Abstract
Background
Advantages associated with the use of cord blood (CB) transplantation include the availability of cryopreserved units, ethnic diversity and lower incidence of graft-versus-host disease when compared to bone marrow or mobilized peripheral blood. However, poor engraftment remains a major obstacle. We and others have found that ex vivo fucosylation can enhance engraftment in murine models and thus ex vivo treatment of CB with fucosyltransferase (FT)-VI prior to transplantation is under clinical evaluation (NCT01471067). However, FT-VII appears to be more relevant to hematopoietic cells and may alter acceptor substrate diversity. In this study, we compare the ability of FT-VI and FT-VII to improve the rapidity, magnitude, multi-lineage and multi-tissue engraftment of human CB hematopoietic stem and progenitor cells (HSPC) in vivo.
Methods
CD34-selected CB HSPC were treated with recombinant FT-VI, FT-VI or mock control, then injected into immunodeficient mice and monitored for multi-lineage and multi-tissue engraftment.
Results
Both FT-VI and FT-VII fucosylated CB CD34+ cells in vitro, and both led to enhanced rates and magnitudes of engraftment when compared to untreated CB CD34+ cells in vivo. Engraftment following treatment with either fucosyltransferase was robust at multiple timepoints, and in multiple tissues, with similar multi-lineage potential. In contrast, only FT-VII was able to fucosylate T- and B-lymphocytes.
Discussion
While we found that FT-VI and FT-VII were similarly able to fucosylate and enhance the engraftment of CB CD34+ cells, differences in their ability to fucosylate lymphocytes cells may modulate graft-versus-tumor and/or graft-versus-host effects and may allow further optimization of CB transplantation.
Introduction
For effective homing and engraftment of hematopoietic stem and progenitor cells (HPSC) to the bone marrow (BM), it is thought that specific cell surface ligands expressed by the HSPC interact with receptors expressed by the endothelial cells lining the blood vessels of the hematopoietic system. While the expression of certain cell surface glycoproteins by HSPC might be sufficient for homing to hematopoietic tissues (1–9), there is evidence that the activity of specific ligands is improved when they are fucosylated (10–17). Fucosylation is the addition of fucose moieties by fucosyltransferase (FT)-directed, site-specific processes. Previous studies have revealed that CB HSPC have consistently lower levels of endogenous fucosylation than BM or mobilized peripheral blood (mPB) progenitor cells and that ex vivo fucosylation using recombinant human FT-VI enhances the adhesion, homing and engraftment of CB HSPC in xenografts (18,19). From these data it appears that ex vivo fucosylation using FT-VI may be used to mitigate the delayed engraftment that is currently associated with CB transplantation. Thus, a clinical trial is underway testing the effect of ex vivo fucosylation of CB using recombinant human FT-VI prior to transplantation (NCT01471067).
However, FT-VI is not normally expressed on hematopoietic cells, but rather in endothelial, epithelial, gastrointestinal and some malignant cells. In contrast, FT-VII is widely expressed on hematopoietic cells including BM CD34+ cells (20). Indeed FT-VII appears to be the dominant fucosyltransferase responsible for producing leukocyte selectin ligand activity (21), and a spontaneous FT-VII mutation impairs selectin binding (22). Importantly FT-VII expression is unexpectedly low in CB HSPC (23), suggesting that fucosylation with FT-VII may provide a more physiologic approach to restoring fucosylated proteins to CB HSPC. The aim of this study was to compare the activities of FT-VI and FT-VII to identify any qualitative differences in the rate, magnitude, multi-lineage and multi-tissue engraftment of human CB HSPC in vivo.
