref M, et al. (2011) Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet 43: 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penn AM, Lee JW, Thuillier P, Wagner M, Maclure KM, et al. (1992) MELAS syndrome with mitochondrial tRNA(Leu)(UUR) mutation: correlation of clinical state, nerve conduction, and muscle 31P magnetic resonance spectroscopy during treatment with nicotinamide and riboflavin. Neurology 42: 2147–2152. [DOI] [PubMed] [Google Scholar]
- 10. Trenell MI, Sue CM, Kemp GJ, Sachinwalla T, Thompson CH (2006) Aerobic exercise and muscle metabolism in patients with mitochondrial myopathy. Muscle Nerve 33: 524–531. [DOI] [PubMed] [Google Scholar]
- 11. Yu-Wai-Man P, Trenell MI, Hollingsworth KG, Griffiths PG, Chinnery PF (2011) OPA1 mutations impair mitochondrial function in both pure and complicated dominant optic atrophy. Brain 134: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleischman A, Johnsen S, Systrom DM, Hrovat M, Farrar CT, et al. (2007) Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab 292: E1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinnwell TM, Sivakumar K, Soueidan S, Jay C, Frank JA, et al. (1995) Metabolic abnormalities in skeletal muscle of patients receiving zidovudine therapy observed by 31P in vivo magnetic resonance spectroscopy. J Clin Invest 96: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hollingsworth KG, Newton JL, Taylor R, McDonald C, Palmer JM, et al. (2008) Pilot study of peripheral muscle function in primary biliary cirrhosis: potential implications for fatigue pathogenesis. Clin Gastroenterol Hepatol 6: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 15. Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D (2001) Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 31: 269–286. [DOI] [PubMed] [Google Scholar]
- 16. Harris RC, Hultman E, Nordesjo LO (1974) Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120. [PubMed] [Google Scholar]
- 17. Brierley EJ, Johnson MA, James OF, Turnbull DM (1996) Effects of physical activity and age on mitochondrial function. Qjm 89: 251–258. [DOI] [PubMed] [Google Scholar]
- 18. Bua E, Johnson J, Herbst A, Delong B, McKenzie D, et al. (2006) Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet 79: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeneson JA, Wiseman RW, Westerhoff HV, Kushmerick MJ (1996) The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem 271: 27995–27998. [DOI] [PubMed] [Google Scholar]
- 20. Payne BA, Hateley CL, Ong EL, Premchand N, Schmid ML, et al. (2013) HIV-associated fatigue in the era of highly active antiretroviral therapy: novel biological mechanisms? HIV Med 14: 247–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phosphorus magnetic resonance data. Calculated 31P-MRS parameters, in resting state and during recovery from sub-maximal exercise.
(DOCX)