Abstract
Improvements in ex vivo generation of enucleated red blood cells are being sought for erythroid biology research, toward the ultimate goal of erythrocyte engineering for clinical use. Based upon the high levels of iron-saturated transferrin in plasma serum, it was hypothesized that terminal differentiation in serum-free media may be highly dependent on the concentration of iron. Here adult human CD34+ cells were cultured in a serum-free medium containing dosed levels of iron-saturated transferrin (holo-Tf, 0.1–1.0 mg/ml). Iron in the culture medium was reduced, but not depleted, with erythroblast differentiation into haemoglobinized cells. At the lowest holo-Tf dose (0.1 mg/ml), terminal differentiation was significantly reduced and the majority of the cells underwent apoptotic death. Cell survival, differentiation and enucleation were enhanced as the holo-Tf dose increased. These data suggest that adequate holo-Tf dosing is critical for terminal differentiation and enucleation of human erythroblasts generated ex vivo in serum-free culture conditions. Published 2013. This article is a US Government work and is in the public domain in the USA.
Keywords: erythropoiesis, serum-free media, holotransferrin, haemoglobin, enucleation, iron
1. Introduction
Erythropoiesis involves the commitment of immature haematopoietic stem cells into erythroblasts, which further differentiate into red blood cells. The maturation process is marked by changes in virtually all aspects of the cells’ metabolism. Erythropoiesis in adult humans, in vivo, occurs almost exclusively in the bone marrow. The erythroid niche of the marrow is composed of cell islands, consisting of a central macrophage encompassed by erythroblasts. The macrophage assists with erythropoiesis in several roles to enhance the differentiation process (An and Mohandas, 2011). Among the proposed functions, macrophages are thought to endocytose the extruded nuclei and provide nutrients, such as iron, to the surrounding erythroblasts. Iron is also supplied by holotransferrin in the blood. Enucleation occurs after the final cell division, where the intracellular organelles undergo autophagy and red blood cells (erythrocytes) are generated. Human red blood cells then circulate in the blood, with a typical half-life of around 50 days.
Although the erythroblastic island niche is a fundamental feature of in vivo erythropoiesis, this process can be replicated ex vivo. The cells are able to differentiate in cultured clot (Lutton et al., 1984), semi-solid (Metcalf, 1981) or liquid (Gartner and Kaplan, 1980) media. Fibach et al. (1989) further advanced the use of liquid suspension cultures of erythroblasts using primary human haematopoietic cells. This innovative technique suggested distinct proliferative advantages for utilizing liquid culture (Fibach et al., 1991). The establishment of a two-phase liquid culture model allowed for the optimization of erythroblast differentiation among mixed cell populations. CD34+ cells from the human host may be purified prior to culture initiation (Wojda et al., 2002), and cultured using defined cytokine mixtures (Drouet et al., 1989; Douay et al., 1994; Leberbauer et al., 2005; Neildez-Nguyen et al., 2002; Miharada et al., 2006). Monitoring of erythroblast differentiation is accomplished in real time using flow cytometry (Miller et al., 1999).
Recently, culture methods have progressed to include enucleation of the cells in the absence of semisolid media or macrophages (Freyssinier et al., 1999; Griffiths et al., 2012; Miharada et al., 2006; Hebiguchi et al., 2008). For this purpose, cytokine combinations including stem cell factor (SCF), thrombopoietin (TPO), granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), interleukin 6 (IL-6), insulin-like growth factor 1 (IGF-1), insulin-like growth factor 2 (IGF-II), vascular endothelial growth factor (VEGF) and feline multicentric sarcoma (fms)-related tyrosine kinase 3 (FLT-3 ligand) are titrated in multi-stage strategies (Neildez-Nguyen et al., 2002; Miharada et al., 2006; Hebiguchi et al., 2008; Griffiths et al., 2012; Giarratana et al., 2005, 2011; Malik et al., 1998; Leberbauer et al., 2005; Freyssinier et al., 1999). The addition of mifepristone at the final stage increases erythroblast enucleation in the presence of human serum (Miharada et al., 2006). Folic acid and vitamin B12 may also advance enucleation frequency (Hebiguchi et al., 2008). Human CD34+ cord blood erythroid progenitors grown in serum-free medium may also be infused into mice to produce erythrocytes (Neildez-Nguyen et al., 2002). Giarratana et al. (2011) described a three-phase culture system containing human plasma that generates cells suitable for transfusion in humans.