Materials and Methods
Two α-(1,3)-fucosyltransferase enzymes, FT-VI and FT-VII (provided by American Stem Cell Inc, Floresville, TX), were compared for their ability to fucosylate CB HSPC in an ex vivo setting. Fucosylation was revealed by flow cytometry through the binding of HECA-452 (BD Biosciences), a directly conjugated (FITC), rat IgM antibody that reacts against fucosylated (sialyl Lewis X (sLeX)-modified) cell surface glycoproteins, including P-selectin glycoprotein ligand (PSGL)-1 (CD162) (24). HECA-452 was originally described as detecting a “cutaneous lymphocyte antigen” (CLA).
Hematopoietic Cells
Fresh CB units were obtained and all animal work conducted under M. D. Anderson Cancer Center Institutional Review Board and Institutional Animal Care and Use Committee approved protocols, respectively. CB mononuclear cells (MNC) were isolated from fresh CB units by Ficoll-Histopaque density separation and CB CD34+ cells enriched by magnetic-activated cell sorting (MACS) according to manufacturer’s instructions (Miltenyi Biotec, Auburn, CA.). MACS-selected CD34+ cells were then pooled and divided into (i) untreated, (ii) FT-VI-treated or (iii) FT-VII-treated fractions. Ex vivo fucosylation was performed as previously described (18). Briefly, CB CD34+ cells were treated at 106 cells/ml for 30 minutes at room temperature with 1 mM GDP β-fucose (EMD Biosciences, San Diego, CA.) in Phosphate Buffered Saline (PBS) containing 1% human serum albumin (HSA, Baxter Healthcare Corp., Westlake Village, CA.) and in the previously optimized concentrations of 100 mU/ml FT-VI, or 75 μg/ml FT-VII. Untreated cells were incubated as above except no enzyme was added. After incubation, cells were washed in PBS containing 1% HSA, cellularity determined by hemocytometer and cells diluted in saline prior to intravenous injection into sublethally-irradiated NOD-SCID IL-2Rγnull (NSG) mice (Jackson Laboratories, Bar Harbor, ME). A 137Cs source delivered a total sublethal radiation dose of 300 cGy over one minute (J. L. Shepherd and Associates Mark I-25 Irradiator, San Fernando, CA). In each group, mice received 105 CB CD34+ cells.
Assessment of human engraftment in peripheral blood (PB), BM and spleen (SP) of NSG recipients
Human engraftment was determined once or twice per week by withdrawal of 40μl of PB from the retro-orbital sinus of anesthetized mice and red blood corpuscles were lysed (Pharm Lyse, BD Pharmingen). At >100 days after transplant, BM and spleen (SP) were also harvested. Samples were assessed for the presence of human and murine CD45 positive cells by flow cytometry (BD FACSCalibur) using PE-conjugated rat anti-mouse CD45 and APC-conjugated mouse anti-human CD45 (both BD Biosciences). Mouse PB, human CB and antibody isotypes provided appropriate controls. Data were acquired and analysis performed using CellQuest Pro software (BD Biosciences). The percentage of human engraftment was calculated as: [Percent human CD45 ÷ (Percent human CD45 + Percent murine CD45)] × 100
Secondary transplantation of human CD34+ cells from the bone marrow of primary CB CD34+ recipients
When recipients of FT-VI-treated or FT-VII-treated CB CD34+ cells were euthanized at >100 days after transplant (as previously described), femoral BM from individuals in each group (‘primary’ recipients) was pooled and transplanted into 3 groups of sublethally-irradiated NSG mice (n=5 mice/gp, ‘secondary’ recipients). Equivalent numbers of nucleated BM cells (approximately 3x107/mouse) were transplanted intravenously and the number of human CD34+ cells (approximately 2x106/mouse) retrospectively assessed by flow cytometry of the pooled BM samples (as previously described). Mice were euthanized >10 weeks after transplantation and human engraftment assessed in PB, BM and SP (as previously described). This would reveal any impact of the initial ex vivo fucosyltransferase treatment on long-term engraftment potential.
