Abstract
Objective
To consolidate the evidence from randomized trials for the use of endovascular therapy (ET) in patients with acute ischemic stroke.
Methods
We searched major databases (MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus) from their inception to February 12, 2013, for randomized trials evaluating the efficacy of ET compared with standard of care for acute ischemic stroke. Pooled absolute and relative risk estimates were synthesized by using a random-effects model. Heterogeneity was assessed by using Q statistic and I2 statistic. Subset analysis was performed for patients with severe stroke (National Institutes of Health Stroke Scale score ≥20). The study was conducted from January 15, 2013 to April 30, 2013.
Results
Of the 1252 retrieved articles, 5 randomized trials enrolling 1197 patients with acute ischemic stroke were included. Seven hundred eleven patients received ET, and 486 received intravenous (IV) tissue plasminogen activator. There was no significant improvement in any of the outcomes in patients receiving ET compared with those receiving IV thrombolysis. On subgroup analysis, ET was found to have better outcomes in patients with severe stroke (National Institutes of Health Stroke Scale score ≥20), showing a dose-response gradient and improving excellent, good, and fair outcomes by an additional 4%, 7%, and 13%, respectively, compared with IV thrombolysis.
Conclusion
Overall, ET is not superior to IV thrombolysis for acute ischemic strokes (level B recommendation). However, ET showed promise and improved outcomes in patients with severe strokes, but the evidence is limited due to sample size. There is a need for further trials evaluating the role of ET in this high-risk group.
Stroke is a leading cause of long-term severe disability and the fourth leading cause of death in the United States. Cost of care, lost productivity, and premature mortality are high for stroke survivors (the estimated cost in the United States in 2008 was $34.3 billion).1 Intravenous (IV) thrombolysis with a recombinant tissue plasminogen activator (tPA) within the first 3 hours of stroke onset is the only therapy approved by the US Food and Drug Administration for acute ischemic stroke (AIS).2 However, recanalization rates with IV tPA are low (14% for internal carotid arteries and 55% for middle cerebral arteries), which led to the exploration of endovascular therapies (ETs) in AIS.3 The benefit of ET in AIS is not clear.4 A meta-analysis found no clear benefit of ET over IV tPA in patients with AIS, but the strength of evidence was limited because of the small sample size of the 2 trials.5 In the present study, we attempted to synthesize the available evidence by including 3 recently published randomized controlled trials (RCTs) of ET for AIS.6–8
The aim of our study was to perform a comprehensive systematic review and meta-analysis of all the published RCTs to compare the efficacy of ET (with or without IV tPA) with IV thrombolysis in patients with AIS by using different clinical outcomes (all-cause mortality, functional outcome, and symptomatic intracranial hemorrhage [sICH] rate).
METHODS
We followed the preferred reporting items for systematic reviews and meta-analyses guidelines to report our study findings.9 A protocol was designed a priori and was registered with PROSPERO.10
Eligibility Criteria
The study’s eligibility criteria were as follows: (1) RCT, (2) comparison of ET with IV throm-bolysis, (3) report of a risk estimate (relative risk, odds ratio, or data from which it could be calculated), and (4) report of the following outcomes: all-cause mortality, functional outcome measured by the modified Rankin Scale (mRS), and/or the sICH rate.
Data Sources and Search Strategies
A comprehensive search was conducted from major databases (Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus) from earliest inception to February 12, 2013, irrespective of any language barrier (Supplemental Appendix, available online at http://www.mayoclinicproceedings.org). The search strategy (controlled vocabulary supplemented with keywords) was designed and conducted by an experienced librarian (L.J.P.) with input from the study team. Conference proceedings of major neurology, neurosurgery, and stroke organizations were searched manually to identify relevant abstracts and potential articles. Content experts were queried, and references of potentially eligible articles were reviewed to identify all potentially eligible articles. In the case of missing data, corresponding authors were contacted for additional information. The study was conducted from January 15, 2013 to April 30, 2013.
Study Selection
Two investigators (B.S. and M.K.M.) independently and in duplicate screened all the abstracts and titles to identify potentially eligible articles for full-text review, and then reviewed the full text of selected articles independently to identify the studies meeting eligibility criteria. The interreviewer agreement was assessed by using Cohen weighted κ,11 and any disagreement was resolved with consensus in the presence of the third investigator (A.K.P.).
Data Collection
A predesigned data abstraction form was used by 2 investigators (B.S. and M.K.M.) to extract data in duplicate from the included studies. For each trial, we extracted the study characteristics (author, year, country, inclusion and exclusion criteria, baseline National Institutes of Health Stroke Scale [NIHSS] score, number of participantsdtotal and in each treatment armdand demographic characteristics of study participants), type of intervention, time to intervention, and outcome measures. Loss of follow-up and missing data were collected for quality assessment.