Despite the major advances in producing enucleated erythroid cells ex vivo, improvements are still being sought for terminal maturation and enucleation of erythroblasts in the absence of animal or human serum (Timmins et al., 2011). Based upon the large amount of holotransferrin in serum when compared to serum-free medium, we hypothesized that adequate iron dosing in the culture medium may be essential for the growth or terminal differentiation of erythroblasts that are cultured in the absence of serum, macrophages or stromal cells. The plasma of adult humans contains 50–150 μg/dl iron. In the setting of iron deficiency, lower plasma iron concentrations are detected and the iron availability in the bone marrow is suboptimal for erythropoiesis. Here we demonstrate that the iron requirements for ex vivo erythropoiesis are similar to those found in the human host. Low iron concentrations in culture result in maturation arrest and a nearly complete absence of enucleation. Once the holotransferrin levels are titrated to levels found comparable to that in adult human serum the differentiation and enucleation levels improve significantly.
2. Materials and methods
2.1. Cell culture
All studies using primary erythroblasts were performed after human subjects review and NIH IRB approval and donor informed consent was obtained. The 21 day ex vivo culture system consisted of two phases. In phase I (culture days 1–7) CD34+ cells were placed in medium containing StemPro-34 complete medium (L-glutamine, pen–strep and StemPro-34 nutrient supplement; Invitrogen, Carlsbad, CA, USA) supplemented with 50 ng/ml SCF (HumanZyme, Chicago, IL, USA), 50 ng/ml FLT3-ligand (HumanZyme) and 10 ng/ml IL-3 (HumanZyme). After 7 days, the cells were transferred at a density of 5 × 104 cells/ml to erythropoietin (EPO; Amgen)-supplemented medium (phase 2; culture days 7–21), which is comprised of the following: StemPro-34 complete medium, 4 U/ml EPO, 3 μM mifepristone (Sigma-Aldrich, St. Louis, MO, USA), 10 μg/ml insulin (Sigma-Aldrich) and dosed titrations of holo-Tf (1.0, 0.8, 0.6, 0.4, 0.2 and 0.1 mg/ml; Sigma-Aldrich) were balanced by transferrin that was not bound to iron (apo-Tf; Sigma-Aldrich) to maintain total transferrin levels of 1.2 mg/ml in all cultures.
2.2. Flow-cytometry analysis
Immunostaining with transferrin receptor (CD71) and glycophorin A (GPA) antibodies (Invitrogen) were performed using the BD FACSAria I flow cytometer (BD Biosciences, San Jose, CA, USA) (Tanno et al., 2009). Positively stained populations of cells were defined by fluorescence of more than two standard deviations (SDs) above the unstained cells. A minimum of 5000 live cell events was recorded. Apoptosis was analysed by flow cytometry, using the Annexin V:PE Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer’s instructions. Cells were also analysed for their RNA content by staining with thiazole orange (Sigma-Aldrich) (Ervasti et al., 2007).
2.3. Cytospin preparation
For morphology studies, cytospins were prepared for each transferrin concentration by centrifugation of the cytoslides at 1000 rpm for 2 min (Thermo Electron Corp., Rockford, IL, USA), followed by staining with Wright–Giemsa reagent (Sigma-Aldrich).
2.4. Iron and transferrin analysis
On culture day 21, 5 ml samples of culture medium from all holo-Tf concentrations were centrifuged at 1000 rpm for 10 min at room temperature. After centrifugation, the supernatants were removed and filtered through a 0.45 μm filter unit. After filtration, the supernatants were analysed on a Dimension Vista 1500 Intelligent Lab System (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). The instrument detection ranges included iron (5–10 000 μg/dl) and transferrin (35–560 mg/dl). Freshly prepared culture media with dosed transferrin titrations were used for comparison.