Additional flow cytometric analyses of CB MNC to compare the activities of FT-VI and FT-VII
While this study was focused primarily on the impact of FT-VI and FT-VII on CB CD34+ cells, limited analysis of the HECA-reactivity of CB MNC following treatment with FT-VI or FT-VII was performed allowing a comparison of fucosylation of non-CD34+ cell types. The HECA-reactivity of total CB MNC (CD45+), CB T-lymphocytes (CD3+), CB B-lymphocytes (CD19/20+) and CB myeloid cells (CD14+) was assessed (all antibodies from BD). Data were acquired using a FACSCalibur (Becton Dickinson) and analysis performed using CellQuest Pro software (BD Biosciences).
Statistical analysis
Data are presented as mean ± SEM and compared using the Student’s T-test with significance assumed at P≤0.05.
Results
Fucosylation of CB CD34+ cells with FT-VI or FT-VII
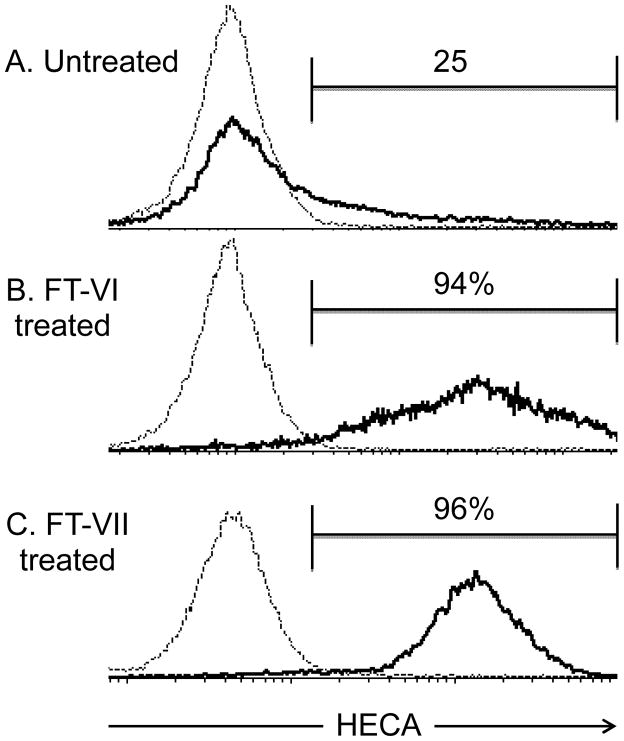
The HECA-452 anti sLex antibody revealed that <25% of CB CD34+ HSPC were ‘fucosylated’ endogenously (Figure 1A). Treatment with either FT-VI or FT-VII, significantly increased (>90%) the proportion of HECA-reactive (‘fucosylated’) CB CD34+ cells (Figures 1B and 1C, respectively). The fucosylation profiles of FT-VI and FT-VII had similar maximal values, although the distribution of fucosylation (HECA-452) intensities was broader for FT-VI, providing a greater range (low to high) of fucosylation on a per cell basis than was observed for FT-VII.
Figure 1. Ex vivo treatment of CB CD34+ cells with FT-VI and FT-VII.
The HECA-452 antibody was used to reveal fucosylation. In a pool of untreated CB CD34+ cells isolated from fresh CB units, approximately 25% were HECA-reactive (Figure 1A). This was increased to 94% (Figure 1B) and 96% (Figure 1C), respectively following ex vivo treatment with FT-VI or FT-VII.