Outcome Measure
The mRS is a tool used to measure the poststroke functional outcome, with scores ranging from 0 to 6 (0, no symptoms at all; 1, no major disability; 2, slight disability; 3, moderate disability requiring some help but able to walk without assistance; 4, moderately severe disability; 5, severe disability; and 6, death). We defined 90-day mRS score of 1 or less as an excellent outcome, 2 or less as a good outcome, and 3 or less as a fair outcome.12
The primary outcome of interest for our study was improvement in the mRS score at 3 months. Secondary outcomes were all-cause mortality and the sICH rate.
Quality Assessment
The quality assessment of the included trials was evaluated by using the Jadad score.13 The Jadad score consists of 3 items: randomization (0–2 points), blinding (0–2 points), and dropouts and withdrawals (0–1 points). Response to each question is either “yes” (1 point) or “no” (0/−1). The score ranges from 0 to 5 points, with higher scores indicating better reporting. Studies with a Jadad score of 2 or less are considered of low quality, and those with a Jadad score of 3 or more are considered of high quality.14 The Cochrane Risk of Bias Tool was used to assess the quality of study methodology of eligible RCTs. Study quality was assessed independently and in duplicate by 2 investigators (B.S. and M.K.M.) following 7 criteria: (1) random sequence generation, (2) allocation concealment (selection bias), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessment (detection bias), (5) incomplete outcome data (attrition bias), (6) selective reporting (reporting bias), and (7) other bias.15 Time from stroke onset to recanalization has been described as an important confounding factor between recanalization and patient outcomes.16,17 Each study was evaluated for adjustment for time from stroke onset to recanalization. Response for each criterion was reported as low risk of bias, high risk of bias, and unclear risk of bias. Any disagreement was resolved with mutual consensus in the presence of the third investigator (A.K.P.).
Subgroup/Subset Analysis
An a priori sensitivity analysis was performed in patients with severe strokes (NIHSS score ≥20) because a favorable trend was seen for ET in this subgroup in the Interventional Management of Stroke (IMS) III trial.6 Further subgroup analyses of studies comparing ET with IV tPA were performed according to the country of origin (US vs non-US), duration of therapy from stroke onset (≤6 hours and >6 hours), and the study design (multicenter vs monocenter) to study the effect of different health care systems on patient outcome.
Levels of Evidence
We classified all the studies into class I to IV by using American Academy of Neurology’s (AAN’s) study classification scheme.18 We further used AAN’s classification of recommendations for the strength of study findings.
Statistical Analyses
Continuous variables were reported as means ± SD or medians with interquartile range, and categorical variables were reported as frequency and proportions. Risk estimate was presented by using risk ratios (RRs) with 95% CI, calculated by using the random Der-Simonian and Laird effects model.19 The heterogeneity among the studies was assessed by using the I2 statistic and the Cochran Q statistic for each outcome.20 A P value of less than .10 of the Cochran Q test suggests that the heterogeneity is beyond random error or chance.20 We calculated the absolute risk difference and number needed to treat with 95% CI for statistically significant outcomes. All other P values were considered significant for P<.05 except for subgroup analysis in which P<.0125 was considered significant after the Bonferroni correction for multiple comparisons. Statistical analyses were performed using Review Manager (RevMan) Version 5.1.