2.5. Statistical analyses
All experiments were performed on three separate donors and compared using paired two-tailed t-tests.
3. Results and discussion
For these studies, mobilized adult human CD34+ cells were cultured using a two-phase serum-free culture technique. During the first phase, which lasted 7 days, a cytokine cocktail (SCF, FLT-3 ligand, IL-3) was added to the serum-free medium (StemPro-34) for proliferation of the haematopoietic progenitor cells. Holo-Tf (0.1 mg/ml) was present in the phase I culture medium. The addition of a higher concentration of holo-Tf during the first culture phase did not significantly affect cellular proliferation or differentiation (data not shown). In the second culture phase (culture days 7–21), EPO, insulin and mifepristone were added to the base medium to promote erythroblast proliferation and differentiation. Holo-Tf titrations in the range of 0.1–1.0 mg/ml were balanced with apo-Tf to maintain equivalent total transferrin concentrations of 1.2 mg/ml. The holo-Tf doses of 0.1–1.0 mg/ml correspond to 20–120 μg/dl iron. Adult blood normally contains 70–190 μg/dl iron (Hoyer, 1944). Therefore, the holo-Tf titration provided iron to the cultured cells at concentrations ranging from iron-deficient (0.1, 0.2 and 0.4 mg/ml) to physiological levels (0.6, 0.8 and 1.0 mg/ml) in human blood.
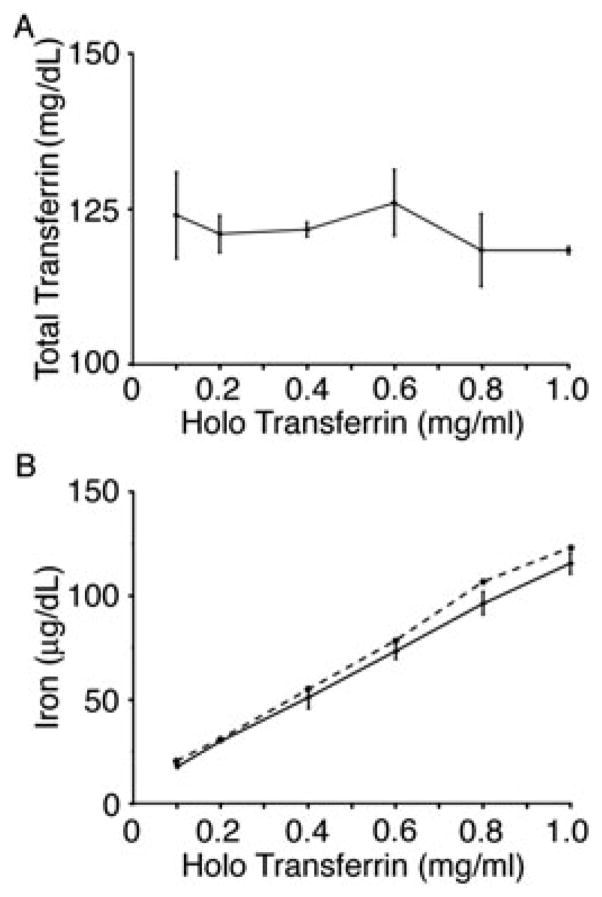
During erythropoiesis in vivo, iron is utilized for cellular proliferation (Gkouvatsos et al., 2012) and haemoglobin synthesis (Shintani et al., 1994). Based upon the amount of haemoglobin iron in erythrocytes (ca. 109 iron molecules/cell), robust iron requirements for ex vivo erythropoiesis were expected. In erythroblasts and other cells, iron is delivered to the cells in its ferric form bound to transferrin. The endocytosed transferrin is recycled back into the extracellular space after delivery of iron (Beaumont and Delaby, 2009). Iron utilization and transferrin recycling were estimated by comparing transferrin and iron levels in fresh and culture day 21 culture media (defined here as ‘conditioned medium’). The iron dose in the culture medium was adjusted by increasing the proportion of holo-Tf, and apo-Tf was used to maintain total transferrin levels at 120 mg/dl for all iron doses. As expected, transferrin levels remained stable over the culture period, with measured levels of 118–126 mg/dl in the conditioned medium (Figure 1A). In contrast to transferrin, the concentration of iron in conditioned medium was decreased when compared to the freshly-prepared medium (Figure 1B). At the lower holo-Tf concentrations (0.1–0.4 mg/ml) the medium was not fully iron-depleted; a relatively minor amount of iron was consumed from the medium at all iron doses. With higher iron concentrations, the difference in iron content between fresh and conditioned culture media was increased. These results suggest that reduced utilization, rather than depletion of iron, occurs at the lower concentrations of holo-Tf.