Human engraftment measured in PB of sublethally-irradiated NSG recipients
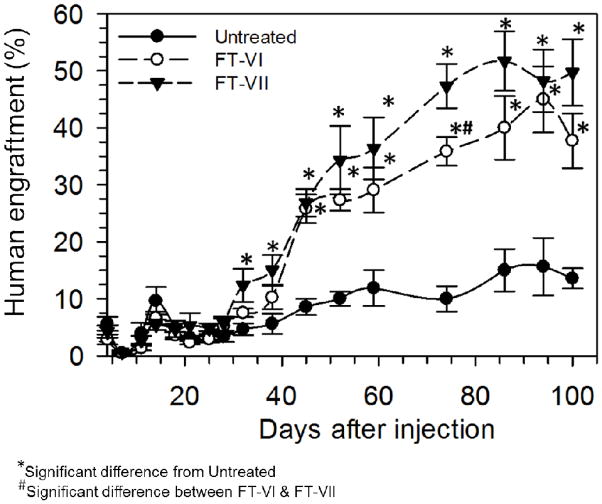
Cohorts of sublethally-irradiated NSG were intravenously injected with 105 untreated, FT-VI-treated or FT-VII-treated CB CD34+ cells (Figure 2). As reported previously, FT-VI treatment significantly enhanced the rate and magnitude of engraftment of CB CD34+ cells in this xenograft assay (18). For the first time, we report here, that FT-VII treatment of CD34+ CB HSPC led to a similarly robust magnitude of engraftment when compared to FT-VI treated CB HSPC. Mice receiving FT-VII-treated CB CD34+ cells demonstrated a significantly higher level of engraftment by day 32 post-transplant compared to recipients of untreated CB CD34+ cells (12±3% vs. 5±1%, P=0.04). Similarly, recipients of FT-VI-treated CB showed a trend toward increased engraftment by day 32 (8±1% vs. 5±1%, P=0.05). Mice receiving either FT-VI or FT-VII-treated CD34+ cells both sustained a significantly improved (approximately 3-fold) engraftment over mice receiving untreated CB CD34+ cells. Although recipients of FT-VII-treated CB CD34+ cells demonstrated consistently greater engraftment than mice receiving FT-VI-treated CB CD34+ cells, this difference was only statistically different on day 74 (“#” in Figure 2). By the peak engraftment at the day 86 time point, recipients of untreated CB CD34+ cells had achieved 15±4% human engraftment, while engraftment was 2.7-fold greater (40±6%, P=0.01) in the recipients of FT-VI-treated CB CD34+ cells and 3.5-fold greater (52±5%, P=0.001) in the recipients of FT-VII-treated CB CD34+ cells. Ex vivo treatment of human CB CD34+ cells with either FT-VI or FT-VII significantly enhanced the rate and magnitude of engraftment in this NSG engraftment model. Magnitude of engraftment trended in favor of FT-VII treatment at most timepoints including early and peak engraftment timepoints.
Figure 2. Human engraftment measured in the peripheral blood (PB), bone marrow (BM) and spleens (SP) of sublethally-irradiated NSG mice following the transplant of untreated, FT-VI-treated or FT-VII-treated CB CD34+ cells.
Significantly improved levels of human engraftment were observed in PB of sublethally-irradiated mice receiving 105 CB CD34+ cells treated with FT-VI or FT-VII as compared to mice receiving 105 untreated CB CD34+ cells.
Human engraftment in BM and spleen
When mice were euthanized, human engraftment in the BM and spleens of the engrafted mice was assessed (Table 1). Flow cytometric assessment of BM of mice receiving untreated CB CD34+ cells revealed 68±4% human engraftment. Human engraftment was significantly (P=0.006), 1.3-fold increased to 88±3% in the recipients of FT-VI-treated CB CD34+ cells and significantly (P=0.0001) 1.4-fold increased to 96±1% in the recipients of FT-VII-treated CB CD34+ cells when compared to recipients of untreated CB CD34+ cells. A significant difference in the human engraftment between recipients of FT-VI or FT-VII-treated CB CD34+ cells was observed (88±3% vs. 96±1%, respectively, P=0.04). Flow cytometric assessment of human engraftment in the spleens of mice receiving untreated CB CD34+ cells revealed 77±3% human engraftment. Human engraftment was not significantly different from this value in the recipients of FT-VI-treated CB CD34+ cells (84±4%, P=0.219), but was significantly (P=0.004) 1.2-fold increased at 91±1%, in the recipients of FT-VII-treated CB CD34+ cells.