RESULTS
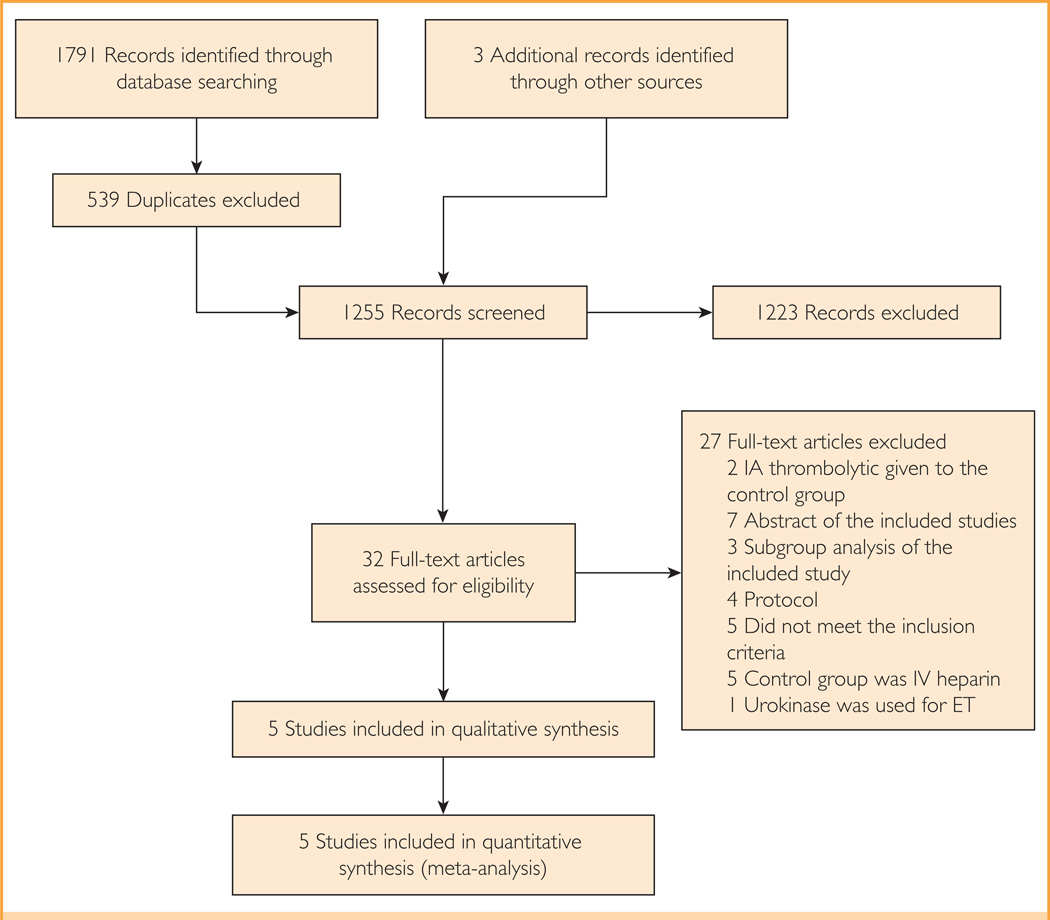
A total of 1252 unique records were identified through comprehensive database search, and 3 additional articles were identified from other sources (Figure 1).6–8 The interobserver agreement was excellent for initial screening of the titles and abstracts (κ 0.93; 95% CI, 0.86–0.99) and full-text review (κ 0.89; 95% CI, 0.68–1.10).
FIGURE 1.
The study flow diagram. ET = endovascular therapy; IV = intravenous.
The 5 studies meeting eligibility criteria included 6 study cohorts (the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy [MR-RESCUE] trial, reported by Kidwell et al,8 was divided into 2 cohorts: penumbra and nonpenumbra), comprising 1197 patients with AIS, of which 711 (59.4%) received ET with or without IV tPA and 486 (40.6%) received a control intervention (IV tPA).6–8,21,22 The study sample size ranged from 7 patients22 to 654 patients.6 The study characteristics are described in Table 1. In the MR-RESCUE trial,8 in which ET was compared with the standard of care, 43.8% of the patients received IV tPA in the ET arm and 29.6% received IV tPA in the control arm. The inclusion, exclusion, and interim analysis criteria are shown in Supplemental Table 1 (available online at http://www.mayoclinicproceedings.org). Two studies were discontinued before their completion.6,21 All studies except 1 had a treatment window of 6 hours or less.8
TABLE 1.
Characteristics of the Included RCTsa
| Reference, year |
Intervention (maximum dosage) |
No. of patients (male %) |
Age (y), mean ± SD or median (range) |
Primary outcome |
NIHSS score, mean/ median |
NIHSS score ≥20 |
Occlusion | Time from symptom onset to treatment (min), median/mean ± SD |
Recanalization (TICI) |
Quality of studiesb |
|---|---|---|---|---|---|---|---|---|---|---|
| Ciccone et al,7 2013 |
I = IA tPA (0.9 mg/kg)c + IV heparin ± MT |
181 (59) | 66±11 | mRS score ≤ 1 at 90 d | 13 | NA | Anterior and posterior circulation— complete data NA |
Median = 225 | NA | + |
| C = IV tPA (0.9 mg/kg) | 181 (57) | 67±11 | 13 | NA | Median = 165 | NA | ||||
| Broderick et al,6 2013 |
I = IVd + IA tPA (22 mg) + IV heparin ± MT |
434 (50) | 69 (23–89) | mRS score ≤2 at 90 d | 17 | 132 | M1 = 135, ICA = 65, single M2 = 61, multiple M2 = 22, basilar occlusion = 4 |
Mean time to IV tPA = 122±38, mean time to groin puncture = 208±47, mean time to IA therapy = 249±51 Mean =121 ±34 |
Grade 2b–3; ICA (38%), M1 (41%), single M2 (44%), multiple M2 (23%) |
+ |
| C = IV tPA (0.9 mg/kg) |
222 (55) | 68 (23–84) | 16 | 72 | NA |
|
NA | |||
| Kidwell et al,8 2013, penumbrae |
I = MT ± IA/IV tPAf(14 mg) |
34 (50) | 66±13 | Improvement in mRS score level |
16 | NA | ICA = 6, M1 = 18, M2 = 10 |
Mean time to groin puncture = 381 ±74 |
Grade 2a–3 at day 7 = 67% |
+ |
| C = standard medical care ± IV tPA |
34 (44) | 66±17 | 16 | NA | ICA = 5, M1 = 23, M2 = 6 |
NA | Grade 2a–3 at day 7 = 93% |
|||
| Kidwell et al,8 2013, nonpenumbra |
I = MT ± IA/IV tPA (14 mg) |
30 (43) | 62±12 | 19 | NA | ICA = 7, M1 = 21, M2 = 2 |
Mean time to groin puncture = 381 ±74 |
Grade 2a–3 at day 7 = 77% |
+ | |
| C = standard medical care ± IV tPA |
20 (60) | 69±16 | 21 | NA | ICA = 2, M1 = 16, M2 = 2 |
NA | Grade 2a–3 at day 7 = 78% |
|||
| Ciccone et al,21 2010 |
I = IA tPA (0.9 mg/kg)g + IV heparin ± MT |
29 (76) | 61 ±14 | mRS score ≤1 at 90 d | 17 | NA | Anterior and posterior circulation— complete data NA |
Median time to start of treatment = 195 |
NA | − |
| C = IV tPA (0.9 mg/kg) |