Figure 1.
Analysis of iron and transferrin. (A) Representative graph of the total amount of transferrin present in culture day 21 conditioned media at the following concentrations: 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml holo-Tf. (B) Representative graph of the amount of iron utilized during the 14 day EPO culture conditioned media (solid line) coinciding with the amount of iron present in the freshly-prepared media (dashed line). Bars represent the standard deviation of the mean values from three separate donors
Cell counts at the end of the culture period (culture day 21) were determined for quantitation of the effects of holo-Tf doses upon cell growth. At the beginning of phase 2 (culture day 7), cell concentrations were adjusted to 5 × 104 cells/ml for all experiments. As shown in Figure 2A, the final cell concentrations were significantly affected according to the holo-Tf dose in the conditioned medium. At iron concentration 1.0 mg/ml there was a 47-fold increase in cells. By comparison, the cell growth was significantly lower as measured by a two-fold increase in culture days 7–21 (phase 2). Based upon the reduced proliferation of 0.1–0.2 mg/ml holo-Tf culture conditions, apoptosis was investigated on culture day 21 by detection of phosphatidylserine on the plasma membrane surface (PS+ cells). Remarkably, 78% of the cells expressed this apoptotic marker at the lowest dose of holo-Tf, 0.1 mg/ml (Figure 2B). At 0.4–1.0mg/ml holo-Tf, a significant reduction in the percentage of PS+ cells was observed. At the highest holo-Tf dose, < 5% PS+ cells were detected. When combined with the cell counts, these results suggest that erythroblast proliferation and survival in EPO-containing medium are highly dependent upon the iron concentration in the culture medium. The low level proliferation combined with high levels of apoptosis suggest that erythroblasts are negatively affected by the lower concentrations of holo-Tf contained in commercial preparations of serum-free medium (usually 0.1 mg/ml), which are normally sufficient to support the growth of other cell types.
Figure 2.
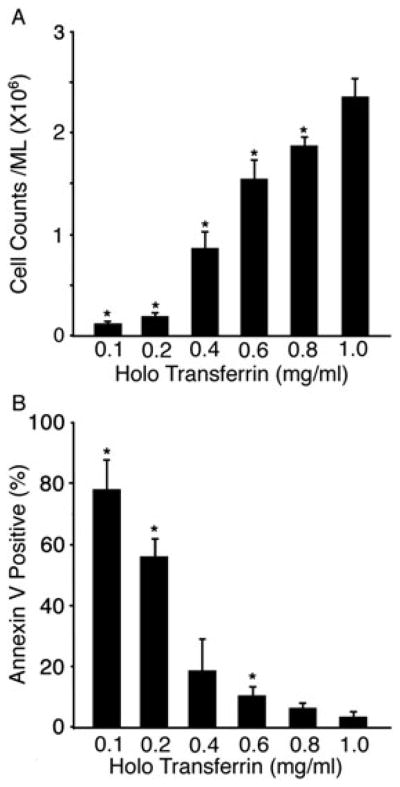
