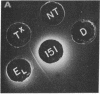
Abstract
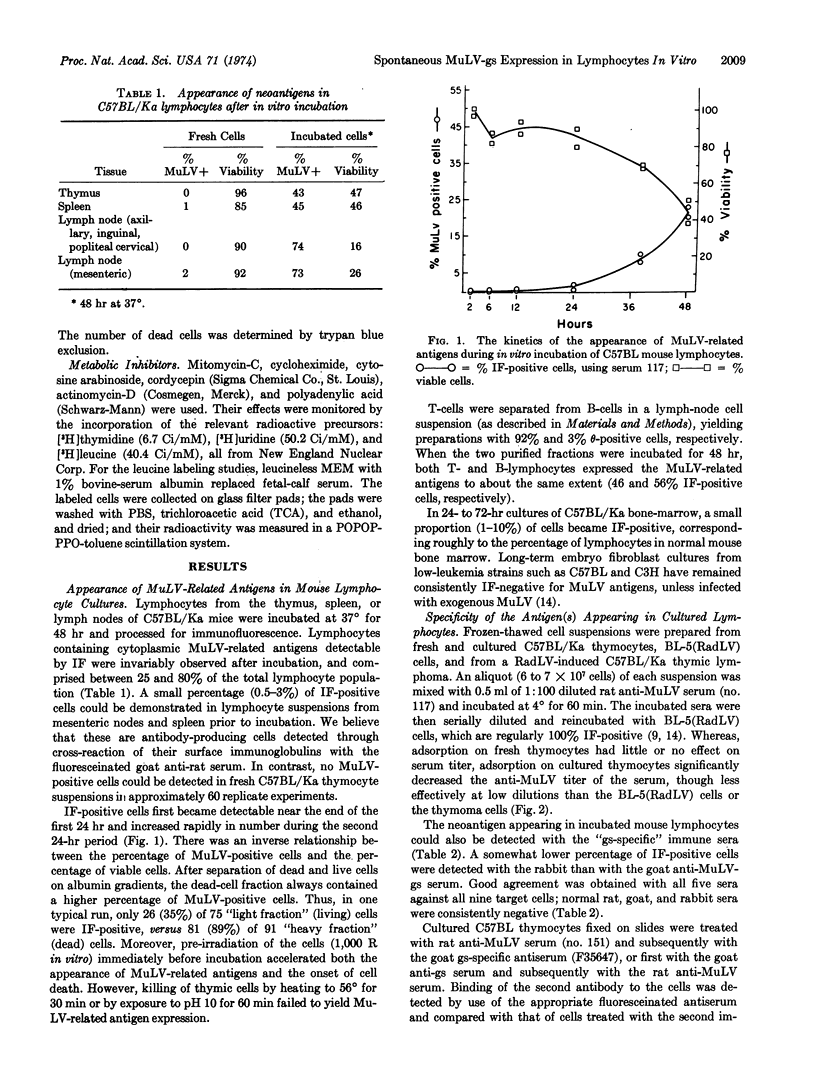
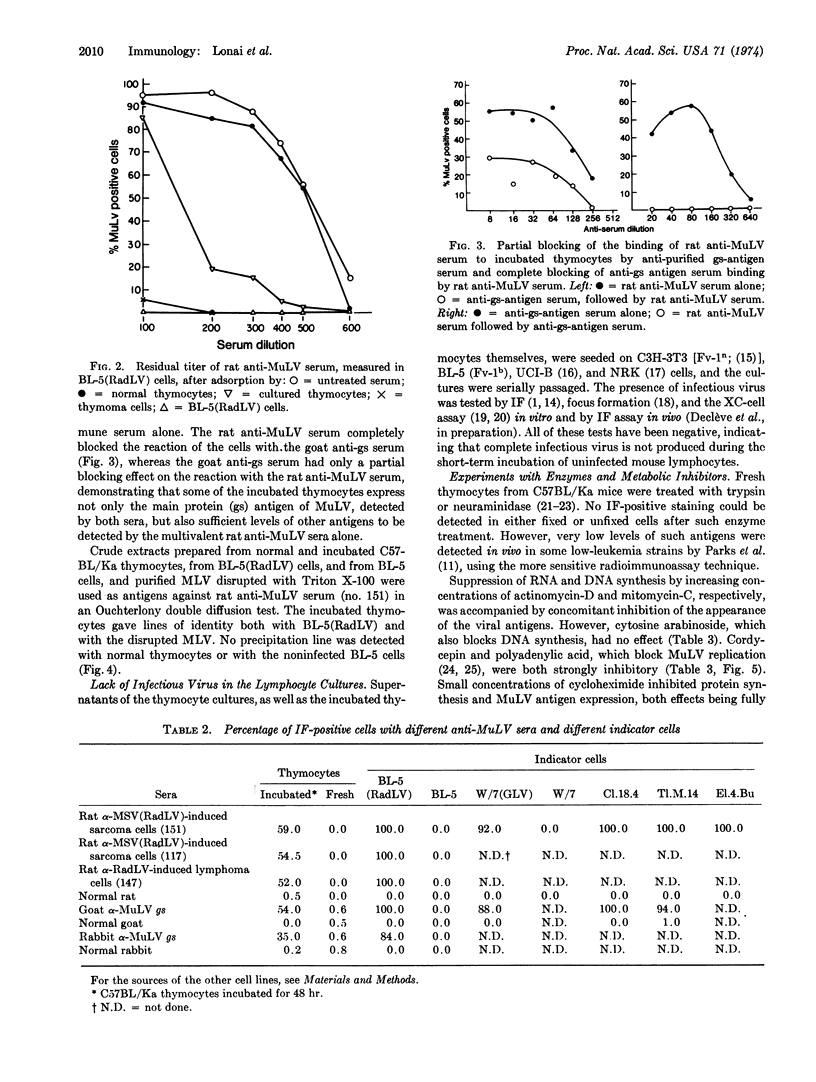
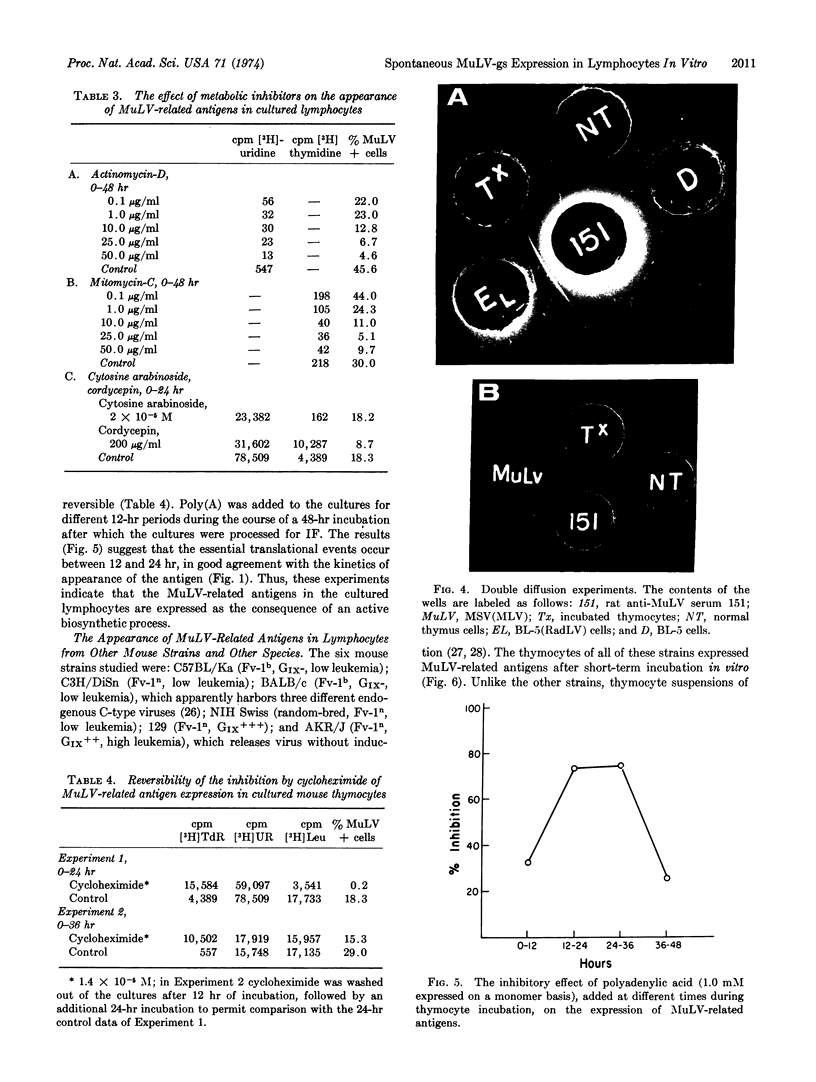
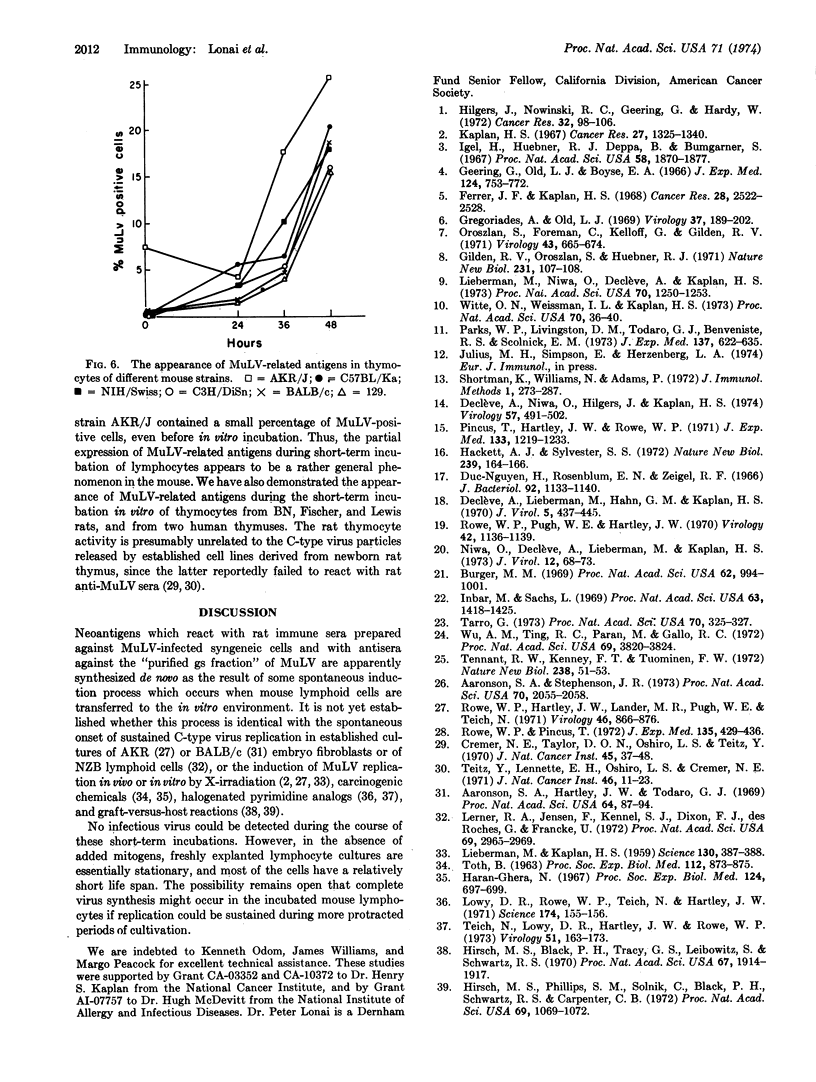
Short-term lymphocyte cultures from mouse thymus, spleen, or lymph nodes were studied for the presence of murine leukemia virus group-specific antigens with an immunofluorescence test using rat immune sera against syngeneic cells infected with the radiation leukemia virus or its pseudotype of murine sarcoma virus and goat and rabbit antisera against purified murine leukemia virus group-specific antigen. Antigens reacting with these sera appeared in the cultured lymphocytes within 24 hr, and the proportion of immunofluorescent-positive cells increased to 25-80% by the second or third day of cultivation, concomitantly with a decrease in cell viability. The appearance of these antigens can be suppressed by inhibitors of DNA (mitomycin-C), RNA (actinomycin-D, cordycepin, and polyadenylic acid), and protein (cycloheximide) synthesis. No infectious virus could be detected by the immunofluorescence and XC-cell tests. The observed phenomenon appears to represent the spontaneous partial derepression of endogenous murine leukemia virus replication in lymphocytes during short-term in vitro cultivation.
Keywords: group-specific antigen, immunofluorescence, murine sarcoma cells, murine leukemia virus
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer N. E., Taylor D. O., Oshiro L. S., Teitz Y. Transformation and virus production in normal rat thymus cells and those infected with Moloney leukemia virus. J Natl Cancer Inst. 1970 Jul;45(1):37–48. [PubMed] [Google Scholar]