25 (75) | 64±12 | 16 | NA | Median time to start of treatment = 195 |
NA | ||||
| Sen et al,22 2009 |
I = IA tPA (22mg) | 3 | 68±16 |
|
16 | NA | M1 = 5, M2 = 1, terminal ICA = 1 |
Mean time to thrombolysis = 236 |
Grade 2a–3 = 3 | + |
| C = IV tPA (0.9 mg/kg ) |
4 | NA | Mean time to thrombolysis = 170 |
Grade 2a–3 = 0 | ||||||
AIS = acute ischemic stroke; C = control; I = intervention; IA = intra-arterial; ICA = internal carotid artery; IQR = interquartile range; IV = intravenous; LVO = large vessel occlusion; MCA = middle cerebral artery; MI = first segment of MCA (horizontal); M2 = second segment of MCA (insular); mRS = modified Rankin Scale; MT = mechanical thrombolysis; NA = not available; NIHSS = National Institutes of Health Stroke Scale; RCT = randomized controlled trial; sICH = symptomatic intracranial hemorrhage; TICI = thrombolysis in cerebral infarction; tPA = tissue plasminogen activator.
Represents quality of studies measured by the Jadad score; + = high quality; − = low quality.
Median tPA dose used = 40 mg (IQR, 20–50 mg).
From March 2006 to June 2011, two thirds of the total approved tPA dose was administered intravenously in the intervention arm (all patients were randomized within 40 min of starting IV tPA); after June 2011, the full dose of IV tPA was used even in the intervention arm. The mean dose of tPA received in the intervention arm = 60.3 ± 14.2 mg (IV tPA = 52.1 ± 12 and IA tPA = 13.3±6.7) and in the control arm = 72.5 ± 14.3 mg.
Penumbra was defined as predicted infarct core of ≤90 mL and a proportion of predicted infarct tissue within the at-risk region of ≤70%.
Mean IA tPA dose = 5.1 mg; overall, 44 (37%) patients received IV tPA.
Median IA tPA dose = 50 mg (IQR, 45–70 mg); median IV tPA dose in the control arm = 66.5 mg (IQR, 58–72 mg).
Quality Assessment
The quality assessment of the included trials using the Jadad score is shown in detail in Supplemental Table 2 (available online at http://www.mayoclinicproceedings.org), and the study quality, given as “high” or “low,” is shown in Table 1. The Supplemental Figure (available online at http://www.mayoclinicproceedings.org) shows the risk-of-bias summary. Performance bias was observed in all studies because none of the studies did a sham procedure in the control arm. Two studies (3 cohorts) had attrition bias,6,8 and 1 study had selective reporting bias.22 None of the studies adjusted the outcome for time from stroke onset to recanalization.16,17
Outcome
No significant difference was found in either primary or secondary outcome when ET (±IV tPA) was compared with IV tPA (Table 2). None to modest heterogeneity was noted for all outcomes (I2, 0% for mRS score ≤3, mortality, and sICH; I2, 1% for mRS score ≤1 and I2, 21% for mRS score ≤2, respectively). The subgroup analysis according to the study enrollment time (within 6 hours vs >6 hours), study location (US vs non-US), or setting (multicenter vs monocenter) did not reveal any difference in patient outcomes (Table 3). A post hoc sensitivity analysis was conducted according to the treatments used in control and intervention groups. We did not observe any difference in outcomes when we compared trials in which only IV tPA was used as the control group6,7,21,22 vs trials in which both IV tPA and standard of care were used as the control group.8 Similarly, no difference was observed in outcomes when trials using ET only (no IV tPA) in the intervention arm7,21,22 were compared with trials using IV tPA (for any number of patients) along with ET.6,8
TABLE 2.