Iron effects upon cell growth and survival. (A) The proliferation of CD34+ cells over a 14 day EPO culture conditioned media period with transferrin titrations at 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml. (B) The level of phosphatidylserine on the surface of the live cell population at culture day 21. Bars represent the standard deviation of the mean values from three separate donors with asterisks signifying p ≤ 0.05 when compared to the 1.0 mg/ml dose
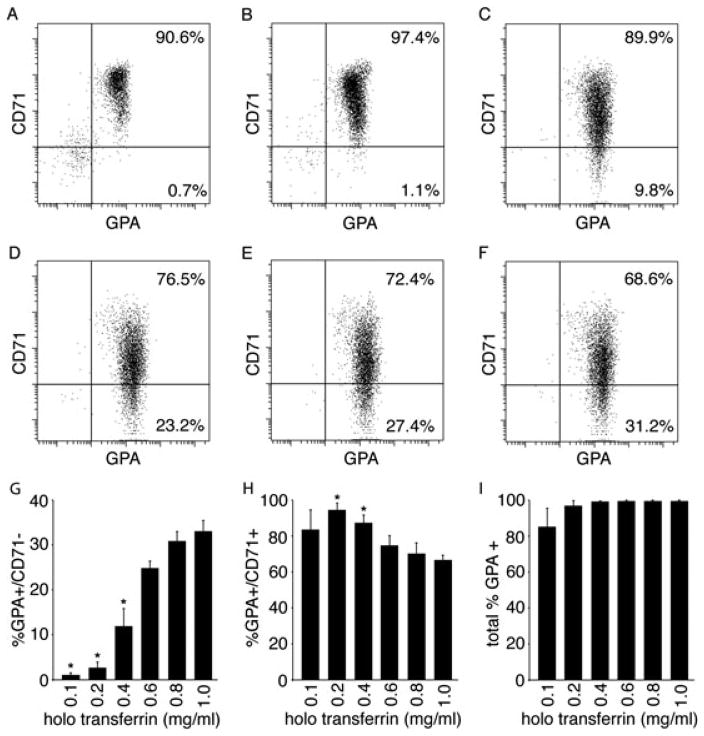
Flow-cytometry studies were performed to determine whether the holo-Tf doses resulted in differences in erythroblast maturation. Human erythropoiesis is characterized by the expression of CD71 and CD235 (GPA, glycophorin A) on the cell surface (Loken et al., 1987). GPA is not detected prior to the pro-erythroblast stage of differentiation. Thereafter, GPA expression is maintained on the more mature cells, including enucleated erythrocytes. In cultured erythroblasts, GPA+ expression does not occur prior to the second culture phase, when erythropoietin is added to the medium. Expression of CD71 increases in response to erythropoietin, but CD71 is lost with terminal maturation (Okumura et al., 1992). CD71 is not present on the surface of mature enucleated erythrocytes (Dertinger et al., 2002). As shown in Figure 3, GPA expression was achieved at all holo-Tf concentrations. However, the loss of CD71 was highly dependent upon the holo-Tf dose. While there was some loss of CD71 among the cellular population at the lower iron concentrations, only a small percentage of these cells matured (GPA+/CD71−; 0.7% and 1.1% for 0.1 mg/ml and 0.2 mg/ml holo-Tf, respectively). The higher holo-Tf concentrations had a dose effect on the increase in the percentage of mature cells that were detected (9.8–31.2% for 0.4–1.0 mg/ml holo-Tf). Hence, lower iron concentrations in the culture medium permit the cells to reach the stage of maturation at which glycophorin A was expressed (pro-erythroblast), but further maturation of the cells was reduced or inhibited. In triplicate experiments, a statistically significant decrease in maturation (GPA+/CD71− phenotype) with a relative increase in less mature erythroblasts (GPA+/CD71+ phenotype) was measured at the holo-Tf dose of 0.4 mg/ml or less (cf. Figure 3G–I).
Figure 3.