Table 1.
Multi-lineage, multi-tissue human engraftment.
| CB CD34+ treatment | Human Engraftment (%)
|
|||||
|---|---|---|---|---|---|---|
| Total (CD45) | B-cell (CD19/20) | Myeloid (CD14) | T-cell (CD3) | |||
| BM | SP | BM | SP | BM | SP | |
|
|
|
|
|
|
||
| Untreated | 68±4 | 77±3 | 10±1 | 15±2 | 10±2 | 1.8±0.2 |
| FT-VI | 88±3* | 84±4 | 14±1* | 30±0.4* | 9±2 | 2.1±0.5 |
| FT-VII | 96±1*# | 91±2* | 15±1* | 33±2* | 17±2*# | 4.3±1.2 |
Significantly different from untreated
Significantly different from FT-VI
Multi-lineage analysis revealed that in recipients of CB CD34+ treated with FT-VI or FT-VII, human B-lymphocyte engraftment was similarly, significantly increased (14–15% of BM and 30–33% of the spleen) as compared to approximately 10% BM and 15% of spleen, in recipients of untreated CB CD34+ cells (Table 1). Outside of human B-lymphocyte engraftment, there were no further differences in the patterns of multi-tissue, multi-lineage human engraftment between recipients of untreated and FT-VI treated CB CD34+ cells. However, recipients of FT-VII treated CB CD34+ cells showed greater (approximately 2-fold) levels of human myeloid cells in BM (approximately 17% vs. 9%) and human T-lymphocytes in the spleen (approximately 4% vs. 2%) than those found in recipients of untreated or FT-VI treated CB CD34+ cells. Therefore, although human engraftment was similarly enhanced when comparing the BM and spleens of recipients of FT-VI and FT-VII treated CB CD34+ cells, FT-VII treatment was associated with elevated human total and myeloid engraftment in the BM and elevated human T cell engraftment in the spleen.
Secondary transplantation of human CD34+ cells from the bone marrow of primary CB CD34+ recipients
Flow cytometric assessment >10 weeks after secondary transplantation revealed that there was no significant difference between the levels of human engraftment measured in the peripheral blood (0.56±0.22% vs. 0.69±0.13, P=0.657), BM (2.86±1.46% vs. 1.08±0.25%, P=0.273) and spleen (2.94±1.20% vs. 1.59±0.34%, P=0.708).
Comparison of FT-VI and FT-VII activities on non-CD34+ CB MNC
The in vivo experiments reported used CD34-selected CB cells to isolate the effects of fucosylation on HSPC homing and engraftment, however, in the majority of clinical CB transplantation protocols transplant the entire CB unit. Thus we compared the effects of FT-VI and FT-VII treatment on total CB MNC which included CD3+ T-cells, CD19+/CD20+ B-cells, and CD14+ myeloid cells in addition to CD34+ cells. Flow cytometric analysis of HECA-reactivity for untreated CB MNC revealed that the majority of CD45+ cells were not endogenously fucosylated (Figure 3A). Consistent with these data, multi-lineage analysis revealed that CD3+ T-cells, CD19+/CD20+ B-cells, and CD14+ myeloid cells (Figures 3D, 3G and 3J, respectively) were not endogenously fucosylated. When CB MNC were treated with FT-VI, two distinct CD45+ peaks were revealed by flow cytometry (Figure 3B) indicating that while a proportion of the CB MNC were fucosylated by FT-VI, a proportion were not. Multi-lineage analysis revealed that while the majority of CD14+ myeloid cells were fucosylated following treatment with FT-VI (Figure 3K), CD3+ T-lymphocytes and CD19+/20+ B-lymphocytes were not (Figures 3E and 3H, respectively). In contrast, the ex vivo treatment of CB MNC with FT-VII yielded a 100% HECA-reactive CD45+ CB MNC product (Figure 3C). Consistent with this observation, multi-lineage analysis revealed that CD14+ myeloid cells, CD3+ T-lymphocytes and CD19+/20+ B-lymphocytes were all fucosylated (Figures 3L, 3F and 3I, respectively).