Outcomes in Patients With Acute Ischemic Stroke Comparing ET vs IV tPA
| Outcome at 90 d | ET vs IV tPA | |||
|---|---|---|---|---|
| ET (n/N) | Control (n/N) | RR (95% CI) | I2 statistic (%) | |
| mRS score ≤1 | 194/685 | 135/478 | 1.02 (0.84–1.23) | 1 |
| mRS score ≤2 | 275/685 | 189/478 | 1.02 (0.84–1.24) | 21 |
| mRS score ≤3 | 397/685 | 270/478 | 1.03 (0.93–1.14) | 0 |
| Mortality | 127/707 | 84/490 | 0.98 (0.76–1.25) | 0 |
| sICH | 42/707 | 30/490 | 0.99 (0.62–1.58) | 0 |
ET = endovascular therapy; IV = intravenous; mRS = modified Rankin Scale; n/N = No. of patients with stroke/total No. of patients with outcome; RR = risk ratio; sICH = symptomatic intracranial hemorrhage; tPA = tissue plasminogen activator.
TABLE 3.
Subgroup Analysisa
| Subgroup analysis | No. of cohorts |
RR | 95% CI |
P value for difference between the subgroupsb |
|---|---|---|---|---|
| Mortality | ||||
| Duration of therapy from stroke onset | ||||
| ≤6 h | 4 | 1.07 | 0.75–1.50 | .39 |
| ≥6 h | 2 | 0.76 | 0.38–1.51 | |
| Study location | ||||
| US | 4 | 0.86 | 0.65–1.15 | .08 |
| Non-US | 2 | 1.43 | 0.87–2.36 | |
| Study setting | ||||
| Multicenter | 5 | 0.98 | 0.76–1.25 | NA |
| Monocenterc | 1 | NA | NA | |
| mRS score ≤1 | ||||
| Duration of therapy from stroke onset | ||||
| ≤6 h | 3 | 1.05 | 0.80–1.38 | .56 |
| >6 h | 2 | 0.73 | 0.23–2.36 | |
| Study location | ||||
| US | 3 | 1.06 | 0.82–1.38 | .85 |
| Non-US | 2 | 1.14 | 0.59–2.19 | |
| Study setting | ||||
| Multicenter | 5 | 1.02 | 0.84–1.23 | NA |
| Monocenterc | 1 | NA | NA | |
| mRS score ≤2 | ||||
| Duration of therapy from stroke onset | ||||
| ≤6 h | 3 | 1.06 | 0.83–1.35 | .38 |
| >6 h | 2 | 0.71 | 0.30–1.69 | |
| Study location | ||||
| US | 3 | 1.04 | 0.86–1.26 | 70 |
| Non-US | 2 | 1.19 | 0.61–2.32 | |
| Study setting | ||||
| Multicenter | 5 | 1.02 | 0.84–1.24 | NA |
| Monocenterc | 1 | NA | NA | |
| mRS score ≤3 | ||||
| Duration of therapy from stroke onset | ||||
| ≤6 h | 3 | 1.05 | 0.94–1.16 | 26 |
| >6 h | 2 | 0.78 | 0.48–1.28 | |
| Study location | ||||
| US | 3 | 1.03 | 0.90–1.18 | .88 |
| Non-US | 2 | 1.03 | 0.89–1.20 | |
| Study setting | ||||
| Multicenter | 5 | 1.03 | 0.93–1.14 | NA |
| Monocenterc | 1 | NA | NA | |
| sICH | ||||
| Duration of therapy from stroke onset | ||||
| ≤6 h | 4 | 0.96 | 0.62–1.58 | .63 |
| >6 h | 2 | 1.50 | 0.27–8.42 | |
| Study location | ||||
| US | 3 | 1.06 | 0.59–1.92 | 77 |
| Non-US | 3 | 0.89 | 0.42–1.89 | |
| Study setting | ||||
| Multicenter | 5 | 1.02 | 0.64–1.63 | .55 |
| Monocenterc | 1 | 0.42 | 0.02–7.71 | |
mRS = modified Rankin scale; NA = not applicable/available; RCT = randomized controlled trial; RR = risk ratio; sICH = symptomatic intracranial hemorrhage.