The effects of a dosed iron titration on cell maturation. Representative flow cytometric dot plots of the differentiation of CD34+ cells cultured in dosed iron titration media at (A) 0.1, (B) 0.2, (C) 0.4, (D) 0.6, (E) 0.8, and (F) 1.0 mg/ml holo-Tf for transferrin receptor (CD71) and glycophorin A (GPA) at culture day 21. Using cells from three separate donors, average percent values for GPA +/CD71−, GPA+/CD71+, total GPA + are shown in panels G, H, I respectively. Bars represent the standard deviation of the mean values from three separate donors with asterisks signifying p ≤0.05 when compared to the 1.0 mg/ml dose
Cytospin preparations were performed 7 and 14 days after the addition of EPO (culture days 14 and 21, respectively). On culture day 14, the predominant population at all iron concentrations was the pro-erythroblast stage (data not shown). By day 21, cytospin preparation demonstrated maturation of the erythroblasts, with enucleated cells being identified at all iron concentrations. Thiazole orange was utilized to quantitate the percentage of enucleation at each holo-Tf dose. Since thiazole orange stains both DNA and RNA, the absence of thiazole orange staining indicates enucleation and terminal maturation of the cells (Lee et al., 1986). The percentage of thiazole orange-negative cells was significantly reduced in the lower iron concentrations (0.1–0.2 mg/ml), with <5% of the cells maturing to red blood cells (Figure 4A). In contrast, as the iron concentrations increased from 0.4 to 1.0 mg/ml, there was a subsequent increase in enucleated cells, which also correlated with the loss of CD71. These results are consistent with the loss of CD71 at similar levels demonstrated in Figure 3. For comparison, enucleated cells cultured in iron concentrations of 0.1 and 1.0 mg/ml were sorted based on the thiazole orange-negative population, indicative of mature red cells. After sorting, the cells were stained with Wright–Giemsa and the 0.1 mg/ml holo-Tf cells were hypochromic (Figure 4B) when compared to the enucleated population sorted from the 1.0 mg/ml holo-Tf cultures (Figure 4C). An adult pattern of haemoglobin production was detected at all concentrations (data not shown).
Figure 4.
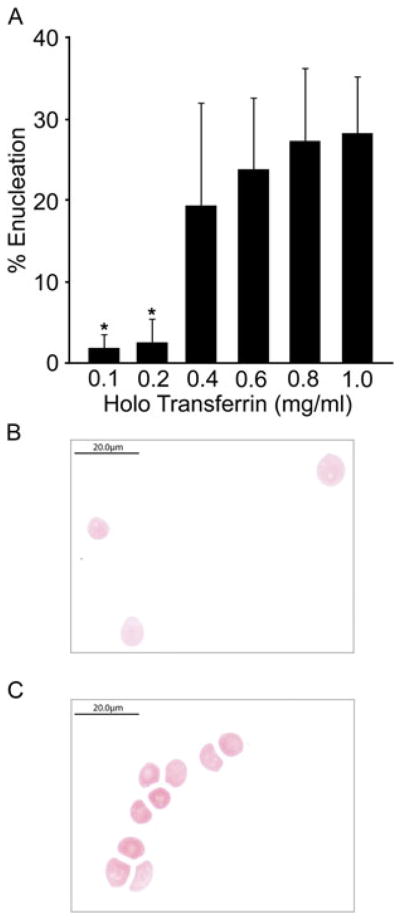
Quantitation of enucleated cells. (A) Thiazole orange analyses were performed on culture day 21 cells from 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml holo-Tf with the representative percentage of enucleated cells. Bars represent the standard deviation of the mean values from three separate donors with asterisks signifying p ≤0.05 when compared to the 1.0 mg/ml dose. (B) Cytospin preparation of culture day 21 thiazole orange negative sorted cells of 0.1 mg/ml followed by Wright-Giemsa staining. Scale bar, 20 μm.(C) Cytospin preparation of culture day 21 thiazole orange negative sorted cells of 1.0 mg/ml holo-Tf followed by Wright-Giemsa staining. Scale bar, 20 μm
Ideally, the erythroid niche in bone marrow should be mimicked to create optimal conditions for the growth, differentiation and enucleation of cultured erythroblasts. Modified strategies may be needed when embryonic stem cells are utilized as the source of erythroblasts (Lu et al., 2008). Additional culture phases may improve erythroblast growth and differentiation (Anstee et al., 2012; Giarratana et al., 2011). Co-culture of the erythroblasts with non-erythroid cells demonstrably increases the percentage of enucleated cells to >50% (Fujimi et al., 2008; Lu et al., 2008). Despite the increases in enucleation with dosed holo-Tf added to the culture medium, the peak enucleation levels achieved here remained around 30%. Therefore, further improvements of the serum-free culture technique were sought to increase the enucleation frequency. Of note, additional holo-Tf (final concentration 1.2 mg/ml) did not result in further improvements in growth, apoptosis or enucleation beyond that achieved at the 1.0 mg/ml concentration (data not shown). While not studied here, ferrous or ferric salts have been utilized in addition to transferrin-bound iron to supplement erythroblast culture medium (Neildez-Nguyen et al., 2002), but the effects upon growth or enucleation were not reported. Achievement of enucleation for >50% of cultured erythroblasts using simple, inexpensive approaches that do not require serum or mixed cell cultures remains as a future challenge.