Figure 3. In vitro comparison of FT-VI and FT-VII activities against non-CB CD34+ cells present in CB MNC.
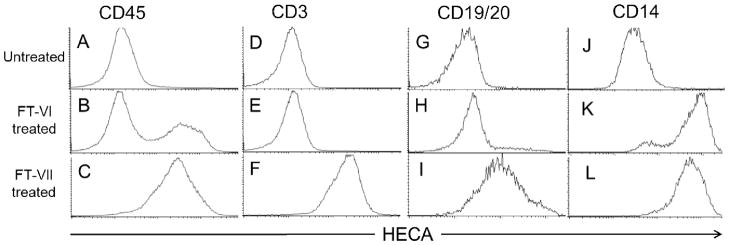
Ex vivo treatment of CB MNC with FT-VI or FT-VII revealed a marked difference between the activities of the two α-(1,3)-fucosyltransferases. Endogenous levels of fucosylation of CD45+, CD3+, CD19/20+ and CD14+ cells contained in CB MNC are shown (3A, 3D, 3G and 3J). FT-VI-treatment fucosylated only part of the CD45+ cell population (4B), which included CD14+ myeloid cells (3K). It did not fucosylate CD3+ (3E) or CD19/20+ (3H) lymphocytes. In contrast, FT-VII fucosylated all CD45+ cells (3C, 3F, 3I and 3L).
Discussion
In this study, we performed a direct comparison of FT-VI and FT-VII treatment of CB HSPC to assess whether any functional and/or qualitative differences would be seen in terms of the rate, magnitude, multi-lineage and multi-tissue engraftment in vivo. We demonstrate that the two enzymes similarly fucosylate CB CD34+ cells ex vivo, and similarly improve the rate and magnitude of engraftment in vivo, with a trend toward higher engraftment following FT-VII treatment. As delayed engraftment and graft loss are significant obstacles for CB transplant patients, this may provide significant benefit. Furthermore, when a multi-lineage, multi-tissue assessment was made of the pattern of human engraftment in recipient mice, there was evidence that the transplantation of FT-VII (rather than FT-VI) treated CB CD34+ cells was associated with improved human myeloid and T-lymphocyte engraftment. These data suggest that the treatment of CB CD34+ cells with FT-VII (rather than FT-VI) may enhance the de novo generation of myeloid and lymphoid cells in the recipient, potentially improving both neutrophil and T-cell recovery post-transplant. Given these results, FT-VII treatment appears to be a promising potential therapeutic approach for further enhancing cord blood transplantation. However, there are limitations to using these in vivo xenograft models to predict engraftment and post-transplant recovery. In particular, the murine hematopoietic microenvironment lacks several critical myeloid growth factors which do not cross species including interleukin (IL)-3, stem cell factor (SCF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). In addition the role of fucosylation on graft-versus-tumor and/or graft-versus-host are not addressed with this CD34-selected/immunodeficient mouse model.
In conclusion, these results further support the potential role of ex vivo fucosylation of CB HSPC to improve engraftment, and reveal differences in the fucosyltransferase activities of FT-VI versus FT-VII on the magnitude of multi-tissue engraftment, including enhanced myeloid and T-cell recovery. Further studies into improving CB transplant through enhanced homing/engraftment are needed.
Acknowledgments
Research performed in this study was supported in part by grants from the National Cancer Institute (P01 CA148600-02 and RO1 CA061508-18) and the Cancer Prevention Research Institute of Texas (RO1 RP100469). We gratefully acknowledge the provision of FT-VI and FT-VII by America Stem Cell, Inc., Floresville, Texas, USA.