P<.10 implies that difference between the 2 subgroups is significant, and it may explain the heterogeneity observed in the overall analysis.
Sen et al22; in this RCT, no patient died and authors did not report the mRS score as an outcome.
Subset Analysis of Patients With Severe Strokes (NIHSS Score ≥20)
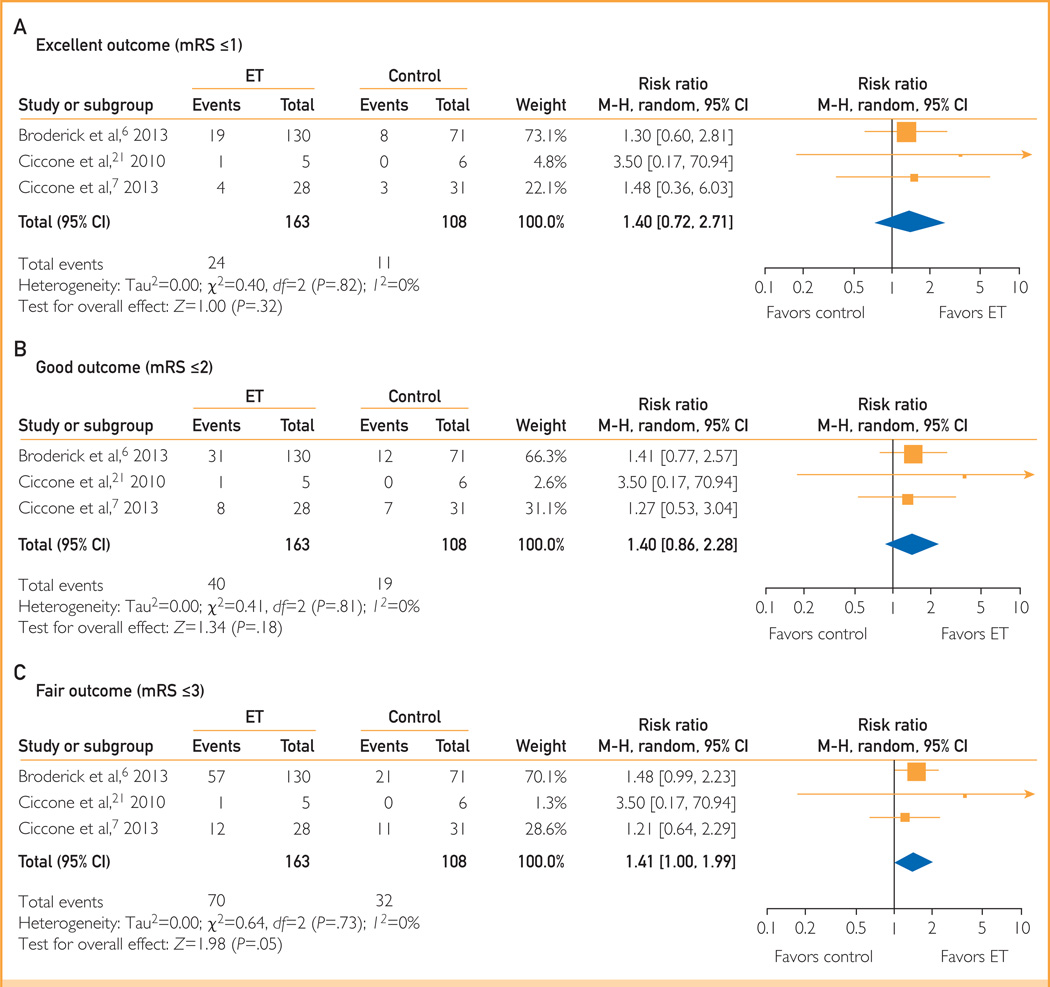
Only 1 study6 had reported outcome data for patients with severe AIS (NIHSS score ≥20) initially. Outcome data were received for 2 additional studies after contacting the corresponding authors.7,21 Of 1163 patients with AIS with reported primary outcome of improvement in the mRS score, 271 (23.3%) had severe stroke. Endovascular therapy was found to have better outcomes in patients with severe stroke (NIHSS score ≥20), with ET showing a dose-response gradient and improving excellent, good, and fair outcomes by an additional 4%, 7%, and 13%, respectively, compared with IV thrombolysis. Compared with IV tPA, ET had favorable fair (RR, 1.41; 95% CI, 1.00–1.99), good (RR, 1.40; 95% CI, 0.86–2.28), and excellent (RR, 1.40; 95% CI, 0.72–2.71) outcomes; however, the effect estimate did not reach statistical significance (Figure 2).