In summary, similar to the clinical setting of iron deficiency, comparatively low concentrations of holo-Tf were not sufficient to fully support erythropoiesis ex vivo. Decreased iron utilization, rather than depletion of iron in the medium, was detected at those low iron doses. Cell growth, survival and terminal differentiation were all diminished significantly by lowering the holo-Tf levels in the culture medium below physiological levels. Adequate amounts of iron are critical for the production of culture-generated erythrocytes in serum-free medium.
Acknowledgments
The Intramural Research Programmes of the National Institute of Diabetes and Digestive and Kidney Diseases supported this study.
Footnotes
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- An X, Mohandas N. ; Erythroblastic islands, terminal erythroid differentiation and reticulocyte maturation. Int J Hematol. 2011;93:139–143. doi: 10.1007/s12185-011-0779-x. [DOI] [PubMed] [Google Scholar]
- Anstee DJ, Gampel A, Toye AM. ; Ex vivo generation of human red cells for transfusion. Curr Opin Hematol. 2012;19:163–169. doi: 10.1097/MOH.0b013e328352240a. [DOI] [PubMed] [Google Scholar]
- Beaumont C, Delaby C. ; Recycling iron in normal and pathological states. Semin Hematol. 2009;46:328–338. doi: 10.1053/j.seminhematol.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Dertinger SD, Torous DK, Hall NE, et al. ; Enumeration of micronucleated CD71-positive human reticulocytes with a single-laser flow cytometer. Mutat Res. 2002;515:3–14. doi: 10.1016/s1383-5718(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Douay L, Giarratana MC, Mary JY, et al. ; Interleukin 2 interacts with myeloid growth factors in serum-free long-term bone marrow culture. Br J Haematol. 1994;86:475–482. doi: 10.1111/j.1365-2141.1994.tb04776.x. [DOI] [PubMed] [Google Scholar]
- Drouet X, Douay L, Giarratana MC, et al. ; Human liquid bone marrow culture in serum-free medium. Br J Haematol. 1989;73:143–147. doi: 10.1111/j.1365-2141.1989.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Ervasti M, Matinlauri I, Punnonen K. ; Quantitative flow cytometric analysis of transferrin receptor expression on reticulocytes. Clin Chim Acta. 2007;383:153–157. doi: 10.1016/j.cca.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Fibach E, Manor D, Oppenheim A, et al. ; Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989;73:100–103. [PubMed] [Google Scholar]
- Fibach E, Manor D, Treves A, et al. ; Growth of human normal erythroid progenitors in liquid culture: a comparison with colony growth in semisolid culture. Int J Cell Cloning. 1991;9:57–64. doi: 10.1002/stem.5530090108. [DOI] [PubMed] [Google Scholar]
- Freyssinier JM, Lecoq-Lafon C, Amsellem S, et al. ; Purification, amplification and characterization of a population of human erythroid progenitors. Br J Haematol. 1999;106:912–922. doi: 10.1046/j.1365-2141.1999.01639.x. [DOI] [PubMed] [Google Scholar]
- Fujimi A, Matsunaga T, Kobune M, et al. ; Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int J Hematol. 2008;87:339–350. doi: 10.1007/s12185-008-0062-y. [DOI] [PubMed] [Google Scholar]