Footnotes
Conflicts of Interest
All authors, with the exception of Dr. Leonard Miller (please see details below), declare no conflict of interest exist. Leonard Miller, Ph.D. is Vice President of Research and co-founder of America Stem Cell, Inc. America Stem Cell, Inc., provided FT-VI and FT-VII used in the studies reported in this manuscript.
References
- 1.Dimitroff CJ, Lee JY, Schor KS, Sandmaier BM, Sackstein R. Differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J Biol Chem. 2001;276:47623–47631. doi: 10.1074/jbc.M105997200. [DOI] [PubMed] [Google Scholar]
- 2.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimitroff CJ, Lee JY, Fuhlbrigge RC, Sackstein R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc Natl Acad Sci USA. 2000;97:13841–13846. doi: 10.1073/pnas.250484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 6.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Invest Dermatol. 2004;122:1061–1069. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 7.Mazo IB, Gutierrez-Ramos JC, Frenette PS, et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama Y, Hidalgo A, Furie BC, et al. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg AW, Kerr WG, Hammer DA. Relationship between selectin-mediated rolling of hematopoietic stem and progenitor cells and progression in hematopoietic development. Blood. 2000;95:478–486. [PubMed] [Google Scholar]
- 10.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 11.Moore KL, Eaton SF, Lyons DE, et al. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- 12.Fuhlbrigge RC, King SL, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J Immunol. 2002;168:5645–5651. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- 13.Kieffer JD, Fuhlbrigge RC, Armerding D, et al. Neutrophils, monocytes, and dendritic cells express the same specialized form of PSGL-1 as do skin-homing memory T cells: cutaneous lymphocyte antigen. Biochem Biophys Res Commun. 2001;285:577–587. doi: 10.1006/bbrc.2001.5230. [DOI] [PubMed] [Google Scholar]
- 14.Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–3096. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo A, Frenette PS. Enforced fucosylation of neonatal CD34+ cells generates selectin ligands that enhance the initial interactions with microvessels but not homing to bone marrow. Blood. 2005;105:567–575. doi: 10.1182/blood-2004-03-1026. [DOI] [PubMed] [Google Scholar]
- 16.Borges E, Pendl G, Eytner R, et al. The binding of T cell-expressed P-selectin glycoprotein ligand-1 to E- and P-selectin is differentially regulated. J Biol Chem. 1997;272:28786–28792. doi: 10.1074/jbc.272.45.28786. [DOI] [PubMed] [Google Scholar]
- 17.Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 18.Robinson SN, Simmons PJ, Thomas MW, et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rgamma(null) mice. Exp Hematol. 2012;40:445–456. doi: 10.1016/j.exphem.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–3096. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- 20.Le MN, Palcic MM, Clarke JL, Davies D, Skacel PO. Developmental regulation of alpha 1,3-fucosyltransferase expression in CD34 positive progenitors and maturing myeloid cells isolated from normal human bone marrow. Glycobiology. 1997;7:357–365. doi: 10.1093/glycob/7.3.357. [DOI] [PubMed] [Google Scholar]
- 21.Maly P, Thall A, Petryniak B, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 22.Bengtson P, Larson C, Lundblad A, Larson G, Pahlsson P. Identification of a missense mutation (G329A;Arg(110)--> GLN) in the human FUT7 gene. J Biol Chem. 2001;276:31575–31582. doi: 10.1074/jbc.M104165200. [DOI] [PubMed] [Google Scholar]
- 23.Anderson H. Thesis dissertation. Finnish Red Cross Blood Service and Faculty of Biosciences, Department of Biological and Environmental Sciences, Division of Genetics, University of Helsinki; Finland: Feb 15, 2008. Transcriptome and Glycome Profiling of Human Hematopoietic CD133+ and CD34+ Cells. https://helda.helsinki.fi/bitstream/handle/10138/22267/transcri.pdf?sequence=1. [Google Scholar]
- 24.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 25.King MA, Covassin L, Brehm MA, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]