Abstract
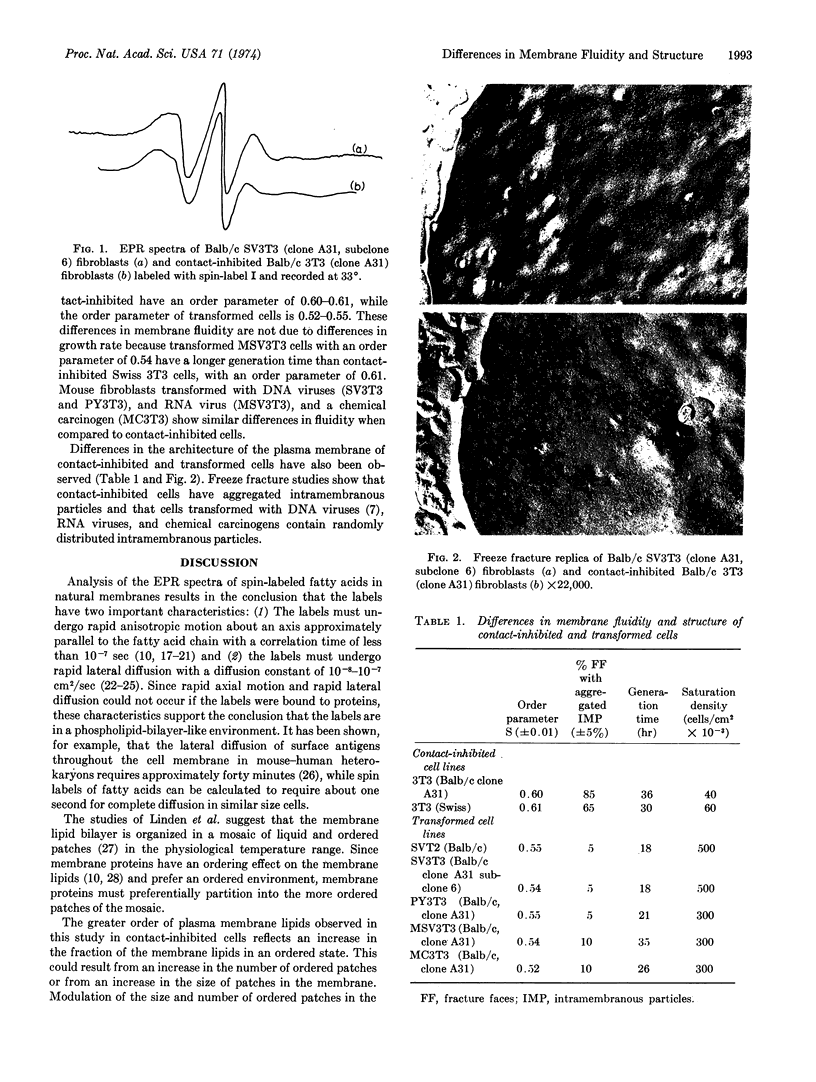
Studies on contact-inhibited mouse embryo fibroblast 3T3 cells and 3T3 cells transformed by oncogenic RNA and DNA viruses and by a chemical carcinogen have demonstrated differences in plasma membrane architecture. Spin-label and freeze-fracture ultrastructural studies have shown that contact-inhibited cells have ordered membrane lipids and aggregated intramembranous particles, whereas transformed cells have fluid membrane lipids and randomly distributed intramembranous particles. These findings suggest a model for how changes in the cell membrane may account for some of the characteristic differences observed between contact-inhibited and transformed cells.
Keywords: electron paramagnetic resonance, freeze fracture electron microscopy, viral transformation, cell proliferation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., AMBROSE E. J. The surface properties of cancer cells: a review. Cancer Res. 1962 Jun;22:525–548. [PubMed] [Google Scholar]
- AUB J. C., TIESLAU C., LANKESTER A. REACTIONS OF NORMAL AND TUMOR CELL SURFACES TO ENZYMES. I. WHEAT-GERM LIPASE AND ASSOCIATED MUCOPOLYSACCHARIDES. Proc Natl Acad Sci U S A. 1963 Oct;50:613–619. doi: 10.1073/pnas.50.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett R. E., Grisham C. M. Spin exchange of spin labeled probes in a natural membrane. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1362–1366. doi: 10.1016/0006-291x(72)90862-5. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Lanyi J. K. Structure of the lipid phase in cell envelope vesicles from Halobacterium cutirubrum. Biochemistry. 1973 May 8;12(10):1933–1939. doi: 10.1021/bi00734a016. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The role of lipid-phase transitions in the regulation of the (sodium + potassium) adenosine triphosphatase. Biochemistry. 1973 Jul 3;12(14):2635–2637. doi: 10.1021/bi00738a013. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Orientation and motion of amphiphilic spin labels in membranes. Proc Natl Acad Sci U S A. 1969 Sep;64(1):20–27. doi: 10.1073/pnas.64.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Canonico P. G., Caspary W. J. Electron spin resonance studies of spin-labeled mammalian cells by detection of surface-membrane signals. Proc Natl Acad Sci U S A. 1973 Jan;70(1):66–70. doi: 10.1073/pnas.70.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella C. J., Devaux P., McConnell H. M. Rapid lateral diffusion of phospholipids in rabbit sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2056–2060. doi: 10.1073/pnas.69.8.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Furcht L. T., Kersey J. H. Changes in membrane structure associated with cell contact. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3631–3635. doi: 10.1073/pnas.70.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J., Hasselbach W. A spin label study of sarcoplasmic vesicles. Eur J Biochem. 1971 Jul 15;21(1):17–21. doi: 10.1111/j.1432-1033.1971.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Venuta S., Rubin H. Sugar transport in normal and Rous sarcoma virus-transformed chick-embryo fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):653–657. doi: 10.1073/pnas.70.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]