- Declève A., Lieberman M., Hahn G. M., Kaplan H. S. Focus formation by a murine sarcoma leukemia virus complex. II. Quantitative aspects of the interaction between radiation leukemia virus and its murine sarcoma virus pseudotype in strain C57BL mouse embryo cells. J Virol. 1970 Apr;5(4):437–445. doi: 10.1128/jvi.5.4.437-445.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Niwa O., Hilgers J., Kaplan H. S. An improved murine leukemia virus immunofluorescence assay. Virology. 1974 Feb;57(2):491–502. doi: 10.1016/0042-6822(74)90188-3. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Kaplan H. S. Antigenic characteristics of lymphomas induced by radiation leukemia virus (RadLV) in mice and rats. Cancer Res. 1968 Dec;28(12):2522–2528. [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S., Huebner R. J. Coexistence of intraspecies and interspecies specific antigenic determinants on the major structural polypeptide of mammalian C-type viruses. Nat New Biol. 1971 May 26;231(21):107–108. doi: 10.1038/newbio231107a0. [DOI] [PubMed] [Google Scholar]
- Gregoriades A., Old L. J. Isolation and some characteristics of a group-specific antigen of the murine leukemia viruses. Virology. 1969 Feb;37(2):189–202. doi: 10.1016/0042-6822(69)90198-6. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Sylvester S. S. Cell line derived from Balb-3T3 that is transformed by murine leukaemia virus: a focus assay for leukaemia virus. Nat New Biol. 1972 Oct 11;239(93):164–166. doi: 10.1038/newbio239164a0. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N. A leukemogenic filtrable agent from chemically-induced lymphoid leukemia in C57BL mice. Proc Soc Exp Biol Med. 1967 Mar;124(3):697–699. doi: 10.3181/00379727-124-31827. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Nowinski R. C., Geering G., Hardy W. Detection of avian and mammalian oncogenic RNA viruses (oncornaviruses) by immunofluorescence. Cancer Res. 1972 Jan;32(1):98–106. [PubMed] [Google Scholar]