FIGURE 2.
Outcomes in patients with severe stroke. A, Excellent outcome (mRS score ≤1). B, Good outcome (mRS score ≤2). C, Fair outcome (mRS score ≤3). ET = endovascular therapy; M-H = Mantel-Haenszel; mRS = modified Rankin Scale.
Publication Bias
Publication bias could not be assessed because of the small number of studies (< 10 studies).23,24
DISCUSSION
This is the first meta-analysis that combined the results from all RCTs6–8,21,22 to date comparing ET (±IV tPA) to IV tPA. By using the AAN classification scheme for therapeutic questions,18 all 5 RCTs comparing ET with IV tPA6–8,21,22 were graded as class II (because of lack of adjustment for time from stroke onset to recanalization, a major confounder).17 On the basis of these 5 class II trials, we found that ET is not superior to IV tPA in improving mortality or functional outcome at 3 months (level B recommendation) with a similar rate of symptomatic hemorrhage (level B recommendation).
Time from stroke onset to intervention is an important factor in the management of patients with AIS, with a decline in favorable outcomes with an increase in picture to puncture time.17 The ideal enrollment time needs to be taken into account in future trials of ET vs IV tPA. The REcanalisation using Combined intravenous Alteplase and Neurointerventional ALgorithm for acute Ischemic StrokE (RECANALISE) study reported that recanalization in less than 210 minutes, in 210 to 260 minutes, and in more than 260 minutes was associated with 93%, 67%, and 37% good outcome (mRS score ≤2) at 90 days, respectively.16 The IMS-III trial reported the mean time from groin puncture to recanalization as 41 minutes.6 On combining the information from these 2 studies, we found that the ideal onset to groin puncture time should be less than 3 hours and the recanalization should be finished within 3.5 hours for maximum benefit and within 4.3 hours for moderate benefit. The outcome data for patients who were treated with ET and underwent recanalization within 3.5 hours were not available from any of the published trials. Subgroup analysis of the IMS-III trial6 and the Local Versus Systemic Thrombolysis for Acute Ischemic Stroke trial7 may help clarify the effect of time from stroke onset to recanalization on patient outcome to some extent; however, it would be limited by sample size. There is much interest in the new stent retrievers that have been reported to have recanalization rates of more than 80%.25,26 Even these studies reported time from onset to groin puncture as 4.7 to 5 hours with good outcome (mRS score ≤2 at 90 days) in only 37% to 40% of patients,25,26 which is similar to the 40% rate reported in Prolyse in Acute Cerebral Thromboembolism II (PROACT-II). This observation suggests that patient outcome is not entirely dependent on the type of ET (intra-arterial thrombolysis or mechanical devices) but that other factors such as time to ET and collateral circulation also play important roles. We need system-based research to evaluate and eliminate the factors causing delay in ET initiation.17
One of the identified causes of delay in ET is multimodal imaging. However, the true value of these imaging techniques is uncertain as evident from the MR-RESCUE trial, which found that penumbra-based ET does not make a difference in patient outcome.8 A retrospective study from 10 US centers reported that a computed tomography-based ET decision may shorten the stroke onset to groin puncture time to 2 hours.27 Immediate transfer of patients to the angiography suite under bridging therapy and use of stent retrievers may also shorten the picture to puncture time,28 and this approach may be explored in future trials. System-based research standardizing the processes to eliminate intercenter variability in multicenter trials may further help to improve time to recanalization.
Endovascular Therapy vs IV tPA for Patients With Severe Stroke (NIHSS Score ≥20)
Around one-fourth of the patients with AIS with reported functional outcomes had severe stroke. Patients with severe AIS (NIHSS score ≥20) had a higher probability of good and excellent outcomes when treated with ET compared with IV tPA; however, these findings did not achieve statistical significance secondary to smaller sample size. These results were based on the pooled estimate of 3 RCTs with small sample size (all class II studies)6,7,21; therefore, they need to be interpreted with caution. However, we observed an internal and external consistency in this subgroup that warrants further exploration in future clinical trials.
Strengths and Limitations
Our meta-analysis had various strengths. We did a comprehensive search of all the major databases and manually searched the abstracts and proceedings of major conferences to avoid selection bias. Authors were contacted for missing data to reduce the attrition bias. Subgroup analyses provided similar results to the overall analysis, explaining the robustness of our study. The majority of the trials were multi-centered, strengthening the generalizability of our meta-analysis findings. Our meta-analysis has a few limitations. There was variability in the definition of “time to therapy” among the studies. Definition of time to ET was not clear and could mean stroke onset to groin puncture, stroke onset to microcatheter placement, or stroke onset to recanalization. Owing to lack of reported data, we could not study the following associations: effect of recanalization on patient outcome, effect of time to recanalization on patient outcome, effect of anterior vs posterior circulation stroke on patient outcome, and effect of ET vs IV tPA on patients’ activities of daily life using the Barthel index. The results of our meta-analysis cannot be generalized to those patients with stroke who are younger than 18 years or older than 85 years.
