Abstract
E. coli cells treated with the bifunctional crosslinking reagents dimethyl malonimidate, succinimidate, adipimidate, suberimidate, and sebacinimidate served for the isolation of rod-shaped “ghosts.” These ghosts proved to be crosslinked over their entire surface; i.e., a macromolecule (resistant to boiling 1% Na dodecyl sulfate) the size of the cell had been created. Also, ghosts could similarly be crosslinked. In both cases, the final “sacs” contained about 60-70% protein, and very little or no lipopolysaccharide. When ghosts from which phospholipid had been removed were crosslinked, the covalently closed ghosts were almost pure protein; 80-90% of their dry mass was accounted for by protein. Ammonolysis of the crosslinked material (whether stemming from crosslinked cells or ghosts) showed that the same four proteins (Na dodecyl sulfate gel bands) had been crosslinked that are found in normally prepared ghosts. These observations practically exclude the hypothesis that a fluid mosaic model of membrane structure can be applied to the outer membrane of the E. coli cell envelope; rather, extensive protein-protein interactions must exist over the whole surface of this membrane. These findings are consistent with the possibility that the ghost polypeptide chains are involved in the determination of cellular shape.
Keywords: envelope proteins, long-range protein-protein interaction
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Green D. E. Membrane proteins and membrane structure. FEBS Lett. 1972 Sep 15;25(2):205–209. doi: 10.1016/0014-5793(72)80486-1. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
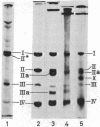
- Henning U., Höhn B., Sonntag I. Cell envelope and shape of Escherichia coli K12. The ghost membrane. Eur J Biochem. 1973 Nov 1;39(1):27–36. doi: 10.1111/j.1432-1033.1973.tb03099.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Braun V., Höhn B. Cell envelope and shape of Escherichia coli K12. Properties of a temperature-sensitive rod mutant. Eur J Biochem. 1972 Apr 24;26(4):570–586. doi: 10.1111/j.1432-1033.1972.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldow C., Robertson J., Rothfield L. Purification of bacterial membrane proteins. The use of guanidinium thiocyanate and urea. J Membr Biol. 1972;10(2):137–152. doi: 10.1007/BF01867850. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. II. Heterogeneity of major outer membrane polypeptides. Arch Biochem Biophys. 1973 Aug;157(2):553–560. doi: 10.1016/0003-9861(73)90674-7. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]