- Hirsch M. S., Black P. H., Tracy G. S., Leibowitz S., Schwartz R. S. Leukemia virus activation in chronic allogeneic disease. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1914–1917. doi: 10.1073/pnas.67.4.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Phillips S. M., Solnik C., Black P. H., Schwartz R. S., Carpenter C. B. Activation of leukemia viruses by graft-versus-host and mixed lymphocyte reactions in vitro. Proc Natl Acad Sci U S A. 1972 May;69(5):1069–1072. doi: 10.1073/pnas.69.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igel H., Huebner R. J., Deppa B., Bumgarner S. Rescue of the defective murine sarcoma virus genome by radiation-induced leukemia virus from C57BL mice. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1870–1877. doi: 10.1073/pnas.58.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Jensen F., Kennel S. J., Dixon F. J., Des Roches G., Francke U. Karyotypic, virologic, and immunologic analyses of two continuous lymphocyte lines established from New Zealand black mice: possible relationship of chromosomal mosaicism to autoimmunity. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2965–2969. doi: 10.1073/pnas.69.10.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Niwa O., Declève A., Kaplan H. S. Continuous propagation of radiation leukemia virus on a C57BL mouse-embryo fibroblast line, with attenuation of leukemogenic activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1250–1253. doi: 10.1073/pnas.70.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Niwa O., Decléve A., Liberman M., Kaplan H. S. Adaptation of plaque assay methods to the in vitro quantitation of the radiation leukemia virus. J Virol. 1973 Jul;12(1):68–73. doi: 10.1128/jvi.12.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Foreman C., Kelloff G., Gilden R. V. The group-specific antigen and other structural proteins of hamster and mouse C-type viruses. Virology. 1971 Mar;43(3):665–674. doi: 10.1016/0042-6822(71)90290-x. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Livingston D. M., Todaro G. J., Benveniste R. E., Scolnick E. M. Radioimmunoassay of mammalian type C viral proteins. 