CONCLUSION
This meta-analysis failed to show any superiority of ET over IV tPA for patients with AIS. Endovascular therapy may lead to a better outcome for patients with severe strokes (NIHSS score ≥20); however, these results should be interpreted with caution and need to be confirmed in a double-blind, large, multicenter RCT.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Alfonso Ciccone and Dr Akira Ogawa for providing us their unpublished data for our analysis.
Grant Support: This work was supported by CTSA grant UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Abbreviations and Acronyms
- AAN
American Academy of Neurology
- AIS
acute ischemic stroke
- ET
endovascular therapy
- IMS
Interventional Management of Stroke
- IV
intravenous
- mRS
modified Rankin Scale
- MR-RESCUE
Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy
- NIHSS
National Institute of Health Stroke Scale
- RCT
randomized controlled trial
- RR
risk ratio
- sICH
symptomatic intracranial hemorrhage
- tPA
tissue plasminogen activator
Footnotes
SUPPLEMENTAL ONLINE MATERIAL
Supplemental material can be found online at http://www.mayoclinicproceedings.org.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;25(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Rha J-H, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 4.Chimowitz MI. Endovascular treatment for acute ischemic stroke—still unproven. N Engl J Med. 2013;368(10):952–955. doi: 10.1056/NEJMe1215730. [DOI] [PubMed] [Google Scholar]
- 5.Nam J, Jing H, O’Reilly D. Intra-arterial thrombolysis vs. standard treatment or intravenous thrombolysis in adults with acute ischemic stroke: a systematic review and meta-analysis [published online ahead of print January 7, 2013] [Accessed August 28, 2013];Int J Stroke. doi: 10.1111/j.1747-4949.2012.00914.x. http://dx.doi.org/10.1111/j.1747-4949.2012.00914.x. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368(10):914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.University of York, Center for Reviews and Dissemination. [Accessed August 2013];PROSPERO: International prospective register of systematic reviews. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013003838.
- 11.Cohen JA. Coefficient of agreement for nominal scales educational and psychological measurement. Educ Psychol Meas. 1960;20(1):37–46. [Google Scholar]
- 12.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135(11):982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 15.JPT Higgins, S Green., editors. The Cochrane Collaboration. [Accessed August 28, 2013];Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011] 2011 http://www.cochrane-handbook.org.
- 16.Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8(9):802–809. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 17.Sun CH, Nogueira RG, Glenn BA, et al. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127(10):1139–1148. doi: 10.1161/CIRCULATIONAHA.112.000506. [DOI] [PubMed] [Google Scholar]
- 18.Gross RA, Johnston KC. Levels of evidence: taking neurology to the next level. Neurology. 2009;72(1):8–10. doi: 10.1212/01.wnl.0000342200.58823.6a. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciccone A, Valvassori L, Ponzio M, et al. Intra-arterial or intravenous thrombolysis for acute ischemic stroke? the SYNTHESIS pilot trial. J Neurointerv Surg. 2010;2(1):74–79. doi: 10.1136/jnis.2009.001388. [DOI] [PubMed] [Google Scholar]
- 22.Sen S, Huang DY, Akhavan O, Wilson S, Verro P, Solander S. IV vs. IA TPA in acute ischemic stroke with CT angiographic evidence of major vessel occlusion: a feasibility study. Neurocrit Care. 2009;11(1):76–81. doi: 10.1007/s12028-009-9204-1. [DOI] [PubMed] [Google Scholar]
- 23.Nordic Cochrane Centre, Cochrane Collaboration. Copenhagen: Nordic Cochrane Centre, Cochrane Collaboration. Review Manager (RevMan). 5.1. 2011 [Google Scholar]
- 24.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 25.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 27.Sheth KN, Terry JB, Nogueira RG, et al. Advanced modality imaging evaluation in acute ischemic stroke may lead to delayed endovascular reperfusion therapy without improvement in clinical outcomes. J Neurointerv Surg. 2013;5(suppl 1):i62–i65. doi: 10.1136/neurintsurg-2012-010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broussalis E, Trinka E, Hitzl W, Wallner A, Chroust V, Killer-Oberpfalzer M. Comparison of stent-retriever devices versus the Merci retriever for endovascular treatment of acute stroke. Am J Neuroradiol. 2013;34(2):366–372. doi: 10.3174/ajnr.A3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.