- Gartner S, Kaplan HS. ; Long-term culture of human bone marrow cells. Proc Natl Acad Sci USA. 1980;77:4756–4759. doi: 10.1073/pnas.77.8.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarratana MC, Kobari L, Lapillonne H, et al. ; Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- Giarratana MC, Rouard H, Dumont A, et al. ; Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkouvatsos K, Papanikolaou G, Pantopoulos K. ; Regulation of iron transport and the role of transferrin. Biochim Biophys Acta. 2012;1820:188–202. doi: 10.1016/j.bbagen.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Griffiths RE, Kupzig S, Cogan N, et al. ; Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis. Blood. 2012;119:6296–6306. doi: 10.1182/blood-2011-09-376475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebiguchi M, Hirokawa M, Guo YM, et al. ; Dynamics of human erythroblast enucleation. Int J Hematol. 2008;88:498–507. doi: 10.1007/s12185-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Hoyer K. ; Physiologic Variations in the iron content of human blood serum. Acta Med Scand. 1944;119:562–576. [Google Scholar]
- Leberbauer C, Boulmé F, Unfried G, et al. ; Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105:85–94. doi: 10.1182/blood-2004-03-1002. [DOI] [PubMed] [Google Scholar]
- Lee LG, Chen CH, Chiu LA. ; Thiazole orange: a new dye for reticulocyte analysis. Cytometry. 1986;7:508–517. doi: 10.1002/cyto.990070603. [DOI] [PubMed] [Google Scholar]
- Loken MR, Shah VO, Dattilio KL, et al. Flow cytometric analysis of human bone marrow: I. Normal erythroid development. Blood. 1987;69:255–263. [PubMed] [Google Scholar]
- Lutton JD, Ibraham NG, Hoffman R, et al. ; Sideroblastic anemia: differences in bone marrow erythroid colony (CFUE) growth responses to erythropoietin in plasma clot and methylcellulose cultures. Am J Hematol. 1984;16:219–226. doi: 10.1002/ajh.2830160303. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Feng Q, Park JS, et al. ; Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik P, Fisher TC, Barsky LL, et al. ; An in vitro model of human red blood cell production from hematopoietic progenitor cells. Blood. 1998;91:2664–2671. [PubMed] [Google Scholar]
- Metcalf D. ; Control of hemopoietic cell proliferation and differentiation. Prog Clin Biol Res. 1981;66:473–486. [PubMed] [Google Scholar]
- Miharada K, Hiroyama T, Sudo K, et al. ; Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- Miller JL, Njoroge JM, Gubin AN, et al. ; Prospective identification of erythroid elements in cultured peripheral blood. Exp Hematol. 1999;27:624–629. doi: 10.1016/s0301-472x(98)00086-1. [DOI] [PubMed] [Google Scholar]
- Neildez-Nguyen TM, Wajcman H, Marden MC, et al. ; Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- Okumura N, Tsuji K, Nakahata T. ; Changes in cell surface antigen expressions during proliferation and differentiation of human erythroid progenitors. Blood. 1992;80:642–650. [PubMed] [Google Scholar]
- Shintani N, Kohgo Y, Kato J, et al. ; Expression and extracellular release of transferrin receptors during peripheral erythroid progenitor cell differentiation in liquid culture. Blood. 1994;83:1209–1215. [PubMed] [Google Scholar]
- Tanno T, Porayette P, Sripichai O, et al. ; Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins NE, Athanasas S, Günther M, et al. ; Ultra-high-yield manufacture of red blood cells from hematopoietic stem cells. Tissue Eng Part C Methods. 2011;17:1131–1137. doi: 10.1089/ten.TEC.2011.0207. [DOI] [PubMed] [Google Scholar]
- Wojda U, Noel P, Miller JL. ; Fetal and adult hemoglobin production during adult erythropoiesis: coordinate expression correlates with cell proliferation. Blood. 2002;99:3005–3013. [PubMed] [Google Scholar]