3. Detection of viral antigen in normal murine cells and tissues. J Exp Med. 1973 Mar 1;137(3):622–635. doi: 10.1084/jem.137.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Shortman K., Williams N., Adams P. The separation of different cell classes from lymphoid organs. V. Simple procedures for the removal of cell debris. Damaged cells and erythroid cells from lymphoid cell suspensions. J Immunol Methods. 1972 May;1(3):273–287. doi: 10.1016/0022-1759(72)90005-1. [DOI] [PubMed] [Google Scholar]
- TOTH B. Development of malignant lymphomas by cell-free filtrates prepared from a chemically induced mouse lymphoma. Proc Soc Exp Biol Med. 1963 Apr;112:873–875. doi: 10.3181/00379727-112-28195. [DOI] [PubMed] [Google Scholar]
- Tarro G. Appearance in trypsinized normal cells of reactivity with antibody presumably specific for malignant cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):325–327. doi: 10.1073/pnas.70.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich N., Lowy D. R., Hartley J. W., Rowe W. P. Studies of the mechanism of induction of infectious murine leukemia virus from AKR mouse embryo cell lines by 5-iododeoxyuridine and 5-bromodeoxyuridine. Virology. 1973 Jan;51(1):163–173. doi: 10.1016/0042-6822(73)90376-0. [DOI] [PubMed] [Google Scholar]
- Teitz Y., Lennette E. H., Oshiro L. S., Cremer N. E. Release of C-type particles from normal rat thymus cultures and those infected with Moloney leukemia virus. J Natl Cancer Inst. 1971 Jan;46(1):11–23. [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L., Kaplan H. S. Structural characteristics of some murine RNA tumor viruses studied by lactoperoxidase iodination. Proc Natl Acad Sci U S A. 1973 Jan;70(1):36–40. doi: 10.1073/pnas.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Paran M., Gallo R. C. Cordycepin inhibits induction of murine leukovirus production by 5-iodo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3820–3824. doi: 10.1073/pnas.69.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]